Strategies for enhancing yield in cell-free protein expression.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-Free Protein Expression Background and Objectives
Cell-free protein expression (CFPE) represents a paradigm shift in biotechnology, emerging from the convergence of biochemistry and synthetic biology. This technology evolved from early cell extract experiments in the 1950s to today's sophisticated systems capable of producing complex proteins outside living cells. The fundamental principle involves extracting cellular machinery necessary for transcription and translation while eliminating cellular components that might interfere with protein production.
The evolution of CFPE systems has been marked by significant milestones, including the development of coupled transcription-translation systems in the 1980s, the commercialization of cell-free lysates in the 1990s, and recent advancements in extract preparation methods that have dramatically improved protein yields. Contemporary CFPE platforms derive from various organisms including Escherichia coli, wheat germ, rabbit reticulocytes, insect cells, and increasingly, cell-free systems based on CHO cells and human cell lines.
The primary objective of enhancing yield in CFPE systems is to achieve protein production levels comparable to or exceeding those of traditional cell-based systems while maintaining cost-effectiveness. This involves optimizing reaction conditions, energy regeneration systems, and translation efficiency to maximize protein output per unit of input material.
Technical goals in this field include developing standardized protocols for extract preparation that ensure consistency across batches, designing energy regeneration systems that extend reaction duration, and creating genetic elements that enhance transcription and translation efficiency. Additionally, there is significant interest in scaling up CFPE reactions from laboratory to industrial volumes while maintaining productivity.
The trajectory of CFPE technology points toward increasingly integrated systems that combine multiple enzymatic pathways to support complex post-translational modifications, particularly important for biopharmaceutical applications. Emerging trends include the development of continuous-flow CFPE systems, the integration of artificial intelligence for reaction optimization, and the creation of freeze-dried cell-free systems that remain stable at ambient temperatures.
Understanding the historical context and technical evolution of CFPE provides essential insights for developing strategies to enhance protein yields. The field continues to advance rapidly, driven by applications in synthetic biology, rapid prototyping of proteins, biosensing, and on-demand production of therapeutics and vaccines.
The evolution of CFPE systems has been marked by significant milestones, including the development of coupled transcription-translation systems in the 1980s, the commercialization of cell-free lysates in the 1990s, and recent advancements in extract preparation methods that have dramatically improved protein yields. Contemporary CFPE platforms derive from various organisms including Escherichia coli, wheat germ, rabbit reticulocytes, insect cells, and increasingly, cell-free systems based on CHO cells and human cell lines.
The primary objective of enhancing yield in CFPE systems is to achieve protein production levels comparable to or exceeding those of traditional cell-based systems while maintaining cost-effectiveness. This involves optimizing reaction conditions, energy regeneration systems, and translation efficiency to maximize protein output per unit of input material.
Technical goals in this field include developing standardized protocols for extract preparation that ensure consistency across batches, designing energy regeneration systems that extend reaction duration, and creating genetic elements that enhance transcription and translation efficiency. Additionally, there is significant interest in scaling up CFPE reactions from laboratory to industrial volumes while maintaining productivity.
The trajectory of CFPE technology points toward increasingly integrated systems that combine multiple enzymatic pathways to support complex post-translational modifications, particularly important for biopharmaceutical applications. Emerging trends include the development of continuous-flow CFPE systems, the integration of artificial intelligence for reaction optimization, and the creation of freeze-dried cell-free systems that remain stable at ambient temperatures.
Understanding the historical context and technical evolution of CFPE provides essential insights for developing strategies to enhance protein yields. The field continues to advance rapidly, driven by applications in synthetic biology, rapid prototyping of proteins, biosensing, and on-demand production of therapeutics and vaccines.
Market Analysis for Cell-Free Protein Production Systems
The cell-free protein expression systems market has been experiencing significant growth, driven by increasing demand for rapid protein production technologies across pharmaceutical, biotechnology, and academic research sectors. The global market for cell-free protein expression systems was valued at approximately $208 million in 2020 and is projected to reach $312 million by 2025, growing at a CAGR of 8.4% during the forecast period.
Pharmaceutical and biotechnology companies represent the largest market segment, accounting for nearly 60% of the total market share. These companies primarily utilize cell-free protein expression systems for drug discovery, protein engineering, and therapeutic protein production. Academic and research institutions constitute the second-largest segment, contributing about 25% of the market revenue, primarily focusing on fundamental research and technology development.
Geographically, North America dominates the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China, Japan, and South Korea, is expected to witness the highest growth rate due to increasing investments in biotechnology research and development infrastructure.
Key market drivers include the advantages of cell-free systems over traditional cell-based methods, such as reduced production time, elimination of cell viability concerns, and the ability to produce difficult-to-express proteins. The growing focus on personalized medicine and biologics has further accelerated market growth, as these systems enable rapid prototyping and production of protein-based therapeutics.
Market restraints include the high cost of reagents and equipment, technical challenges in scaling up production, and regulatory uncertainties surrounding products developed using cell-free systems. Additionally, limited yield compared to traditional cell-based systems remains a significant challenge that industry players are actively addressing through technological innovations.
The competitive landscape features established players like Thermo Fisher Scientific, Merck KGaA, and New England Biolabs, alongside emerging companies specializing in cell-free technology such as Sutro Biopharma and Arbor Biotechnologies. Strategic collaborations between academic institutions and industry partners are increasingly common, accelerating technology transfer and commercialization of novel cell-free expression platforms.
Customer segments are diversifying beyond traditional research applications, with growing interest from diagnostic companies, synthetic biology startups, and contract manufacturing organizations. This expansion indicates the versatility and increasing acceptance of cell-free protein expression as a mainstream technology in the biotechnology industry.
Pharmaceutical and biotechnology companies represent the largest market segment, accounting for nearly 60% of the total market share. These companies primarily utilize cell-free protein expression systems for drug discovery, protein engineering, and therapeutic protein production. Academic and research institutions constitute the second-largest segment, contributing about 25% of the market revenue, primarily focusing on fundamental research and technology development.
Geographically, North America dominates the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China, Japan, and South Korea, is expected to witness the highest growth rate due to increasing investments in biotechnology research and development infrastructure.
Key market drivers include the advantages of cell-free systems over traditional cell-based methods, such as reduced production time, elimination of cell viability concerns, and the ability to produce difficult-to-express proteins. The growing focus on personalized medicine and biologics has further accelerated market growth, as these systems enable rapid prototyping and production of protein-based therapeutics.
Market restraints include the high cost of reagents and equipment, technical challenges in scaling up production, and regulatory uncertainties surrounding products developed using cell-free systems. Additionally, limited yield compared to traditional cell-based systems remains a significant challenge that industry players are actively addressing through technological innovations.
The competitive landscape features established players like Thermo Fisher Scientific, Merck KGaA, and New England Biolabs, alongside emerging companies specializing in cell-free technology such as Sutro Biopharma and Arbor Biotechnologies. Strategic collaborations between academic institutions and industry partners are increasingly common, accelerating technology transfer and commercialization of novel cell-free expression platforms.
Customer segments are diversifying beyond traditional research applications, with growing interest from diagnostic companies, synthetic biology startups, and contract manufacturing organizations. This expansion indicates the versatility and increasing acceptance of cell-free protein expression as a mainstream technology in the biotechnology industry.
Current Challenges in Cell-Free Protein Yield Optimization
Despite significant advancements in cell-free protein expression systems, several persistent challenges continue to limit optimal protein yield. One of the primary obstacles is the rapid depletion of energy resources during the reaction process. ATP and other high-energy molecules are consumed quickly, leading to premature termination of protein synthesis before maximum yield can be achieved. This energy limitation represents a fundamental constraint that affects all cell-free protein expression platforms.
Extract preparation inconsistency presents another significant challenge. The quality and composition of cell extracts can vary considerably between batches, affecting reproducibility and scalability of protein production. This variability stems from differences in cell growth conditions, harvesting times, and lysis methods, all of which can significantly impact the final protein yield and quality.
Proteolytic degradation remains a persistent issue in cell-free systems. Without the protective compartmentalization found in living cells, expressed proteins are exposed to proteases present in the cell extract, leading to degradation before sufficient quantities can accumulate. This problem is particularly pronounced for complex or unstable protein targets.
The limited duration of cell-free reactions represents another key challenge. Most systems remain active for only 4-8 hours before components become depleted or inhibitory byproducts accumulate. This temporal constraint limits the total protein yield that can be achieved in a single reaction, necessitating either larger reaction volumes or repeated batch processes.
Post-translational modifications present significant hurdles for certain protein classes. While prokaryotic cell-free systems are efficient for basic protein expression, they lack the machinery for complex eukaryotic modifications such as glycosylation, phosphorylation, and disulfide bond formation, limiting their utility for many therapeutic proteins and complex enzymes.
Inhibition by reaction byproducts constitutes another yield-limiting factor. As protein synthesis proceeds, the accumulation of inorganic phosphate and other metabolic byproducts can inhibit translation machinery and reduce overall system efficiency. This feedback inhibition becomes increasingly problematic as reaction scale increases.
Cost considerations remain a significant barrier to widespread adoption. High-quality reagents, especially nucleotides and energy regeneration components, contribute substantially to the overall expense of cell-free protein production. This economic constraint limits industrial scalability and commercial applications despite the technical advantages of cell-free systems.
Optimizing translation efficiency continues to challenge researchers, as the complex interplay between ribosomes, mRNA stability, and translation factors is not fully understood or controllable in cell-free environments. This knowledge gap hampers rational design approaches to yield enhancement.
Extract preparation inconsistency presents another significant challenge. The quality and composition of cell extracts can vary considerably between batches, affecting reproducibility and scalability of protein production. This variability stems from differences in cell growth conditions, harvesting times, and lysis methods, all of which can significantly impact the final protein yield and quality.
Proteolytic degradation remains a persistent issue in cell-free systems. Without the protective compartmentalization found in living cells, expressed proteins are exposed to proteases present in the cell extract, leading to degradation before sufficient quantities can accumulate. This problem is particularly pronounced for complex or unstable protein targets.
The limited duration of cell-free reactions represents another key challenge. Most systems remain active for only 4-8 hours before components become depleted or inhibitory byproducts accumulate. This temporal constraint limits the total protein yield that can be achieved in a single reaction, necessitating either larger reaction volumes or repeated batch processes.
Post-translational modifications present significant hurdles for certain protein classes. While prokaryotic cell-free systems are efficient for basic protein expression, they lack the machinery for complex eukaryotic modifications such as glycosylation, phosphorylation, and disulfide bond formation, limiting their utility for many therapeutic proteins and complex enzymes.
Inhibition by reaction byproducts constitutes another yield-limiting factor. As protein synthesis proceeds, the accumulation of inorganic phosphate and other metabolic byproducts can inhibit translation machinery and reduce overall system efficiency. This feedback inhibition becomes increasingly problematic as reaction scale increases.
Cost considerations remain a significant barrier to widespread adoption. High-quality reagents, especially nucleotides and energy regeneration components, contribute substantially to the overall expense of cell-free protein production. This economic constraint limits industrial scalability and commercial applications despite the technical advantages of cell-free systems.
Optimizing translation efficiency continues to challenge researchers, as the complex interplay between ribosomes, mRNA stability, and translation factors is not fully understood or controllable in cell-free environments. This knowledge gap hampers rational design approaches to yield enhancement.
Current Yield Enhancement Methodologies and Approaches
01 Optimization of cell-free expression systems
Cell-free protein expression systems can be optimized by modifying reaction conditions such as temperature, pH, and component concentrations. These optimizations can significantly increase protein yield by creating an ideal environment for translation machinery. Strategies include adjusting energy regeneration systems, optimizing amino acid concentrations, and balancing transcription and translation rates to maximize protein production efficiency.- Optimization of cell-free expression systems: Various strategies can be employed to optimize cell-free protein expression systems for increased yield. These include modifying reaction conditions such as temperature, pH, and incubation time, as well as optimizing the composition of the reaction mixture. The addition of specific components like energy regeneration systems, nucleotides, and amino acids can significantly enhance protein production efficiency in cell-free environments.
- Genetic engineering approaches for yield improvement: Genetic modifications of template DNA and expression vectors can substantially improve cell-free protein expression yields. These approaches include codon optimization, incorporation of strong promoters, enhancers, and efficient translation initiation sites. Additionally, engineering the genetic elements to reduce secondary structures in mRNA can facilitate better translation efficiency, resulting in higher protein yields.
- Supplementation with chaperones and folding enhancers: The addition of molecular chaperones, folding enhancers, and disulfide bond isomerases to cell-free expression systems can improve the yield of correctly folded, functional proteins. These additives help prevent protein aggregation and facilitate proper protein folding, particularly for complex or difficult-to-express proteins, thereby increasing the overall yield of biologically active proteins.
- Continuous-exchange cell-free systems: Continuous-exchange cell-free (CECF) systems involve the continuous supply of substrates and removal of inhibitory byproducts during the protein synthesis reaction. This approach extends the duration of active protein synthesis and significantly increases yield compared to batch reactions. Various dialysis and flow-based systems have been developed to implement this concept, resulting in multi-fold improvements in protein production.
- Alternative energy sources and metabolic engineering: Incorporating alternative energy sources and metabolic engineering strategies can enhance the energy efficiency of cell-free protein expression systems. These approaches include using non-conventional energy-rich compounds, engineering metabolic pathways to reduce energy consumption, and implementing regenerative energy systems. Such modifications help overcome energy limitations that typically constrain protein synthesis yields in cell-free environments.
02 Enhanced extract preparation methods
The preparation method of cell extracts significantly impacts protein expression yield. Advanced extraction techniques that preserve enzymatic activities and remove inhibitory components can substantially improve yields. These methods include optimized cell lysis protocols, extract clarification procedures, and supplementation with specific cofactors that maintain the integrity of the translation machinery, resulting in higher protein production capacity.Expand Specific Solutions03 Genetic template optimization
Optimizing the genetic templates used in cell-free protein expression systems can lead to increased yields. This includes codon optimization for the cell-free system, designing efficient promoters and ribosome binding sites, and engineering mRNA stability elements. Template modifications that enhance transcription efficiency and mRNA stability contribute to higher protein production rates in cell-free systems.Expand Specific Solutions04 Supplementation with expression enhancers
Adding specific components to cell-free reaction mixtures can enhance protein expression yields. These include molecular chaperones that assist in protein folding, protease inhibitors that prevent protein degradation, and specific ions or cofactors that optimize enzymatic activities. Supplementation strategies can be tailored to specific protein targets, addressing bottlenecks in the expression process and improving overall yields.Expand Specific Solutions05 Continuous-exchange cell-free systems
Continuous-exchange cell-free systems allow for the replenishment of nutrients and removal of inhibitory byproducts during protein synthesis. These systems utilize dialysis membranes or microfluidic devices to maintain optimal reaction conditions over extended periods. By addressing resource limitations and inhibitory feedback mechanisms, continuous-exchange systems can achieve significantly higher protein yields compared to batch reactions.Expand Specific Solutions
Leading Companies and Research Institutions in Cell-Free Technology
Cell-free protein expression technology is currently in a growth phase, with increasing market adoption driven by its advantages in rapid prototyping and complex protein production. The global market is expanding steadily, estimated to reach significant value as applications diversify across pharmaceutical, diagnostic, and research sectors. Technologically, the field shows varying maturity levels among key players. Companies like Sutro Biopharma and Lonza have established advanced platforms with clinical applications, while Leniobio and QIAGEN offer commercially viable systems. Research institutions including Tsinghua University and Northwestern University continue to push boundaries in yield optimization. Emerging players such as Kangma Biological Technology and PAEAN Biotechnology are developing novel approaches to address efficiency challenges, indicating a competitive landscape with both established leaders and innovative newcomers driving technological advancement.
Lonza Ltd.
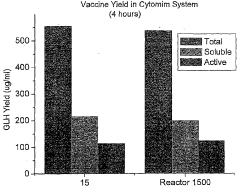
Technical Solution: Lonza has developed a proprietary cell-free protein expression platform called ExpiCHO™ that focuses on enhancing yield through optimized reaction conditions. Their strategy involves a multi-faceted approach including: 1) Engineered CHO cell extracts with reduced nuclease activity to preserve template DNA integrity [1]; 2) Customized energy regeneration systems that maintain ATP levels throughout the reaction; 3) Supplementation with specific amino acids and cofactors to prevent resource depletion; 4) Optimized transcription-translation coupling mechanisms that improve protein folding efficiency; and 5) Post-translational modification capabilities that mimic in vivo conditions. Lonza's platform incorporates continuous-exchange cell-free (CECF) technology that allows for extended reaction times by continuously supplying fresh substrates while removing inhibitory byproducts [3], resulting in reported yield improvements of up to 10-fold compared to batch reactions.
Strengths: Superior scalability from microliter to liter scale while maintaining consistent protein quality; excellent post-translational modification capabilities mimicking mammalian systems; reduced batch-to-batch variability through standardized extract preparation. Weaknesses: Higher cost compared to E. coli-based systems; complex setup requirements for continuous exchange systems; limited shelf-life of reaction components requiring cold chain logistics.
Sutro Biopharma, Inc.
Technical Solution: Sutro Biopharma has pioneered XpressCF®, an industrialized cell-free protein synthesis platform specifically engineered for therapeutic protein production. Their yield enhancement strategy centers on extract optimization and reaction engineering: 1) Development of proprietary E. coli strains with reduced protease activity and enhanced translation machinery [2]; 2) Implementation of semi-continuous flow systems that maintain optimal reagent concentrations throughout extended reaction times; 3) Site-specific incorporation of non-natural amino acids (nnAAs) through orthogonal tRNA/synthetase pairs, enabling precise conjugation points for antibody-drug conjugates; 4) Customized energy regeneration systems utilizing phosphoenolpyruvate (PEP) and creatine phosphate to sustain ATP levels; and 5) Optimization of transcriptional and translational elements including promoters, UTRs, and ribosome binding sites. Sutro's platform has demonstrated protein yields exceeding 1 g/L for certain products [4], representing a significant advancement over traditional cell-free systems that typically produce in the mg/L range.
Strengths: Exceptional scalability (demonstrated up to 100L scale); rapid production timelines (24-48 hours from gene to protein); precise control over site-specific modifications enabling novel conjugated therapeutics; elimination of cell viability concerns. Weaknesses: Higher cost of goods compared to cell-based systems; challenges with complex post-translational modifications; intellectual property constraints limiting broader application; dependency on specialized equipment for large-scale production.
Key Innovations in Cell-Free Protein Expression Components
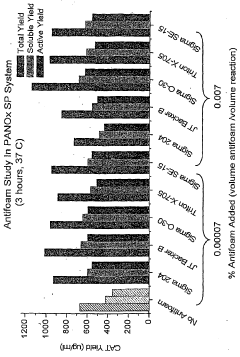
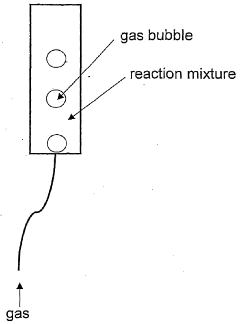
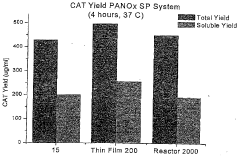
Protein expression yield enhancement in cell-free protein synthesis systems by addition of antifoam agents
PatentInactiveNZ549523A
Innovation
- The addition of antifoam agents to cell-free protein synthesis reactions, particularly at concentrations between 0.00007% and 0.007% by weight, enhances protein yields by preventing foaming and optimizing reaction conditions, especially in bubble-column reactors under aerobic conditions.
Methods to increase protein production yield of a yeast and human cell-free lysate
PatentWO2025170531A1
Innovation
- Supplementing the CFPS system with compounds such as kinase inhibitors (e.g., bosutinib, cerdulatinib, dasatinib, afatinib) and gonadotropin-releasing hormone receptor agonists (e.g., nafarelin) to increase protein expression by reducing ATP consumption in non-essential metabolic processes, thereby channeling more ATP into protein expression.
Scale-up Considerations for Industrial Applications
Scaling up cell-free protein expression systems from laboratory to industrial scale presents significant challenges that must be addressed to maintain high yields and economic viability. The transition requires careful consideration of bioreactor design, as traditional stirred-tank reactors may not provide optimal conditions for cell-free systems. Novel bioreactor configurations, such as continuous-flow or microfluidic systems, offer promising alternatives by enabling better control over reaction parameters and extending reaction lifetimes.
Raw material sourcing becomes increasingly critical at industrial scales. The cost and quality consistency of key components—including energy sources, nucleotides, amino acids, and enzymes—directly impact both economic feasibility and expression yields. Establishing robust supply chains and developing standardized quality control protocols for these inputs is essential for successful scale-up operations.
Process monitoring and control systems require substantial adaptation when moving to industrial production. Real-time analytics for substrate consumption, protein synthesis rates, and byproduct accumulation enable dynamic adjustments to maintain optimal reaction conditions. Advanced sensor technologies coupled with automated feedback systems can significantly enhance process stability and reproducibility across production batches.
Energy regeneration systems represent a particular challenge at scale. The ATP and GTP requirements of cell-free protein synthesis necessitate efficient regeneration mechanisms that remain effective in larger volumes. Engineering more robust energy regeneration pathways or implementing continuous energy supply strategies can help overcome the energy limitations often observed in scaled-up reactions.
Downstream processing considerations must be integrated into scale-up strategies from the outset. The absence of cellular debris in cell-free systems offers advantages in purification, but larger reaction volumes introduce new separation challenges. Developing scalable, continuous purification methods specifically optimized for cell-free expression products can improve overall process economics.
Regulatory and quality assurance frameworks for industrial-scale cell-free protein production remain underdeveloped compared to traditional cell-based manufacturing. Establishing appropriate GMP guidelines and validation protocols specific to cell-free systems will be crucial for commercial applications, particularly for therapeutic proteins where regulatory requirements are stringent.
Economic modeling indicates that achieving cost-effective production at industrial scale requires optimization across multiple parameters simultaneously. Sensitivity analyses suggest that extending reaction duration, increasing protein yields per unit of extract, and reducing extract preparation costs offer the highest potential returns on investment for industrial implementation of cell-free protein expression technologies.
Raw material sourcing becomes increasingly critical at industrial scales. The cost and quality consistency of key components—including energy sources, nucleotides, amino acids, and enzymes—directly impact both economic feasibility and expression yields. Establishing robust supply chains and developing standardized quality control protocols for these inputs is essential for successful scale-up operations.
Process monitoring and control systems require substantial adaptation when moving to industrial production. Real-time analytics for substrate consumption, protein synthesis rates, and byproduct accumulation enable dynamic adjustments to maintain optimal reaction conditions. Advanced sensor technologies coupled with automated feedback systems can significantly enhance process stability and reproducibility across production batches.
Energy regeneration systems represent a particular challenge at scale. The ATP and GTP requirements of cell-free protein synthesis necessitate efficient regeneration mechanisms that remain effective in larger volumes. Engineering more robust energy regeneration pathways or implementing continuous energy supply strategies can help overcome the energy limitations often observed in scaled-up reactions.
Downstream processing considerations must be integrated into scale-up strategies from the outset. The absence of cellular debris in cell-free systems offers advantages in purification, but larger reaction volumes introduce new separation challenges. Developing scalable, continuous purification methods specifically optimized for cell-free expression products can improve overall process economics.
Regulatory and quality assurance frameworks for industrial-scale cell-free protein production remain underdeveloped compared to traditional cell-based manufacturing. Establishing appropriate GMP guidelines and validation protocols specific to cell-free systems will be crucial for commercial applications, particularly for therapeutic proteins where regulatory requirements are stringent.
Economic modeling indicates that achieving cost-effective production at industrial scale requires optimization across multiple parameters simultaneously. Sensitivity analyses suggest that extending reaction duration, increasing protein yields per unit of extract, and reducing extract preparation costs offer the highest potential returns on investment for industrial implementation of cell-free protein expression technologies.
Economic Feasibility and Cost-Benefit Analysis
The economic viability of cell-free protein expression systems represents a critical consideration for both research institutions and commercial entities. Current cost analyses indicate that cell-free protein synthesis (CFPS) systems require significant initial investment, with reagent costs ranging from $0.03 to $1.00 per microliter of reaction volume, depending on the specific system employed. High-yield commercial kits can cost upwards of $500-1,000 for relatively small reaction volumes, presenting a substantial barrier to widespread adoption.
When comparing CFPS to traditional cell-based expression methods, the economic equation becomes more nuanced. While cell-based systems benefit from economies of scale in large production scenarios, CFPS demonstrates superior cost-effectiveness for small-scale protein production, particularly for difficult-to-express proteins or those requiring rapid turnaround times. Recent advancements in extract preparation protocols have reduced production costs by approximately 60% over the past decade.
The cost-benefit analysis must account for multiple factors beyond direct reagent expenses. Time savings represent a significant economic advantage, with CFPS reducing production timelines from weeks to hours compared to cell-based methods. This acceleration translates to reduced labor costs and faster research-to-market pathways, particularly valuable in therapeutic protein development where time-to-market critically impacts competitive positioning.
Energy efficiency metrics further enhance the economic case for CFPS. Studies indicate that optimized cell-free systems can achieve 2-5 fold higher energy conversion efficiency compared to whole-cell systems, resulting in improved yield-per-resource ratios. Additionally, the simplified downstream processing requirements of CFPS systems reduce purification costs by an estimated 30-40% compared to traditional methods.
Return on investment calculations suggest that for specialized applications, particularly in pharmaceutical development, the higher initial costs of CFPS are offset by reduced development cycles and increased success rates. Sensitivity analysis indicates that as production scales increase beyond 100 liters, traditional cell-based systems regain economic advantage unless significant breakthroughs in CFPS scalability are achieved.
Future economic projections appear promising, with technological improvements expected to reduce CFPS costs by 5-10% annually over the next five years. The development of reusable components, such as immobilized enzymes and regeneration systems for energy sources, could potentially reduce operational costs by up to 70%, dramatically altering the economic feasibility landscape for large-scale applications.
When comparing CFPS to traditional cell-based expression methods, the economic equation becomes more nuanced. While cell-based systems benefit from economies of scale in large production scenarios, CFPS demonstrates superior cost-effectiveness for small-scale protein production, particularly for difficult-to-express proteins or those requiring rapid turnaround times. Recent advancements in extract preparation protocols have reduced production costs by approximately 60% over the past decade.
The cost-benefit analysis must account for multiple factors beyond direct reagent expenses. Time savings represent a significant economic advantage, with CFPS reducing production timelines from weeks to hours compared to cell-based methods. This acceleration translates to reduced labor costs and faster research-to-market pathways, particularly valuable in therapeutic protein development where time-to-market critically impacts competitive positioning.
Energy efficiency metrics further enhance the economic case for CFPS. Studies indicate that optimized cell-free systems can achieve 2-5 fold higher energy conversion efficiency compared to whole-cell systems, resulting in improved yield-per-resource ratios. Additionally, the simplified downstream processing requirements of CFPS systems reduce purification costs by an estimated 30-40% compared to traditional methods.
Return on investment calculations suggest that for specialized applications, particularly in pharmaceutical development, the higher initial costs of CFPS are offset by reduced development cycles and increased success rates. Sensitivity analysis indicates that as production scales increase beyond 100 liters, traditional cell-based systems regain economic advantage unless significant breakthroughs in CFPS scalability are achieved.
Future economic projections appear promising, with technological improvements expected to reduce CFPS costs by 5-10% annually over the next five years. The development of reusable components, such as immobilized enzymes and regeneration systems for energy sources, could potentially reduce operational costs by up to 70%, dramatically altering the economic feasibility landscape for large-scale applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!