Sodium Acetate’s Role in the Symbiosis of Bio‑Based Chemicals
Sodium Acetate Symbiosis Background and Objectives
Sodium acetate has emerged as a pivotal compound in the symbiosis of bio-based chemicals, marking a significant shift towards sustainable and eco-friendly industrial processes. This organic salt, formed by the reaction of acetic acid with sodium hydroxide, has been known for centuries but has recently gained renewed attention in the context of bioeconomy and circular chemistry.
The evolution of sodium acetate's role in bio-based chemical symbiosis can be traced back to the increasing global emphasis on reducing carbon footprints and developing renewable resources. As industries seek alternatives to petrochemical-based products, sodium acetate has become a focal point due to its versatile applications and potential as a platform chemical in various bio-based processes.
The primary objective of exploring sodium acetate's symbiotic role is to establish interconnected bio-based production systems where the byproduct or waste from one process serves as a valuable input for another. This approach aims to maximize resource efficiency, minimize waste, and create closed-loop systems that align with circular economy principles.
One of the key drivers behind the growing interest in sodium acetate is its potential to act as a bridge between different bio-based chemical processes. For instance, it can be derived from the fermentation of organic waste and subsequently used as a feedstock for the production of various chemicals, including bioplastics, solvents, and pharmaceuticals.
The technological evolution in this field has been marked by significant advancements in fermentation processes, purification techniques, and the development of novel catalysts that enable the efficient conversion of sodium acetate into high-value products. These innovations have paved the way for more economically viable and environmentally sustainable production methods.
As research progresses, the symbiotic role of sodium acetate is expected to expand further, potentially revolutionizing the bio-based chemical industry. The ultimate goal is to create a network of interconnected processes where sodium acetate serves as a central hub, facilitating the efficient utilization of resources and minimizing environmental impact.
Understanding the background and objectives of sodium acetate symbiosis is crucial for predicting future trends and identifying potential breakthroughs in the field of bio-based chemicals. This knowledge will guide further research and development efforts, ultimately contributing to the transition towards a more sustainable and circular bioeconomy.
Market Analysis for Bio-Based Chemicals
The bio-based chemicals market has been experiencing significant growth in recent years, driven by increasing environmental concerns and the push for sustainable alternatives to petroleum-based products. Sodium acetate, a versatile compound with applications in various industries, plays a crucial role in this expanding market.
The global bio-based chemicals market is projected to reach substantial value in the coming years, with a compound annual growth rate (CAGR) outpacing many traditional chemical sectors. This growth is fueled by factors such as government regulations promoting sustainable practices, consumer demand for eco-friendly products, and technological advancements in bio-based production processes.
Sodium acetate, as a key player in the bio-based chemicals landscape, contributes to this market expansion through its diverse applications. In the food industry, it serves as a preservative and flavoring agent, aligning with the growing trend of clean label and natural ingredients. The pharmaceutical sector utilizes sodium acetate in various formulations, benefiting from its biocompatibility and safety profile.
The textile industry represents another significant market for sodium acetate, where it is used in dyeing processes and as a buffering agent. As the demand for sustainable textiles rises, the role of bio-based chemicals like sodium acetate becomes increasingly important. Additionally, the compound finds applications in de-icing solutions, offering an environmentally friendly alternative to traditional road salts.
The symbiotic relationship between sodium acetate and other bio-based chemicals creates opportunities for integrated production processes and circular economy models. This synergy not only enhances the economic viability of bio-based chemical production but also contributes to reducing overall environmental impact.
Market trends indicate a shift towards bio-based alternatives across various industries, with sodium acetate well-positioned to capitalize on this movement. The compound's versatility and compatibility with other bio-based chemicals make it an attractive option for manufacturers looking to transition away from petroleum-based products.
Regionally, North America and Europe lead in the adoption of bio-based chemicals, including sodium acetate, due to stringent environmental regulations and consumer awareness. However, emerging economies in Asia-Pacific and Latin America are expected to witness rapid growth in the bio-based chemicals market, presenting new opportunities for sodium acetate producers and related industries.
Challenges in the market include the need for cost-competitive production methods and the establishment of reliable supply chains for bio-based feedstocks. However, ongoing research and development efforts are addressing these issues, potentially leading to more efficient and economical production of sodium acetate and other bio-based chemicals.
Current Challenges in Sodium Acetate Symbiosis
The symbiosis of sodium acetate in bio-based chemical production faces several significant challenges that hinder its widespread adoption and optimal utilization. One of the primary obstacles is the high production cost associated with sodium acetate synthesis. Traditional methods often involve energy-intensive processes or rely on petrochemical feedstocks, which contradicts the sustainability goals of bio-based chemical production.
Another challenge lies in the purification and separation of sodium acetate from complex biological mixtures. The presence of various organic compounds and impurities in fermentation broths or other bio-based processes makes it difficult to isolate high-purity sodium acetate efficiently. This issue not only affects the quality of the final product but also increases the overall production costs.
The stability of sodium acetate under varying process conditions poses another significant challenge. In many bio-based chemical processes, pH fluctuations, temperature changes, and the presence of other ions can affect the stability and functionality of sodium acetate. This instability can lead to reduced efficiency in its role as a buffer, pH regulator, or intermediate in various biochemical reactions.
Furthermore, the limited understanding of sodium acetate's interactions with other bio-based chemicals and microorganisms in complex systems presents a challenge. While its role in certain processes is well-documented, its potential synergies or antagonistic effects in novel bio-based chemical production pathways remain largely unexplored. This knowledge gap hampers the development of optimized processes that fully leverage sodium acetate's capabilities.
The scalability of sodium acetate production and integration into large-scale bio-based chemical processes is another hurdle. Many promising lab-scale results fail to translate effectively to industrial-scale operations due to challenges in maintaining consistent quality, managing waste streams, and optimizing process parameters.
Additionally, the environmental impact of sodium acetate production and use in bio-based chemical symbiosis needs further assessment. While it is generally considered a more sustainable option compared to many petrochemical alternatives, concerns remain about energy consumption, water usage, and potential environmental effects of large-scale production and application.
Lastly, regulatory challenges and market acceptance pose significant obstacles to the widespread adoption of sodium acetate in bio-based chemical symbiosis. Stringent regulations on chemical use in various industries, coupled with the need for extensive safety and efficacy data, can slow down the integration of sodium acetate into new applications. Moreover, convincing industries to shift from established processes to those incorporating sodium acetate requires demonstrating clear economic and environmental benefits, which can be challenging given the complexities of bio-based production systems.
Existing Sodium Acetate Symbiosis Solutions
01 Sodium acetate in chemical processes
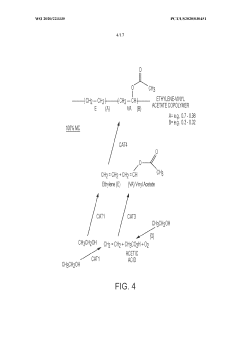
Sodium acetate is widely used in various chemical processes, including as a catalyst, pH regulator, and reagent in organic synthesis. It plays a crucial role in industrial applications such as textile manufacturing, food processing, and pharmaceutical production.- Use of sodium acetate in chemical processes: Sodium acetate is widely used in various chemical processes as a reagent, catalyst, or buffer. It plays a role in reactions such as acetylation, esterification, and pH control. Its properties make it valuable in industrial applications and laboratory settings.
- Application in heat storage and thermal management: Sodium acetate trihydrate is utilized in heat storage systems and thermal management applications. It undergoes phase changes that allow it to store and release heat effectively, making it useful in heating pads, hand warmers, and energy storage solutions.
- Use in food and beverage industry: Sodium acetate serves as a food additive and preservative in the food and beverage industry. It acts as a flavoring agent, acidity regulator, and helps extend the shelf life of various products. Its use is regulated and approved by food safety authorities.
- Application in textile and leather processing: In the textile and leather industries, sodium acetate is used in dyeing processes, as a mordant, and for pH adjustment. It helps improve color fastness and overall quality of the treated materials. Its properties make it suitable for various fabric and leather treatments.
- Use in environmental and waste treatment: Sodium acetate finds applications in environmental and waste treatment processes. It can be used in wastewater treatment, as a deicer for roads, and in certain pollution control methods. Its biodegradability and relatively low environmental impact make it suitable for these purposes.
02 Sodium acetate in heat storage applications
Sodium acetate trihydrate is utilized as a phase change material for thermal energy storage. It has a high latent heat of fusion and can store and release heat at specific temperatures, making it suitable for applications in heating systems, temperature-controlled packaging, and thermal management in buildings.Expand Specific Solutions03 Sodium acetate in food and beverage industry
Sodium acetate is used as a food additive and preservative. It acts as a acidity regulator, flavoring agent, and antimicrobial agent in various food products. It helps extend shelf life, enhance taste, and maintain product quality in processed foods and beverages.Expand Specific Solutions04 Sodium acetate in water treatment
Sodium acetate is employed in water treatment processes for pH adjustment and as a dechlorinating agent. It helps neutralize acidic water and remove residual chlorine from treated water, making it useful in municipal water treatment plants and industrial wastewater management systems.Expand Specific Solutions05 Sodium acetate in medical and pharmaceutical applications
Sodium acetate is used in medical and pharmaceutical formulations as a buffering agent and electrolyte replacement. It is utilized in intravenous fluids, dialysis solutions, and as an excipient in various drug formulations. Additionally, it has applications in diagnostic tests and as a component in some medical devices.Expand Specific Solutions
Key Players in Bio-Based Chemical Industry
The symbiosis of bio-based chemicals, including sodium acetate, is an emerging field in the transition towards sustainable industrial processes. The market is in its early growth stage, with increasing demand driven by environmental concerns and regulatory pressures. While the global market size is expanding, it remains relatively small compared to traditional chemical industries. Technologically, the field is rapidly evolving, with companies like Abbott Laboratories, Amgen, and BASF leading research efforts. Universities such as Rice University and the National University of Singapore are also contributing significantly to advancing the technology. However, the overall maturity level varies across different applications and processes, indicating a dynamic and competitive landscape with substantial room for innovation and market growth.
PTT Global Chemical Plc
Xyleco, Inc.
Core Innovations in Sodium Acetate Utilization
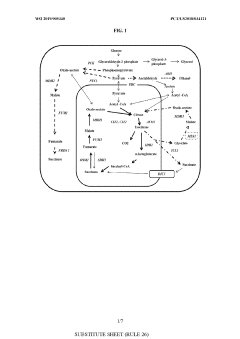
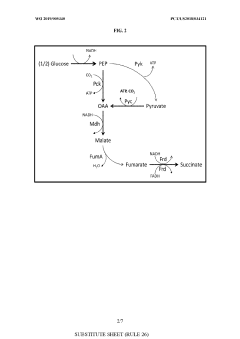
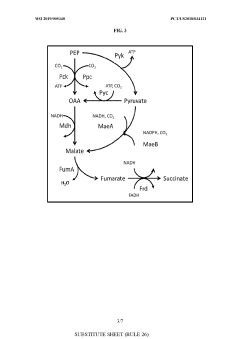
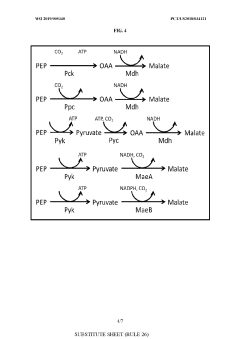
- Genetically modified yeast cells with altered metabolic pathways, including a reductive succinic acid pathway operational in the cytoplasm, enhanced pentose phosphate pathway activity, and a modified glyoxylate shunt, to facilitate succinic acid production and redox balance, utilizing enzymes like pyruvate kinase, malic enzyme, fumarase, and formate dehydrogenase to produce succinate in the cytoplasm.
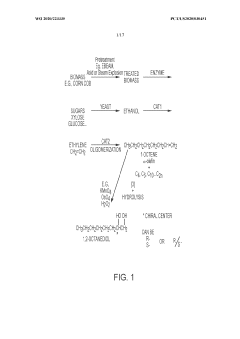
- Bio-based ethylene is produced from biomass sources such as lignocellulosic or starchy materials through ethanol dehydration, allowing for the creation of a variety of bio-based chemical compounds and polymers with high modern carbon content, reducing fossil fuel consumption and greenhouse gas emissions.
Environmental Impact Assessment
The environmental impact assessment of sodium acetate's role in the symbiosis of bio-based chemicals reveals both positive and negative implications. On the positive side, the use of sodium acetate as a platform chemical in bio-based production processes can contribute to reducing reliance on fossil fuel-derived chemicals, thereby potentially lowering greenhouse gas emissions and mitigating climate change impacts.
Sodium acetate's production from renewable resources, such as biomass or waste streams, aligns with circular economy principles and can help reduce overall environmental footprint compared to traditional petrochemical routes. Its biodegradability also means less persistent pollution in ecosystems when released into the environment.
However, the environmental benefits are not without caveats. The production of sodium acetate through bio-based routes still requires energy inputs and may involve the use of chemicals or enzymes that have their own environmental impacts. The sourcing of biomass feedstocks for sodium acetate production must be carefully managed to avoid competition with food crops or negative land-use changes.
Water consumption and potential eutrophication risks associated with the production and use of sodium acetate in bio-based chemical processes need to be carefully assessed and mitigated. Proper waste management strategies must be implemented to handle byproducts and residues from sodium acetate-related processes.
Life cycle assessments (LCAs) comparing sodium acetate-based symbiotic systems with conventional chemical production routes are crucial for quantifying the net environmental benefits. These assessments should consider factors such as energy efficiency, water usage, land use changes, and potential for carbon sequestration.
The scalability of sodium acetate-centered symbiotic systems also plays a role in their overall environmental impact. As production scales up, efficiencies may improve, potentially enhancing the environmental benefits. However, increased scale may also lead to more significant localized impacts on ecosystems and communities near production facilities.
Regulatory frameworks and industry standards will be essential in ensuring that the environmental benefits of sodium acetate in bio-based chemical symbiosis are maximized while potential negative impacts are minimized. This includes setting guidelines for sustainable feedstock sourcing, emissions control, and waste management practices.
Regulatory Framework for Bio-Based Chemicals
The regulatory framework for bio-based chemicals, including sodium acetate, is a complex and evolving landscape that significantly impacts the development and commercialization of these sustainable alternatives. At the global level, organizations such as the United Nations Environment Programme (UNEP) and the Organisation for Economic Co-operation and Development (OECD) provide guidelines and recommendations for the sustainable management of chemicals, including bio-based ones.
In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating bio-based chemicals under the Toxic Substances Control Act (TSCA). The TSCA requires manufacturers to submit premanufacture notices for new chemical substances, including those derived from biological sources. The EPA evaluates these notices to determine potential risks to human health and the environment.
The European Union has implemented the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation, which applies to all chemicals, including bio-based ones. REACH requires companies to register chemical substances and provide safety data, promoting the safe use of chemicals throughout their lifecycle.
In addition to these overarching regulations, specific standards and certifications have been developed for bio-based products. For instance, the USDA BioPreferred Program in the United States promotes the purchase and use of bio-based products through federal procurement preferences and voluntary labeling.
The International Organization for Standardization (ISO) has also developed standards for bio-based products, such as ISO 16620 for the determination of bio-based carbon content. These standards help ensure consistency and quality in the bio-based chemicals industry.
Regulatory frameworks also address the environmental impact of bio-based chemicals. Life Cycle Assessment (LCA) methodologies are increasingly being incorporated into regulations to evaluate the overall environmental footprint of these products. This approach considers factors such as raw material sourcing, production processes, and end-of-life disposal.
As the bio-based chemicals industry continues to grow, regulatory bodies are adapting their frameworks to address new challenges and opportunities. This includes developing guidelines for novel production methods, such as synthetic biology, and addressing concerns related to genetic modification and biosecurity.
The regulatory landscape for bio-based chemicals also intersects with broader sustainability initiatives, such as the United Nations Sustainable Development Goals (SDGs) and circular economy strategies. These frameworks encourage the development of bio-based chemicals as part of a more sustainable and resource-efficient economy.