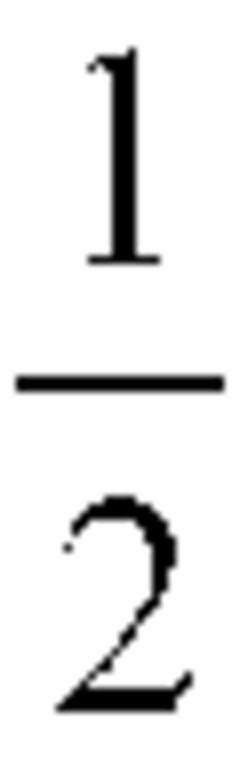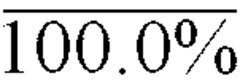Sodium Acetate: Pioneering the Path in Chemical Stability
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Acetate Evolution
Sodium acetate has undergone a remarkable evolution since its discovery in the early 19th century. Initially recognized as a byproduct of various chemical processes, it gradually gained prominence as a versatile compound with numerous applications. The timeline of sodium acetate's development can be traced through several key stages, each marked by significant advancements in understanding its properties and potential uses.
In the early stages, sodium acetate was primarily utilized in textile manufacturing as a mordant for dyeing and printing fabrics. This application laid the foundation for further exploration of its chemical properties. As analytical techniques improved in the mid-20th century, researchers began to uncover the unique characteristics of sodium acetate, particularly its ability to form supersaturated solutions and undergo rapid crystallization.
The 1960s and 1970s saw a surge in research focused on sodium acetate's thermal properties. Scientists discovered its exceptional heat storage capacity, leading to the development of sodium acetate trihydrate as a phase change material. This breakthrough opened up new avenues for applications in thermal energy storage and temperature regulation systems.
The late 20th century marked a significant shift in sodium acetate's evolution, as its potential in food preservation and flavor enhancement became apparent. Food scientists explored its use as a acidity regulator and preservative, leading to its widespread adoption in the food industry. Concurrently, the pharmaceutical sector began investigating sodium acetate's role in buffering solutions and as a potential therapeutic agent.
In recent decades, the focus has shifted towards enhancing the stability and performance of sodium acetate in various applications. Advanced manufacturing techniques have been developed to produce high-purity sodium acetate with consistent properties. Researchers have also explored novel formulations and composites to improve its functionality in specific applications, such as extended-release heat packs and advanced cooling systems.
The evolution of sodium acetate has been characterized by continuous innovation and cross-disciplinary research. From its humble beginnings as a chemical curiosity to its current status as a multi-functional compound, sodium acetate has demonstrated remarkable versatility. Recent developments have focused on sustainable production methods and exploring its potential in emerging fields such as nanotechnology and advanced materials science.
As we look to the future, the evolution of sodium acetate continues to unfold. Ongoing research is exploring its potential in cutting-edge applications, including smart materials, energy storage solutions, and biomedical technologies. The journey of sodium acetate serves as a testament to the power of scientific inquiry and the potential for seemingly simple compounds to revolutionize multiple industries.
In the early stages, sodium acetate was primarily utilized in textile manufacturing as a mordant for dyeing and printing fabrics. This application laid the foundation for further exploration of its chemical properties. As analytical techniques improved in the mid-20th century, researchers began to uncover the unique characteristics of sodium acetate, particularly its ability to form supersaturated solutions and undergo rapid crystallization.
The 1960s and 1970s saw a surge in research focused on sodium acetate's thermal properties. Scientists discovered its exceptional heat storage capacity, leading to the development of sodium acetate trihydrate as a phase change material. This breakthrough opened up new avenues for applications in thermal energy storage and temperature regulation systems.
The late 20th century marked a significant shift in sodium acetate's evolution, as its potential in food preservation and flavor enhancement became apparent. Food scientists explored its use as a acidity regulator and preservative, leading to its widespread adoption in the food industry. Concurrently, the pharmaceutical sector began investigating sodium acetate's role in buffering solutions and as a potential therapeutic agent.
In recent decades, the focus has shifted towards enhancing the stability and performance of sodium acetate in various applications. Advanced manufacturing techniques have been developed to produce high-purity sodium acetate with consistent properties. Researchers have also explored novel formulations and composites to improve its functionality in specific applications, such as extended-release heat packs and advanced cooling systems.
The evolution of sodium acetate has been characterized by continuous innovation and cross-disciplinary research. From its humble beginnings as a chemical curiosity to its current status as a multi-functional compound, sodium acetate has demonstrated remarkable versatility. Recent developments have focused on sustainable production methods and exploring its potential in emerging fields such as nanotechnology and advanced materials science.
As we look to the future, the evolution of sodium acetate continues to unfold. Ongoing research is exploring its potential in cutting-edge applications, including smart materials, energy storage solutions, and biomedical technologies. The journey of sodium acetate serves as a testament to the power of scientific inquiry and the potential for seemingly simple compounds to revolutionize multiple industries.
Market Demand Analysis
The market demand for sodium acetate has been steadily growing, driven by its versatile applications across various industries. In the food and beverage sector, sodium acetate serves as a crucial preservative and flavor enhancer, particularly in snack foods, baked goods, and dairy products. The increasing consumer preference for convenience foods and extended shelf life has bolstered the demand for this chemical compound.
The pharmaceutical industry represents another significant market for sodium acetate. Its use as a buffering agent in intravenous fluids and dialysis solutions has become indispensable in medical settings. The global expansion of healthcare services and the rising prevalence of chronic diseases requiring frequent medical interventions have contributed to the sustained demand in this sector.
In the textile industry, sodium acetate plays a vital role in dyeing processes, acting as a pH regulator and dye-fixing agent. The growth of the fashion industry, coupled with the increasing demand for high-quality, durable textiles, has further propelled the market for sodium acetate in this sector.
The chemical industry utilizes sodium acetate in various processes, including as a catalyst in organic synthesis and as a raw material for producing other acetate compounds. The ongoing innovation in chemical manufacturing and the development of new materials have maintained a steady demand for sodium acetate in this sector.
Environmental applications of sodium acetate, particularly its use as a de-icing agent for roads and runways, have gained traction in regions prone to harsh winters. The shift towards more environmentally friendly de-icing solutions has opened up new market opportunities for sodium acetate-based products.
The global sodium acetate market is expected to experience continued growth in the coming years. Factors such as urbanization, industrialization, and the expansion of end-use industries in emerging economies are likely to drive this growth. Additionally, the increasing focus on sustainable and bio-based chemicals may create new avenues for sodium acetate production and application.
However, the market also faces challenges, including price volatility of raw materials and stringent regulations on chemical usage in certain applications. Manufacturers are responding by investing in research and development to improve production efficiency and explore new applications, ensuring the long-term stability and growth of the sodium acetate market.
The pharmaceutical industry represents another significant market for sodium acetate. Its use as a buffering agent in intravenous fluids and dialysis solutions has become indispensable in medical settings. The global expansion of healthcare services and the rising prevalence of chronic diseases requiring frequent medical interventions have contributed to the sustained demand in this sector.
In the textile industry, sodium acetate plays a vital role in dyeing processes, acting as a pH regulator and dye-fixing agent. The growth of the fashion industry, coupled with the increasing demand for high-quality, durable textiles, has further propelled the market for sodium acetate in this sector.
The chemical industry utilizes sodium acetate in various processes, including as a catalyst in organic synthesis and as a raw material for producing other acetate compounds. The ongoing innovation in chemical manufacturing and the development of new materials have maintained a steady demand for sodium acetate in this sector.
Environmental applications of sodium acetate, particularly its use as a de-icing agent for roads and runways, have gained traction in regions prone to harsh winters. The shift towards more environmentally friendly de-icing solutions has opened up new market opportunities for sodium acetate-based products.
The global sodium acetate market is expected to experience continued growth in the coming years. Factors such as urbanization, industrialization, and the expansion of end-use industries in emerging economies are likely to drive this growth. Additionally, the increasing focus on sustainable and bio-based chemicals may create new avenues for sodium acetate production and application.
However, the market also faces challenges, including price volatility of raw materials and stringent regulations on chemical usage in certain applications. Manufacturers are responding by investing in research and development to improve production efficiency and explore new applications, ensuring the long-term stability and growth of the sodium acetate market.
Technical Challenges
Sodium acetate, despite its widespread use and seemingly simple chemical structure, presents several significant technical challenges in its production, storage, and application. One of the primary issues is maintaining its chemical stability under varying environmental conditions. The hygroscopic nature of sodium acetate makes it susceptible to moisture absorption, which can lead to clumping and degradation of the product. This necessitates the development of advanced packaging and storage solutions to preserve its integrity.
Another challenge lies in the purification process of sodium acetate. Achieving high levels of purity, essential for many industrial and pharmaceutical applications, requires sophisticated separation techniques. Impurities, even in trace amounts, can significantly affect the performance and safety of sodium acetate in critical applications. The development of cost-effective and efficient purification methods remains an ongoing challenge for manufacturers.
The production of sodium acetate also faces environmental concerns. Traditional manufacturing processes often involve the use of petrochemical-derived acetic acid, raising sustainability issues. The industry is under pressure to develop greener production methods, such as utilizing bio-based acetic acid or exploring alternative synthesis routes that reduce carbon footprint and minimize waste generation.
In terms of application, sodium acetate's use as a phase change material for thermal energy storage presents unique technical hurdles. While its high latent heat of fusion makes it an attractive candidate for heat storage applications, issues such as supercooling and phase segregation during repeated melting-freezing cycles need to be addressed. Engineers are working on developing additives and encapsulation techniques to enhance the reliability and efficiency of sodium acetate-based thermal energy storage systems.
The use of sodium acetate in food preservation and as a flavoring agent also faces regulatory challenges. Ensuring compliance with evolving food safety standards across different regions requires continuous research and development efforts. Additionally, there is a growing demand for natural and clean-label ingredients, pushing the industry to explore bio-based production methods for sodium acetate that can meet these consumer preferences without compromising on functionality or cost-effectiveness.
Lastly, the scaling up of sodium acetate production to meet increasing global demand presents its own set of challenges. Optimizing reactor designs, improving heat transfer efficiency, and developing continuous flow processes are areas of ongoing research. These efforts aim to enhance production capacity while maintaining product quality and reducing energy consumption.
Another challenge lies in the purification process of sodium acetate. Achieving high levels of purity, essential for many industrial and pharmaceutical applications, requires sophisticated separation techniques. Impurities, even in trace amounts, can significantly affect the performance and safety of sodium acetate in critical applications. The development of cost-effective and efficient purification methods remains an ongoing challenge for manufacturers.
The production of sodium acetate also faces environmental concerns. Traditional manufacturing processes often involve the use of petrochemical-derived acetic acid, raising sustainability issues. The industry is under pressure to develop greener production methods, such as utilizing bio-based acetic acid or exploring alternative synthesis routes that reduce carbon footprint and minimize waste generation.
In terms of application, sodium acetate's use as a phase change material for thermal energy storage presents unique technical hurdles. While its high latent heat of fusion makes it an attractive candidate for heat storage applications, issues such as supercooling and phase segregation during repeated melting-freezing cycles need to be addressed. Engineers are working on developing additives and encapsulation techniques to enhance the reliability and efficiency of sodium acetate-based thermal energy storage systems.
The use of sodium acetate in food preservation and as a flavoring agent also faces regulatory challenges. Ensuring compliance with evolving food safety standards across different regions requires continuous research and development efforts. Additionally, there is a growing demand for natural and clean-label ingredients, pushing the industry to explore bio-based production methods for sodium acetate that can meet these consumer preferences without compromising on functionality or cost-effectiveness.
Lastly, the scaling up of sodium acetate production to meet increasing global demand presents its own set of challenges. Optimizing reactor designs, improving heat transfer efficiency, and developing continuous flow processes are areas of ongoing research. These efforts aim to enhance production capacity while maintaining product quality and reducing energy consumption.
Current Stabilization Methods
01 Thermal stability of sodium acetate
Sodium acetate exhibits good thermal stability, making it suitable for applications involving high temperatures. Its stability allows for use in various industrial processes and products where heat resistance is required. The compound maintains its chemical structure and properties under elevated temperatures, which is beneficial for many manufacturing and chemical processes.- Thermal stability of sodium acetate: Sodium acetate exhibits good thermal stability, making it suitable for applications involving high temperatures. Its stability allows it to maintain its chemical properties and effectiveness in various thermal conditions, which is beneficial for industrial processes and product formulations.
- Chemical compatibility of sodium acetate: Sodium acetate demonstrates compatibility with a wide range of chemicals, enhancing its versatility in different formulations. This compatibility allows for its use in various chemical processes and product compositions without significant degradation or unwanted reactions.
- pH stability of sodium acetate solutions: Sodium acetate solutions show good pH stability, making them useful as buffer solutions in various applications. This stability helps maintain consistent pH levels in chemical processes, biological systems, and product formulations where pH control is critical.
- Moisture resistance of sodium acetate: Sodium acetate exhibits resistance to moisture, which contributes to its stability in various environmental conditions. This property makes it suitable for use in products and processes where moisture sensitivity could be an issue, helping to maintain product integrity and effectiveness.
- Long-term storage stability of sodium acetate: Sodium acetate demonstrates good long-term storage stability under proper conditions. This characteristic ensures that the compound maintains its chemical properties and effectiveness over extended periods, which is important for product shelf life and industrial applications requiring consistent performance over time.
02 Chemical compatibility of sodium acetate
Sodium acetate demonstrates good chemical compatibility with various substances, making it versatile in different formulations and mixtures. Its stability in the presence of other chemicals allows for its use in diverse applications, including as a buffering agent, food additive, and in pharmaceutical preparations. This compatibility enhances its utility in complex chemical systems and products.Expand Specific Solutions03 pH stability of sodium acetate solutions
Sodium acetate solutions exhibit pH stability, making them useful as buffer solutions in various applications. The compound helps maintain a stable pH in aqueous environments, which is crucial in many biological, chemical, and industrial processes. This property makes sodium acetate valuable in applications where pH control is essential for product stability or process efficiency.Expand Specific Solutions04 Long-term storage stability of sodium acetate
Sodium acetate demonstrates good long-term storage stability under proper conditions. It can maintain its chemical properties and effectiveness over extended periods, making it suitable for various commercial and industrial applications. Proper storage conditions, such as protection from moisture and extreme temperatures, help ensure the compound's stability during storage and transportation.Expand Specific Solutions05 Stability of sodium acetate in different physical forms
Sodium acetate exhibits stability in various physical forms, including anhydrous, trihydrate, and solution forms. This stability across different physical states enhances its versatility in various applications and manufacturing processes. The compound's ability to maintain its properties in different forms allows for flexibility in product formulation and storage.Expand Specific Solutions
Key Industry Players
The sodium acetate market is in a mature stage, with established players and stable demand across various industries. The global market size is estimated to be in the hundreds of millions of dollars, driven by applications in food preservation, pharmaceuticals, and industrial processes. Technologically, sodium acetate production is well-established, but innovation continues in areas like purity enhancement and sustainable manufacturing. Key players such as Nantong Alchemy Biotech, Fuso Pharmaceutical, and QIAGEN are investing in R&D to improve product quality and expand applications. Emerging companies like Ceradis BV are exploring novel formulations and bio-based alternatives, indicating potential for future market disruption and growth in eco-friendly solutions.
Bayer AG
Technical Solution: Bayer AG has pioneered the use of sodium acetate in agrochemical formulations to improve the stability and efficacy of crop protection products. Their research has led to the development of a novel controlled-release system that utilizes sodium acetate as a core component. This system allows for the gradual release of active ingredients, reducing the frequency of application and minimizing environmental impact[2]. Bayer has also incorporated sodium acetate into their seed treatment technologies, where it serves as a stabilizing agent for sensitive biological control agents. In their latest innovation, they have combined sodium acetate with biodegradable polymers to create a smart delivery system that responds to environmental triggers such as soil pH or moisture levels, optimizing the release of agrochemicals[4].
Strengths: Improved product efficacy, reduced environmental impact, innovative delivery systems. Weaknesses: Higher initial product costs, potential regulatory challenges for novel formulations.
Unilever NV
Technical Solution: Unilever NV has leveraged sodium acetate's properties in personal care and home products to enhance stability and performance. Their research has focused on using sodium acetate as a pH buffer and preservative booster in cosmetic formulations. By incorporating sodium acetate into their patented "Stability Plus" technology, Unilever has developed a range of skincare products with extended shelf life and improved texture stability[6]. In their home care division, sodium acetate has been utilized in laundry detergents as a water softener and pH regulator, contributing to better cleaning performance in hard water conditions. Unilever's latest innovation involves a microencapsulation technique that uses sodium acetate to create time-release fragrance capsules for fabric care products, providing long-lasting freshness[8].
Strengths: Enhanced product stability, improved performance in diverse applications, consumer-friendly innovations. Weaknesses: Potential cost increases, challenges in natural product formulations.
Innovative Acetate Research
Stabilizing of acetate systems
PatentInactiveEP0623642A2
Innovation
- Incorporating specific strong acids or acid-releasing compounds, such as sulfuric acid or alkali metal hydrogen sulfates, into the silicone paste formulation to enhance storage stability, allowing for effective neutralization and maintaining functionality over extended periods.
Bleaching composition
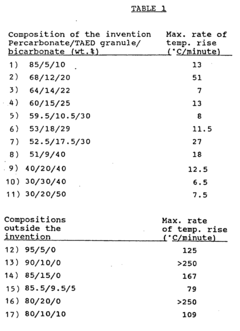
PatentInactiveEP0427314B2
Innovation
- A bleaching composition comprising 10-90% sodium percarbonate, 4-40% bleach activator, and 5-85% alkali metal bicarbonate or sesquicarbonate, without the need for heat treatment or acidic compounds, with the ratio of sodium percarbonate to bleach activator being at least 4:5 and bicarbonate to bleach activator at least 5:4, and optional detergent ingredients, minimizing self-heating risks and maintaining stability.
Environmental Impact
Sodium acetate, a versatile compound with applications ranging from food preservation to industrial processes, has garnered attention for its potential environmental impacts. As the use of this chemical compound continues to expand, it is crucial to assess its effects on ecosystems and human health.
One of the primary environmental considerations for sodium acetate is its biodegradability. Unlike many synthetic chemicals, sodium acetate is readily biodegradable, breaking down into harmless components in natural environments. This characteristic significantly reduces its long-term environmental persistence and potential for bioaccumulation in food chains.
However, the production and disposal of sodium acetate can still pose environmental challenges. The manufacturing process may involve energy-intensive steps and the use of other chemicals, contributing to carbon emissions and potential pollution if not properly managed. Additionally, improper disposal of sodium acetate-containing products or industrial waste can lead to localized environmental issues.
In aquatic environments, sodium acetate can have both positive and negative effects. While it can serve as a carbon source for certain microorganisms, potentially supporting biodiversity, excessive concentrations may lead to eutrophication in water bodies. This process can result in algal blooms and oxygen depletion, negatively impacting aquatic ecosystems.
The use of sodium acetate in de-icing applications, particularly for airport runways, has raised concerns about its impact on soil and groundwater. Although less corrosive than traditional salt-based de-icers, sodium acetate can still alter soil chemistry and potentially affect plant growth in surrounding areas. Proper application and runoff management are essential to mitigate these effects.
From an air quality perspective, sodium acetate generally has a low impact. It does not contribute significantly to volatile organic compound (VOC) emissions or particulate matter formation. However, dust from dry sodium acetate handling in industrial settings should be controlled to prevent localized air quality issues.
As sustainability becomes increasingly important in chemical manufacturing and use, efforts are being made to improve the environmental profile of sodium acetate production. This includes exploring renewable feedstocks, optimizing manufacturing processes to reduce energy consumption, and developing more efficient recycling and waste management strategies.
In conclusion, while sodium acetate presents a relatively favorable environmental profile compared to many other chemical compounds, its growing use necessitates ongoing monitoring and research to fully understand and mitigate potential ecological impacts. Balancing its benefits in chemical stability with environmental stewardship remains a key challenge for industries utilizing this compound.
One of the primary environmental considerations for sodium acetate is its biodegradability. Unlike many synthetic chemicals, sodium acetate is readily biodegradable, breaking down into harmless components in natural environments. This characteristic significantly reduces its long-term environmental persistence and potential for bioaccumulation in food chains.
However, the production and disposal of sodium acetate can still pose environmental challenges. The manufacturing process may involve energy-intensive steps and the use of other chemicals, contributing to carbon emissions and potential pollution if not properly managed. Additionally, improper disposal of sodium acetate-containing products or industrial waste can lead to localized environmental issues.
In aquatic environments, sodium acetate can have both positive and negative effects. While it can serve as a carbon source for certain microorganisms, potentially supporting biodiversity, excessive concentrations may lead to eutrophication in water bodies. This process can result in algal blooms and oxygen depletion, negatively impacting aquatic ecosystems.
The use of sodium acetate in de-icing applications, particularly for airport runways, has raised concerns about its impact on soil and groundwater. Although less corrosive than traditional salt-based de-icers, sodium acetate can still alter soil chemistry and potentially affect plant growth in surrounding areas. Proper application and runoff management are essential to mitigate these effects.
From an air quality perspective, sodium acetate generally has a low impact. It does not contribute significantly to volatile organic compound (VOC) emissions or particulate matter formation. However, dust from dry sodium acetate handling in industrial settings should be controlled to prevent localized air quality issues.
As sustainability becomes increasingly important in chemical manufacturing and use, efforts are being made to improve the environmental profile of sodium acetate production. This includes exploring renewable feedstocks, optimizing manufacturing processes to reduce energy consumption, and developing more efficient recycling and waste management strategies.
In conclusion, while sodium acetate presents a relatively favorable environmental profile compared to many other chemical compounds, its growing use necessitates ongoing monitoring and research to fully understand and mitigate potential ecological impacts. Balancing its benefits in chemical stability with environmental stewardship remains a key challenge for industries utilizing this compound.
Regulatory Compliance
Regulatory compliance plays a crucial role in the development, production, and distribution of sodium acetate, ensuring its safe and responsible use across various industries. The regulatory landscape for this chemical compound is complex and multifaceted, involving numerous agencies and standards at both national and international levels.
In the United States, the Food and Drug Administration (FDA) oversees the use of sodium acetate in food and pharmaceutical applications. The compound is classified as Generally Recognized as Safe (GRAS) for use as a food additive, subject to specific limitations and conditions. For pharmaceutical use, sodium acetate must comply with the standards set forth in the United States Pharmacopeia (USP) and adhere to Good Manufacturing Practices (GMP) guidelines.
The Environmental Protection Agency (EPA) regulates the environmental impact of sodium acetate production and disposal under the Toxic Substances Control Act (TSCA). Manufacturers must comply with reporting requirements and adhere to guidelines for handling, storage, and transportation of the chemical.
Internationally, the European Chemicals Agency (ECHA) governs the use of sodium acetate within the European Union under the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation. Companies producing or importing sodium acetate in quantities exceeding one tonne per year must register the substance and provide detailed safety information.
The United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards. Sodium acetate manufacturers and distributors must comply with GHS requirements for labeling and safety data sheets, ensuring consistent hazard communication across different countries and regions.
In the context of chemical stability, regulatory bodies often require extensive stability testing and documentation. This includes accelerated stability studies, long-term stability studies, and stress testing to evaluate the compound's behavior under various environmental conditions. These studies are essential for determining shelf life, storage conditions, and potential degradation products.
Compliance with these regulations necessitates robust quality management systems, comprehensive documentation, and regular audits. Companies must invest in training programs to ensure that all personnel involved in the production, handling, and distribution of sodium acetate are well-versed in the relevant regulatory requirements and best practices.
As the regulatory landscape continues to evolve, staying abreast of changes and emerging requirements is crucial for maintaining compliance. This may involve engaging with regulatory agencies, participating in industry associations, and continuously updating internal processes and procedures to align with the latest standards and guidelines.
In the United States, the Food and Drug Administration (FDA) oversees the use of sodium acetate in food and pharmaceutical applications. The compound is classified as Generally Recognized as Safe (GRAS) for use as a food additive, subject to specific limitations and conditions. For pharmaceutical use, sodium acetate must comply with the standards set forth in the United States Pharmacopeia (USP) and adhere to Good Manufacturing Practices (GMP) guidelines.
The Environmental Protection Agency (EPA) regulates the environmental impact of sodium acetate production and disposal under the Toxic Substances Control Act (TSCA). Manufacturers must comply with reporting requirements and adhere to guidelines for handling, storage, and transportation of the chemical.
Internationally, the European Chemicals Agency (ECHA) governs the use of sodium acetate within the European Union under the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation. Companies producing or importing sodium acetate in quantities exceeding one tonne per year must register the substance and provide detailed safety information.
The United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards. Sodium acetate manufacturers and distributors must comply with GHS requirements for labeling and safety data sheets, ensuring consistent hazard communication across different countries and regions.
In the context of chemical stability, regulatory bodies often require extensive stability testing and documentation. This includes accelerated stability studies, long-term stability studies, and stress testing to evaluate the compound's behavior under various environmental conditions. These studies are essential for determining shelf life, storage conditions, and potential degradation products.
Compliance with these regulations necessitates robust quality management systems, comprehensive documentation, and regular audits. Companies must invest in training programs to ensure that all personnel involved in the production, handling, and distribution of sodium acetate are well-versed in the relevant regulatory requirements and best practices.
As the regulatory landscape continues to evolve, staying abreast of changes and emerging requirements is crucial for maintaining compliance. This may involve engaging with regulatory agencies, participating in industry associations, and continuously updating internal processes and procedures to align with the latest standards and guidelines.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!