Sodium Acetate’s Potential in Purifying Water Systems
Sodium Acetate Background
Sodium acetate, a chemical compound with the formula CH3COONa, has a long history of use in various industries. Its potential in water purification systems has gained increasing attention in recent years due to its unique properties and environmental friendliness. This organic salt is formed by the combination of acetic acid and sodium hydroxide, resulting in a stable, non-toxic substance that is highly soluble in water.
The exploration of sodium acetate's role in water treatment can be traced back to the early 20th century when researchers began investigating its properties as a buffering agent. However, it wasn't until the late 1980s that its potential in water purification systems started to gain significant traction. The compound's ability to regulate pH levels and its capacity to act as a mild reducing agent made it an attractive option for water treatment processes.
In the context of water purification, sodium acetate has demonstrated remarkable versatility. Its primary function lies in its ability to neutralize acidic water, making it particularly useful in areas where water sources are affected by acid rain or industrial runoff. Additionally, sodium acetate has shown promise in removing heavy metals from contaminated water through a process known as precipitation.
The compound's effectiveness in water treatment is largely attributed to its chemical structure. The acetate ion (CH3COO-) can form complexes with various metal ions, facilitating their removal from water. This property has led to investigations into sodium acetate's potential for treating industrial wastewater, where heavy metal contamination is a common concern.
Another significant aspect of sodium acetate's background in water purification is its role in biological treatment processes. The compound serves as a readily biodegradable carbon source for microorganisms involved in wastewater treatment. This characteristic has made it valuable in enhancing the efficiency of biological treatment systems, particularly in situations where the organic content of wastewater is low.
The environmental implications of using sodium acetate in water purification systems have also contributed to its growing popularity. Unlike some traditional water treatment chemicals, sodium acetate is biodegradable and does not persist in the environment. This aligns well with the increasing global focus on sustainable and eco-friendly water treatment solutions.
Recent advancements in nanotechnology have opened up new avenues for sodium acetate's application in water purification. Research has shown that nanoparticles synthesized using sodium acetate as a precursor can exhibit enhanced adsorption properties, potentially revolutionizing the removal of contaminants from water.
Water Purification Market
The water purification market has experienced significant growth in recent years, driven by increasing global concerns over water quality and scarcity. This market encompasses a wide range of technologies and solutions designed to remove contaminants from water, making it safe for consumption and various industrial applications. The demand for clean water is universal, spanning residential, commercial, and industrial sectors.
In the residential segment, there is a growing awareness among consumers about the importance of water quality, leading to increased adoption of home water purification systems. This includes point-of-use devices such as faucet filters, pitcher filters, and under-sink systems. The commercial sector, including hotels, restaurants, and office buildings, is also investing in water purification technologies to ensure safe drinking water for employees and customers.
The industrial sector represents a substantial portion of the water purification market, with applications in manufacturing, power generation, and food and beverage production. These industries require large-scale water treatment solutions to meet regulatory standards and operational needs. The pharmaceutical and electronics industries, in particular, demand ultra-pure water for their processes, driving innovation in advanced purification technologies.
Geographically, the water purification market shows varying levels of maturity and growth potential. Developed regions like North America and Europe have established markets with a focus on upgrading existing infrastructure and adopting more efficient technologies. In contrast, emerging economies in Asia-Pacific and Africa present significant growth opportunities due to rapid industrialization, urbanization, and increasing water stress.
The market is characterized by a mix of large multinational corporations and smaller, specialized companies. Key players in the industry are continuously investing in research and development to improve purification efficiency, reduce energy consumption, and develop more sustainable solutions. There is a notable trend towards the integration of smart technologies and IoT-enabled systems in water purification, allowing for real-time monitoring and optimization of water treatment processes.
Environmental regulations and public health concerns are major drivers of market growth. Governments worldwide are implementing stricter water quality standards, compelling industries and municipalities to invest in advanced purification technologies. Additionally, the growing focus on sustainability is pushing the market towards more eco-friendly purification methods, such as membrane filtration and UV disinfection.
The potential of sodium acetate in water purification systems represents an emerging area of interest within this dynamic market. As a novel approach, it could address specific challenges in water treatment, potentially offering advantages in terms of efficiency, cost-effectiveness, or environmental impact. The integration of sodium acetate-based solutions into existing water purification technologies could open new avenues for market expansion and technological advancement in the industry.
Current Challenges
The use of sodium acetate in water purification systems faces several significant challenges that need to be addressed for its widespread adoption. One of the primary obstacles is the limited research and development in this specific application. While sodium acetate has been extensively studied in other fields, its potential in water treatment remains relatively unexplored, leading to a lack of comprehensive data on its effectiveness and long-term impacts.
Another challenge lies in the scalability of sodium acetate-based water purification systems. Current research primarily focuses on small-scale applications, and there is a need for extensive testing and optimization to determine its viability for large-scale water treatment facilities. This scaling issue is closely tied to cost considerations, as the economic feasibility of using sodium acetate in industrial-scale water purification remains uncertain.
The environmental impact of sodium acetate in water systems is another area of concern. While it is generally considered safe and biodegradable, the long-term effects of its continuous use in water treatment on aquatic ecosystems and downstream environments are not fully understood. This lack of comprehensive environmental impact studies poses a challenge to its widespread implementation.
Technical challenges also exist in the integration of sodium acetate into existing water treatment infrastructures. Many current systems are designed for traditional purification methods, and incorporating sodium acetate may require significant modifications or redesigns of treatment plants. This could lead to high initial investment costs and potential disruptions in water supply during transitions.
Furthermore, there are regulatory hurdles to overcome. The use of new chemicals in water treatment often requires extensive testing and approval from regulatory bodies. The process of obtaining these approvals can be time-consuming and costly, potentially slowing down the adoption of sodium acetate-based purification methods.
Lastly, there is a challenge in public perception and acceptance. Introducing a new chemical into water treatment processes may face resistance from consumers concerned about water safety. Overcoming this requires not only proving the safety and efficacy of sodium acetate but also engaging in public education and awareness campaigns to build trust in this new purification method.
Existing Purification
01 Crystallization and recrystallization techniques
Sodium acetate can be purified through crystallization and recrystallization processes. This involves dissolving the impure sodium acetate in water, filtering out insoluble impurities, and then allowing the solution to cool and crystallize. The resulting crystals can be separated from the mother liquor and dried to obtain purified sodium acetate.- Crystallization and recrystallization techniques: Purification of sodium acetate often involves crystallization and recrystallization processes. These techniques exploit the solubility differences of sodium acetate and impurities in various solvents, typically water or alcohol. The process may include controlled cooling, seeding, or solvent addition to promote crystal formation and growth, followed by filtration to separate the purified crystals from the mother liquor.
- Ion exchange purification: Ion exchange resins can be used to remove impurities from sodium acetate solutions. This method involves passing the impure solution through a column containing ion exchange resin, which selectively removes unwanted ions while allowing sodium acetate to pass through. The process may include multiple stages or different types of resins to target specific impurities.
- Membrane filtration and separation: Advanced membrane technologies, such as nanofiltration or reverse osmosis, can be employed to purify sodium acetate solutions. These methods use semi-permeable membranes to separate sodium acetate from impurities based on molecular size or charge. The process may involve multiple filtration stages or combined with other purification techniques for optimal results.
- Electrodialysis purification: Electrodialysis can be used to purify sodium acetate by selectively removing ions based on their electrical charge. This process involves applying an electric field across a series of ion-selective membranes, allowing for the separation of sodium acetate from impurities. The method can be particularly effective for removing inorganic contaminants from sodium acetate solutions.
- Continuous flow purification systems: Continuous flow systems can be designed for the efficient purification of sodium acetate on an industrial scale. These systems may incorporate multiple purification techniques in a single process, such as crystallization, filtration, and ion exchange. The continuous nature of the process allows for higher throughput and potentially improved efficiency compared to batch processes.
02 Distillation and evaporation methods
Purification of sodium acetate can be achieved through distillation and evaporation techniques. These methods involve heating the sodium acetate solution to remove volatile impurities and concentrate the solution. The purified sodium acetate can then be recovered through crystallization or spray drying.Expand Specific Solutions03 Ion exchange and adsorption processes
Ion exchange resins and adsorbents can be used to remove impurities from sodium acetate solutions. This process involves passing the solution through a column containing the ion exchange resin or adsorbent material, which selectively removes specific impurities. The purified solution can then be further processed to obtain solid sodium acetate.Expand Specific Solutions04 Membrane filtration techniques
Membrane filtration methods, such as ultrafiltration or nanofiltration, can be employed to purify sodium acetate solutions. These techniques use semi-permeable membranes to separate impurities based on molecular size or charge, allowing for the production of high-purity sodium acetate.Expand Specific Solutions05 Chemical treatment and precipitation
Chemical treatments can be used to remove specific impurities from sodium acetate solutions. This may involve adding reagents to precipitate impurities, adjusting pH to optimize purification, or using oxidizing or reducing agents to convert impurities into more easily removable forms. The treated solution can then be filtered and processed to obtain purified sodium acetate.Expand Specific Solutions
Key Industry Players
The sodium acetate water purification technology market is in its early growth stage, with increasing interest due to its potential for sustainable water treatment. The market size is expanding, driven by growing water scarcity concerns and stricter environmental regulations. While the technology is still evolving, several key players are advancing its development. Companies like Ecolab USA, Inc. and PristineHydro Development, Inc. are at the forefront, focusing on innovative water treatment solutions. Established chemical giants such as China Petroleum & Chemical Corp. and Solvay SA are also exploring this field, leveraging their extensive resources and expertise. Academic institutions like the Indian Institute of Technology Madras are contributing to research and development, further enhancing the technology's maturity and potential applications.
Ecolab USA, Inc.
PristineHydro Development, Inc.
Sodium Acetate Innovations
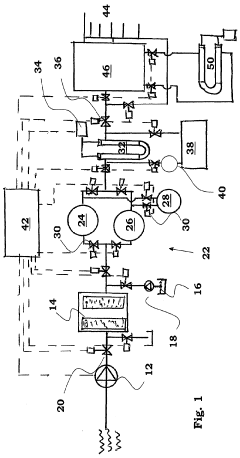
- A water purification system with conduits, a pump, a filter, an ion exchange unit, and a photocatalytic treatment unit, featuring a control system with monitoring and sensor means for automatic operation, including parallel ion exchange vessels for regeneration during low water demand and back-flushing of filters, and a buffer tank for continuous treatment and storage.
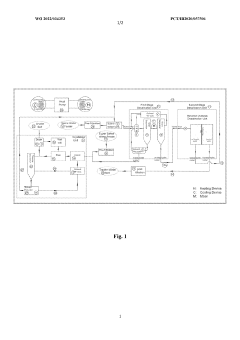
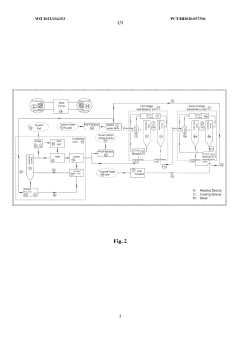
- A solvent extraction water treatment process that uses low-polarity organic solvents for desalination and hydrophilic solvents for salt crystallization, employing a temperature swing method to achieve zero liquid waste discharge, with a heat exchanger and heat pump system for efficient energy transfer, and recycling solvents to treat a wide range of salinity levels from low to ultra-high concentrations.
Environmental Impact
The use of sodium acetate in water purification systems presents both potential benefits and environmental concerns that require careful consideration. As a chemical compound, sodium acetate's impact on the environment is multifaceted and depends largely on its application methods and disposal practices.
One of the primary environmental advantages of using sodium acetate in water treatment is its potential to reduce the reliance on more harmful chemicals. Traditional water purification often involves the use of chlorine-based compounds, which can lead to the formation of toxic byproducts when reacting with organic matter in water. Sodium acetate, being a relatively benign substance, may offer a safer alternative that minimizes the risk of introducing harmful substances into aquatic ecosystems.
However, the increased use of sodium acetate in water systems could lead to elevated sodium levels in treated water and surrounding environments. This may pose challenges for freshwater ecosystems and agricultural lands sensitive to sodium content. Careful monitoring and management of sodium levels would be necessary to prevent potential negative impacts on soil structure and plant growth in areas where treated water is used for irrigation.
The production and transportation of sodium acetate also carry environmental implications. While it can be synthesized through relatively simple processes, large-scale production may contribute to industrial emissions and energy consumption. Sustainable manufacturing practices and efficient transportation methods would be crucial in mitigating these environmental costs.
From a waste management perspective, sodium acetate's biodegradability offers an advantage over some persistent chemicals used in water treatment. It can be broken down by microorganisms in the environment, potentially reducing long-term accumulation in ecosystems. However, improper disposal or accidental release of concentrated sodium acetate solutions could still lead to localized environmental disturbances, particularly in aquatic habitats sensitive to pH and mineral balance changes.
The environmental impact of sodium acetate in water purification systems also extends to its potential role in reducing the need for extensive water treatment infrastructure. If effective, it could lead to more decentralized and energy-efficient water purification methods, potentially decreasing the overall environmental footprint of water treatment processes.
In conclusion, while sodium acetate shows promise as a more environmentally friendly option for water purification, its widespread adoption would require comprehensive environmental impact assessments. These should address potential effects on aquatic ecosystems, soil health, and broader environmental systems. Balancing the benefits of improved water quality against potential ecological risks will be crucial in determining the overall environmental viability of sodium acetate-based water purification technologies.
Regulatory Framework
The regulatory framework surrounding the use of sodium acetate in water purification systems is complex and multifaceted, involving various governmental agencies and international bodies. In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating water treatment chemicals and processes. The EPA's National Primary Drinking Water Regulations set legally enforceable standards for contaminants in drinking water, which any purification method using sodium acetate must adhere to.
The Food and Drug Administration (FDA) also has oversight in this area, particularly concerning the use of sodium acetate as an indirect food additive when used in water treatment processes that may affect food production or processing. The FDA's regulations ensure that the use of such chemicals does not result in harmful residues in food products.
Internationally, the World Health Organization (WHO) provides guidelines for drinking water quality, which many countries use as a basis for their national standards. These guidelines address the potential health impacts of various water treatment chemicals, including sodium acetate, and provide recommendations for their safe use and maximum allowable concentrations.
In the European Union, the European Chemicals Agency (ECHA) regulates the use of chemicals under the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation. This framework requires manufacturers and importers of chemicals, including those used in water purification, to assess and manage the risks associated with their substances.
Many countries have their own regulatory bodies responsible for water quality and treatment standards. For example, in Canada, Health Canada works with the provinces, territories, and other federal departments to develop the Guidelines for Canadian Drinking Water Quality. These guidelines would need to be considered when implementing sodium acetate-based purification systems in Canadian water treatment facilities.
The regulatory landscape also includes industry standards and best practices, such as those developed by the American Water Works Association (AWWA) and the Water Environment Federation (WEF). These organizations provide guidance on the selection, application, and management of water treatment technologies, which would encompass the use of sodium acetate in purification processes.
As research into sodium acetate's potential in water purification advances, it is likely that regulatory frameworks will evolve to address any new findings regarding its efficacy and safety. This may involve updates to existing regulations or the development of new standards specific to sodium acetate-based purification methods.