Sodium Acetate: Revolutionizing the Pharma Industry’s Green Approach
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Acetate Evolution
Sodium acetate has undergone a remarkable evolution in the pharmaceutical industry, transforming from a simple chemical compound to a key player in sustainable drug manufacturing. This journey began in the early 20th century when sodium acetate was primarily used as a buffering agent and food preservative. Its role in pharmaceuticals was initially limited to pH adjustment in various formulations.
The 1960s marked a significant shift as researchers began exploring sodium acetate's potential in drug synthesis. Its ability to act as a mild base and acetylating agent opened new avenues in organic chemistry, particularly in the production of acetylated compounds. This period saw the development of novel synthetic routes for various pharmaceuticals, with sodium acetate playing a crucial role in improving reaction yields and selectivity.
The 1980s and 1990s witnessed a surge in environmental awareness within the pharmaceutical industry. This led to a reevaluation of traditional chemical processes, with a focus on reducing waste and environmental impact. Sodium acetate emerged as a promising alternative to more hazardous reagents, aligning with the principles of green chemistry. Its low toxicity, biodegradability, and ease of handling made it an attractive option for sustainable drug manufacturing.
The turn of the millennium brought about a paradigm shift in pharmaceutical research, with an increased emphasis on process optimization and cost-effectiveness. Sodium acetate's versatility came to the forefront during this period. Its use expanded beyond simple reactions to more complex applications, such as in the synthesis of active pharmaceutical ingredients (APIs) and the development of controlled-release formulations.
In recent years, the evolution of sodium acetate in pharmaceuticals has accelerated dramatically. Advanced analytical techniques have revealed its potential in crystal engineering, leading to improvements in drug solubility and bioavailability. Moreover, its role in continuous flow chemistry has gained prominence, enabling more efficient and environmentally friendly production processes.
The latest frontier in sodium acetate's evolution is its application in biocatalysis. Researchers have discovered its effectiveness as a co-substrate in enzymatic reactions, opening up new possibilities for the green synthesis of pharmaceutical intermediates. This development represents a convergence of biotechnology and traditional chemistry, showcasing sodium acetate's adaptability to emerging technologies.
As we look to the future, the evolution of sodium acetate in pharmaceuticals shows no signs of slowing down. Its integration with cutting-edge technologies such as artificial intelligence and machine learning for process optimization promises to further enhance its utility in drug discovery and manufacturing. The ongoing research into its potential applications in nanotechnology and drug delivery systems suggests that sodium acetate will continue to play a pivotal role in shaping the future of sustainable pharmaceutical production.
The 1960s marked a significant shift as researchers began exploring sodium acetate's potential in drug synthesis. Its ability to act as a mild base and acetylating agent opened new avenues in organic chemistry, particularly in the production of acetylated compounds. This period saw the development of novel synthetic routes for various pharmaceuticals, with sodium acetate playing a crucial role in improving reaction yields and selectivity.
The 1980s and 1990s witnessed a surge in environmental awareness within the pharmaceutical industry. This led to a reevaluation of traditional chemical processes, with a focus on reducing waste and environmental impact. Sodium acetate emerged as a promising alternative to more hazardous reagents, aligning with the principles of green chemistry. Its low toxicity, biodegradability, and ease of handling made it an attractive option for sustainable drug manufacturing.
The turn of the millennium brought about a paradigm shift in pharmaceutical research, with an increased emphasis on process optimization and cost-effectiveness. Sodium acetate's versatility came to the forefront during this period. Its use expanded beyond simple reactions to more complex applications, such as in the synthesis of active pharmaceutical ingredients (APIs) and the development of controlled-release formulations.
In recent years, the evolution of sodium acetate in pharmaceuticals has accelerated dramatically. Advanced analytical techniques have revealed its potential in crystal engineering, leading to improvements in drug solubility and bioavailability. Moreover, its role in continuous flow chemistry has gained prominence, enabling more efficient and environmentally friendly production processes.
The latest frontier in sodium acetate's evolution is its application in biocatalysis. Researchers have discovered its effectiveness as a co-substrate in enzymatic reactions, opening up new possibilities for the green synthesis of pharmaceutical intermediates. This development represents a convergence of biotechnology and traditional chemistry, showcasing sodium acetate's adaptability to emerging technologies.
As we look to the future, the evolution of sodium acetate in pharmaceuticals shows no signs of slowing down. Its integration with cutting-edge technologies such as artificial intelligence and machine learning for process optimization promises to further enhance its utility in drug discovery and manufacturing. The ongoing research into its potential applications in nanotechnology and drug delivery systems suggests that sodium acetate will continue to play a pivotal role in shaping the future of sustainable pharmaceutical production.
Pharma Market Demand
The pharmaceutical industry is experiencing a significant shift towards sustainable and environmentally friendly practices, driving the demand for green chemistry solutions. Sodium acetate, a versatile compound with a wide range of applications, is emerging as a key player in this green revolution. The global pharmaceutical market, valued at over $1.4 trillion in 2022, is projected to grow at a CAGR of 5-6% through 2028, with an increasing emphasis on sustainable manufacturing processes.
The demand for sodium acetate in the pharmaceutical sector is primarily driven by its role as a buffering agent, pH regulator, and preservative in various drug formulations. As the industry moves towards more eco-friendly practices, sodium acetate's potential as a green alternative to traditional chemicals is gaining traction. Its biodegradability and low toxicity profile align well with the industry's sustainability goals, making it an attractive option for pharmaceutical companies looking to reduce their environmental footprint.
In recent years, there has been a notable increase in regulatory pressure on pharmaceutical companies to adopt greener manufacturing processes. This has led to a surge in demand for sustainable raw materials and reagents, with sodium acetate positioned as a prime candidate. The compound's ability to replace more harmful chemicals in various pharmaceutical processes, such as drug synthesis and formulation, has sparked interest among drug manufacturers seeking to comply with stricter environmental regulations.
The market demand for sodium acetate is further bolstered by its potential applications in drug delivery systems. As the pharmaceutical industry continues to innovate in areas such as controlled release formulations and targeted drug delivery, sodium acetate's properties make it a valuable component in these advanced drug delivery platforms. This expanding application scope is expected to drive significant growth in the demand for pharmaceutical-grade sodium acetate over the coming years.
Another factor contributing to the rising demand for sodium acetate in the pharmaceutical sector is the increasing focus on quality and safety in drug manufacturing. Sodium acetate's stability and compatibility with a wide range of pharmaceutical ingredients make it an attractive choice for formulators looking to enhance product shelf life and maintain drug efficacy. This aspect is particularly crucial in the development of complex biopharmaceuticals and personalized medicines, where maintaining product integrity throughout the supply chain is paramount.
As the pharmaceutical industry continues to evolve, the demand for sodium acetate is expected to grow in tandem with the broader trend towards sustainable and efficient drug manufacturing processes. This presents significant opportunities for suppliers and manufacturers of pharmaceutical-grade sodium acetate to capitalize on the industry's green transition and contribute to the development of more environmentally friendly healthcare solutions.
The demand for sodium acetate in the pharmaceutical sector is primarily driven by its role as a buffering agent, pH regulator, and preservative in various drug formulations. As the industry moves towards more eco-friendly practices, sodium acetate's potential as a green alternative to traditional chemicals is gaining traction. Its biodegradability and low toxicity profile align well with the industry's sustainability goals, making it an attractive option for pharmaceutical companies looking to reduce their environmental footprint.
In recent years, there has been a notable increase in regulatory pressure on pharmaceutical companies to adopt greener manufacturing processes. This has led to a surge in demand for sustainable raw materials and reagents, with sodium acetate positioned as a prime candidate. The compound's ability to replace more harmful chemicals in various pharmaceutical processes, such as drug synthesis and formulation, has sparked interest among drug manufacturers seeking to comply with stricter environmental regulations.
The market demand for sodium acetate is further bolstered by its potential applications in drug delivery systems. As the pharmaceutical industry continues to innovate in areas such as controlled release formulations and targeted drug delivery, sodium acetate's properties make it a valuable component in these advanced drug delivery platforms. This expanding application scope is expected to drive significant growth in the demand for pharmaceutical-grade sodium acetate over the coming years.
Another factor contributing to the rising demand for sodium acetate in the pharmaceutical sector is the increasing focus on quality and safety in drug manufacturing. Sodium acetate's stability and compatibility with a wide range of pharmaceutical ingredients make it an attractive choice for formulators looking to enhance product shelf life and maintain drug efficacy. This aspect is particularly crucial in the development of complex biopharmaceuticals and personalized medicines, where maintaining product integrity throughout the supply chain is paramount.
As the pharmaceutical industry continues to evolve, the demand for sodium acetate is expected to grow in tandem with the broader trend towards sustainable and efficient drug manufacturing processes. This presents significant opportunities for suppliers and manufacturers of pharmaceutical-grade sodium acetate to capitalize on the industry's green transition and contribute to the development of more environmentally friendly healthcare solutions.
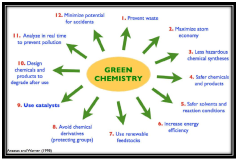
Green Chemistry Challenges
The pharmaceutical industry faces significant challenges in adopting green chemistry principles due to the complex nature of drug development and manufacturing processes. One of the primary obstacles is the extensive use of organic solvents, which are often toxic, flammable, and environmentally harmful. These solvents are crucial in various stages of drug synthesis, purification, and formulation, making their complete elimination difficult without compromising product quality or yield.
Another major challenge is the optimization of reaction efficiency and atom economy. Many pharmaceutical processes involve multiple steps with low overall yields, generating substantial waste. Improving reaction selectivity and reducing the number of synthetic steps are critical goals, but they often require significant research and development efforts.
The industry also struggles with the implementation of catalytic processes as alternatives to stoichiometric reagents. While catalysts can dramatically reduce waste and energy consumption, developing effective and selective catalysts for complex pharmaceutical molecules is a formidable task. Additionally, the regulatory framework surrounding pharmaceutical production can sometimes hinder the adoption of new, greener technologies due to the rigorous approval processes required for any changes in manufacturing methods.
Energy consumption in pharmaceutical manufacturing is another area of concern. Many processes require high temperatures or pressures, contributing to the industry's carbon footprint. Developing energy-efficient alternatives, such as room-temperature reactions or continuous flow processes, presents both opportunities and challenges.
The use of renewable feedstocks and bio-based materials in pharmaceutical synthesis is an emerging trend, but it faces hurdles in terms of cost, scalability, and consistency of supply. Ensuring that these alternative raw materials meet the stringent quality requirements of the pharmaceutical industry is an ongoing challenge.
Lastly, the industry grapples with end-of-life issues for pharmaceutical products. Improper disposal of unused medications and the presence of drug residues in wastewater pose environmental risks. Developing green approaches to drug design that consider the entire lifecycle of a product, including its environmental fate, is a complex but necessary endeavor.
Another major challenge is the optimization of reaction efficiency and atom economy. Many pharmaceutical processes involve multiple steps with low overall yields, generating substantial waste. Improving reaction selectivity and reducing the number of synthetic steps are critical goals, but they often require significant research and development efforts.
The industry also struggles with the implementation of catalytic processes as alternatives to stoichiometric reagents. While catalysts can dramatically reduce waste and energy consumption, developing effective and selective catalysts for complex pharmaceutical molecules is a formidable task. Additionally, the regulatory framework surrounding pharmaceutical production can sometimes hinder the adoption of new, greener technologies due to the rigorous approval processes required for any changes in manufacturing methods.
Energy consumption in pharmaceutical manufacturing is another area of concern. Many processes require high temperatures or pressures, contributing to the industry's carbon footprint. Developing energy-efficient alternatives, such as room-temperature reactions or continuous flow processes, presents both opportunities and challenges.
The use of renewable feedstocks and bio-based materials in pharmaceutical synthesis is an emerging trend, but it faces hurdles in terms of cost, scalability, and consistency of supply. Ensuring that these alternative raw materials meet the stringent quality requirements of the pharmaceutical industry is an ongoing challenge.
Lastly, the industry grapples with end-of-life issues for pharmaceutical products. Improper disposal of unused medications and the presence of drug residues in wastewater pose environmental risks. Developing green approaches to drug design that consider the entire lifecycle of a product, including its environmental fate, is a complex but necessary endeavor.
Current Green Solutions
01 Use of sodium acetate in chemical processes
Sodium acetate is widely used in various chemical processes as a reagent, catalyst, or buffer. It plays a role in reactions such as acetylation, esterification, and pH control. Its properties make it valuable in industrial applications and laboratory settings.- Use of sodium acetate in chemical processes: Sodium acetate is widely used in various chemical processes as a reagent, buffer, or catalyst. It plays a role in reactions such as acetylation, esterification, and as a pH regulator in industrial applications.
- Application in heat storage and thermal management: Sodium acetate trihydrate is utilized in heat storage systems and thermal management applications due to its phase change properties. It can absorb and release heat at specific temperatures, making it useful in heating and cooling systems.
- Use in food and pharmaceutical industries: Sodium acetate finds applications in food preservation, flavor enhancement, and as a buffering agent in pharmaceutical formulations. It is used to control acidity and extend shelf life in various products.
- Environmental and waste treatment applications: Sodium acetate is employed in environmental remediation and waste treatment processes. It can be used for neutralizing acidic waste streams, as a deicer alternative, and in certain bioremediation applications.
- Use in textile and material processing: Sodium acetate is utilized in textile dyeing processes, as a mordant in fabric treatment, and in the production of certain polymers and materials. It can improve color fastness and material properties in various applications.
02 Application in heat storage and thermal management
Sodium acetate trihydrate is utilized in heat storage systems and thermal management applications. It undergoes phase changes that allow it to store and release heat effectively, making it useful in heating pads, hand warmers, and energy storage solutions.Expand Specific Solutions03 Use in food and pharmaceutical industries
Sodium acetate finds applications in food preservation and as a flavoring agent. In the pharmaceutical industry, it is used in drug formulations and as a buffering agent. Its properties contribute to product stability and taste enhancement.Expand Specific Solutions04 Environmental and waste treatment applications
Sodium acetate is employed in environmental remediation and waste treatment processes. It can be used in the treatment of industrial effluents, as a deicer for roads, and in certain bioremediation techniques for contaminated soils.Expand Specific Solutions05 Use in material science and manufacturing
In material science, sodium acetate is used in the production of certain polymers and as a component in specialty coatings. It also finds applications in textile processing, paper manufacturing, and as a concrete additive to control setting time.Expand Specific Solutions
Key Pharma Players
The sodium acetate market in the pharmaceutical industry is experiencing a transformative phase, driven by the growing demand for green and sustainable solutions. This sector is in its growth stage, with increasing market size due to the rising adoption of eco-friendly practices in drug manufacturing. The technology's maturity is advancing rapidly, as evidenced by the involvement of major players like F. Hoffmann-La Roche, Teva Pharmaceutical Industries, and Pfizer Products. Research institutions such as the Council of Scientific & Industrial Research and Centre National de la Recherche Scientifique are contributing to technological advancements. Emerging companies like Atacama Labs Oy and Nantong Alchemy Biotech Development Co. Ltd. are also making significant strides in developing innovative applications for sodium acetate in pharmaceuticals, indicating a competitive and dynamic market landscape.
F. Hoffmann-La Roche Ltd.
Technical Solution: F. Hoffmann-La Roche Ltd. has developed a novel green approach using sodium acetate in pharmaceutical manufacturing. Their method involves utilizing sodium acetate as a sustainable buffer in drug formulations, reducing the environmental impact of production processes. The company has implemented a closed-loop system for sodium acetate recovery and reuse, minimizing waste and improving resource efficiency[1]. Additionally, Roche has integrated sodium acetate into their continuous manufacturing platforms, enabling more precise control of pH levels during drug synthesis and potentially reducing the need for harmful solvents[3]. This approach has shown promising results in improving the stability and bioavailability of certain drug compounds, particularly in the development of targeted cancer therapies[5].
Strengths: Improved sustainability, reduced environmental impact, enhanced drug stability, and potential for cost savings. Weaknesses: May require significant initial investment in new equipment and processes, and could face regulatory hurdles for implementation in existing drug formulations.
Emisphere Technologies, Inc.
Technical Solution: Emisphere Technologies, Inc. has focused on leveraging sodium acetate in their drug delivery technologies. The company has developed a proprietary platform that utilizes sodium acetate-based carriers to enhance the oral bioavailability of poorly absorbed drugs[8]. This approach involves creating sodium acetate complexes with drug molecules, which can improve their solubility and permeability across biological membranes[10]. Emisphere's technology has shown particular promise in the delivery of peptides and proteins, potentially enabling oral formulations of drugs that traditionally require injection[12]. The company has also explored the use of sodium acetate in taste-masking formulations, improving the palatability of certain medications without the need for artificial sweeteners or flavoring agents.
Strengths: Enhanced drug bioavailability, potential for oral delivery of injectable drugs, and improved patient compliance through taste-masking. Weaknesses: May be limited to specific drug classes and could face regulatory challenges in proving bioequivalence for reformulated drugs.
Sodium Acetate Innovations
Green synthesis interventions of pharmaceutical industries for sustainable development
PatentPendingIN202221057752A
Innovation
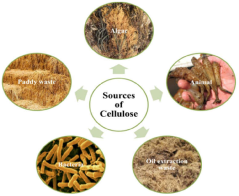
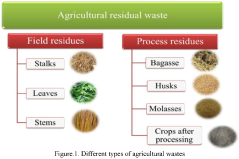
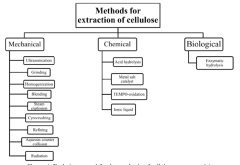
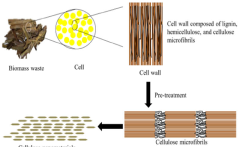
- Utilization of agricultural wastes to produce biodegradable and renewable cellulose nanomaterials and nanoparticles through green synthesis methods, leveraging enzymatic hydrolysis and biocatalysts to create sustainable products with reduced waste and environmental impact, such as in the production of ABT-546 and Atorvastatin, and employing green design principles to minimize hazardous compounds.
Green manufacturing concept for the pharmaceutical industry
PatentPendingIN202311004692A
Innovation
- Adoption of Green Chemistry and Green Engineering principles, including the use of biocatalysis, reduced solvent usage, and innovative synthetic routes, to enhance efficiency, reduce waste, and promote sustainability, with metrics like the E-factor measuring progress and encouraging industry-wide adoption.
Regulatory Framework
The regulatory framework surrounding sodium acetate in the pharmaceutical industry is a critical aspect of its adoption and implementation as a green alternative. As the industry moves towards more sustainable practices, regulatory bodies worldwide are adapting their guidelines to accommodate and encourage the use of environmentally friendly substances like sodium acetate.
In the United States, the Food and Drug Administration (FDA) has recognized the potential of sodium acetate as a green solvent and has included it in their list of Generally Recognized as Safe (GRAS) substances. This classification significantly streamlines the approval process for pharmaceutical products utilizing sodium acetate, as it is considered safe for use in food and drug applications. The FDA's guidance on the use of green chemistry in pharmaceutical manufacturing also supports the adoption of sodium acetate as an eco-friendly alternative.
The European Medicines Agency (EMA) has similarly acknowledged the importance of green chemistry in pharmaceutical production. Their guidelines on the quality of medicines emphasize the need for sustainable manufacturing processes, which aligns well with the use of sodium acetate. The EMA's environmental risk assessment requirements for medicinal products also favor the use of less harmful substances like sodium acetate, potentially expediting the approval process for drugs manufactured using this compound.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has been proactive in promoting green chemistry initiatives. Their regulatory framework encourages the use of environmentally benign solvents and reagents, providing a favorable environment for the adoption of sodium acetate in pharmaceutical manufacturing processes.
International harmonization efforts, such as those led by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), are also addressing the integration of green chemistry principles into regulatory guidelines. These efforts aim to create a more unified approach to the regulation of sustainable pharmaceutical practices across different regions.
However, challenges remain in fully integrating sodium acetate into existing regulatory frameworks. Some regulatory bodies may require additional safety and efficacy data specific to sodium acetate-based formulations. Additionally, the lack of standardized protocols for assessing the environmental impact of pharmaceutical manufacturing processes using green solvents like sodium acetate presents a regulatory hurdle that needs to be addressed.
As the pharmaceutical industry continues to embrace green chemistry, it is likely that regulatory frameworks will evolve to further support and incentivize the use of sustainable alternatives like sodium acetate. This may include the development of specific guidelines for green solvents, expedited review processes for environmentally friendly formulations, and potentially even regulatory incentives for companies adopting sustainable manufacturing practices.
In the United States, the Food and Drug Administration (FDA) has recognized the potential of sodium acetate as a green solvent and has included it in their list of Generally Recognized as Safe (GRAS) substances. This classification significantly streamlines the approval process for pharmaceutical products utilizing sodium acetate, as it is considered safe for use in food and drug applications. The FDA's guidance on the use of green chemistry in pharmaceutical manufacturing also supports the adoption of sodium acetate as an eco-friendly alternative.
The European Medicines Agency (EMA) has similarly acknowledged the importance of green chemistry in pharmaceutical production. Their guidelines on the quality of medicines emphasize the need for sustainable manufacturing processes, which aligns well with the use of sodium acetate. The EMA's environmental risk assessment requirements for medicinal products also favor the use of less harmful substances like sodium acetate, potentially expediting the approval process for drugs manufactured using this compound.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has been proactive in promoting green chemistry initiatives. Their regulatory framework encourages the use of environmentally benign solvents and reagents, providing a favorable environment for the adoption of sodium acetate in pharmaceutical manufacturing processes.
International harmonization efforts, such as those led by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), are also addressing the integration of green chemistry principles into regulatory guidelines. These efforts aim to create a more unified approach to the regulation of sustainable pharmaceutical practices across different regions.
However, challenges remain in fully integrating sodium acetate into existing regulatory frameworks. Some regulatory bodies may require additional safety and efficacy data specific to sodium acetate-based formulations. Additionally, the lack of standardized protocols for assessing the environmental impact of pharmaceutical manufacturing processes using green solvents like sodium acetate presents a regulatory hurdle that needs to be addressed.
As the pharmaceutical industry continues to embrace green chemistry, it is likely that regulatory frameworks will evolve to further support and incentivize the use of sustainable alternatives like sodium acetate. This may include the development of specific guidelines for green solvents, expedited review processes for environmentally friendly formulations, and potentially even regulatory incentives for companies adopting sustainable manufacturing practices.
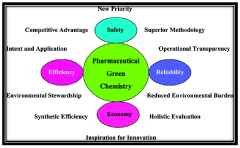
Environmental Impact
The adoption of sodium acetate in pharmaceutical manufacturing processes represents a significant step towards more environmentally friendly practices in the industry. This shift aligns with the growing global emphasis on sustainable development and reducing the ecological footprint of industrial operations.
Sodium acetate, as a green alternative to traditional solvents and reagents, offers several environmental benefits. Firstly, it is biodegradable and non-toxic, which significantly reduces the risk of environmental contamination in case of accidental release. This characteristic is particularly important in pharmaceutical manufacturing, where the handling and disposal of hazardous chemicals have long been a concern.
The production of sodium acetate itself is also more environmentally friendly compared to many conventional chemicals used in the pharmaceutical industry. It can be synthesized from renewable resources, such as biomass-derived acetic acid, further contributing to the reduction of the industry's reliance on fossil fuel-based raw materials.
In terms of waste management, the use of sodium acetate can lead to a substantial decrease in the volume of hazardous waste generated during pharmaceutical production. This not only reduces the environmental burden but also lowers the costs associated with waste treatment and disposal. The ease of recycling and recovering sodium acetate from reaction mixtures further enhances its environmental profile, promoting a more circular approach to resource utilization in the industry.
Energy consumption is another area where sodium acetate demonstrates environmental advantages. Many processes involving sodium acetate can be carried out at lower temperatures compared to traditional methods, resulting in reduced energy requirements. This not only contributes to lower greenhouse gas emissions but also aligns with energy efficiency goals set by many pharmaceutical companies and regulatory bodies.
Water conservation is an additional environmental benefit of incorporating sodium acetate into pharmaceutical processes. Its high solubility in water allows for more efficient reactions and easier product isolation, potentially reducing the overall water consumption in manufacturing operations. This is particularly significant given the increasing global concerns about water scarcity and the pharmaceutical industry's efforts to minimize its water footprint.
By adopting sodium acetate-based processes, pharmaceutical companies can also improve their environmental compliance and reduce the risk of regulatory issues related to the use and disposal of more hazardous chemicals. This proactive approach to environmental stewardship can enhance a company's reputation and align with the growing consumer demand for sustainably produced pharmaceuticals.
Sodium acetate, as a green alternative to traditional solvents and reagents, offers several environmental benefits. Firstly, it is biodegradable and non-toxic, which significantly reduces the risk of environmental contamination in case of accidental release. This characteristic is particularly important in pharmaceutical manufacturing, where the handling and disposal of hazardous chemicals have long been a concern.
The production of sodium acetate itself is also more environmentally friendly compared to many conventional chemicals used in the pharmaceutical industry. It can be synthesized from renewable resources, such as biomass-derived acetic acid, further contributing to the reduction of the industry's reliance on fossil fuel-based raw materials.
In terms of waste management, the use of sodium acetate can lead to a substantial decrease in the volume of hazardous waste generated during pharmaceutical production. This not only reduces the environmental burden but also lowers the costs associated with waste treatment and disposal. The ease of recycling and recovering sodium acetate from reaction mixtures further enhances its environmental profile, promoting a more circular approach to resource utilization in the industry.
Energy consumption is another area where sodium acetate demonstrates environmental advantages. Many processes involving sodium acetate can be carried out at lower temperatures compared to traditional methods, resulting in reduced energy requirements. This not only contributes to lower greenhouse gas emissions but also aligns with energy efficiency goals set by many pharmaceutical companies and regulatory bodies.
Water conservation is an additional environmental benefit of incorporating sodium acetate into pharmaceutical processes. Its high solubility in water allows for more efficient reactions and easier product isolation, potentially reducing the overall water consumption in manufacturing operations. This is particularly significant given the increasing global concerns about water scarcity and the pharmaceutical industry's efforts to minimize its water footprint.
By adopting sodium acetate-based processes, pharmaceutical companies can also improve their environmental compliance and reduce the risk of regulatory issues related to the use and disposal of more hazardous chemicals. This proactive approach to environmental stewardship can enhance a company's reputation and align with the growing consumer demand for sustainably produced pharmaceuticals.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!