Understanding Sodium Acetate's Role in Chemical Reactions
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Acetate Overview
Sodium acetate, a versatile compound with the chemical formula CH3COONa, plays a significant role in various chemical reactions and industrial applications. This salt of acetic acid and sodium is characterized by its white crystalline appearance and mild, vinegar-like odor. Its unique properties make it an essential component in numerous chemical processes and products.
In chemical reactions, sodium acetate often serves as a buffer, helping to maintain a stable pH in solutions. This buffering capacity is particularly useful in biochemical experiments and industrial processes where pH control is crucial. The compound's ability to regulate acidity stems from its composition, which includes both a weak acid (acetate) and its conjugate base (sodium).
Sodium acetate's role extends beyond pH regulation. It is frequently employed as a source of acetate ions in organic synthesis reactions. These acetate ions can act as nucleophiles, participating in various substitution and elimination reactions. This property makes sodium acetate a valuable reagent in the production of esters, certain pharmaceuticals, and other organic compounds.
In the field of analytical chemistry, sodium acetate finds application in chromatography techniques. It is used in the preparation of mobile phases for high-performance liquid chromatography (HPLC) and as a component in buffer solutions for gel electrophoresis. These applications leverage sodium acetate's ability to influence the separation and migration of molecules based on their chemical properties.
The compound's hygroscopic nature and its ability to form a supersaturated solution are exploited in the creation of heat packs and hand warmers. When a supersaturated solution of sodium acetate is triggered to crystallize, it releases heat, demonstrating an exothermic process that has practical applications in consumer products.
In the textile industry, sodium acetate serves as a mordant, enhancing the binding of dyes to fabrics. It also finds use in the leather tanning process, where it helps to neutralize acids and stabilize the leather. These applications showcase the compound's versatility in material science and manufacturing.
Sodium acetate's role in food preservation and flavoring is noteworthy. As an approved food additive (E262), it acts as a preservative and acidity regulator in various food products. Its mild, salty flavor profile makes it a suitable ingredient in snack foods and as a vinegar flavoring agent.
Understanding the multifaceted role of sodium acetate in chemical reactions is crucial for researchers and industry professionals alike. Its diverse applications across chemistry, biochemistry, and material science underscore its importance as a fundamental compound in both laboratory and industrial settings. As research continues, new applications and reactions involving sodium acetate are likely to emerge, further expanding its significance in the field of chemistry.
In chemical reactions, sodium acetate often serves as a buffer, helping to maintain a stable pH in solutions. This buffering capacity is particularly useful in biochemical experiments and industrial processes where pH control is crucial. The compound's ability to regulate acidity stems from its composition, which includes both a weak acid (acetate) and its conjugate base (sodium).
Sodium acetate's role extends beyond pH regulation. It is frequently employed as a source of acetate ions in organic synthesis reactions. These acetate ions can act as nucleophiles, participating in various substitution and elimination reactions. This property makes sodium acetate a valuable reagent in the production of esters, certain pharmaceuticals, and other organic compounds.
In the field of analytical chemistry, sodium acetate finds application in chromatography techniques. It is used in the preparation of mobile phases for high-performance liquid chromatography (HPLC) and as a component in buffer solutions for gel electrophoresis. These applications leverage sodium acetate's ability to influence the separation and migration of molecules based on their chemical properties.
The compound's hygroscopic nature and its ability to form a supersaturated solution are exploited in the creation of heat packs and hand warmers. When a supersaturated solution of sodium acetate is triggered to crystallize, it releases heat, demonstrating an exothermic process that has practical applications in consumer products.
In the textile industry, sodium acetate serves as a mordant, enhancing the binding of dyes to fabrics. It also finds use in the leather tanning process, where it helps to neutralize acids and stabilize the leather. These applications showcase the compound's versatility in material science and manufacturing.
Sodium acetate's role in food preservation and flavoring is noteworthy. As an approved food additive (E262), it acts as a preservative and acidity regulator in various food products. Its mild, salty flavor profile makes it a suitable ingredient in snack foods and as a vinegar flavoring agent.
Understanding the multifaceted role of sodium acetate in chemical reactions is crucial for researchers and industry professionals alike. Its diverse applications across chemistry, biochemistry, and material science underscore its importance as a fundamental compound in both laboratory and industrial settings. As research continues, new applications and reactions involving sodium acetate are likely to emerge, further expanding its significance in the field of chemistry.
Market Applications
Sodium acetate, a versatile compound formed by the reaction of sodium hydroxide and acetic acid, finds extensive applications across various industries due to its unique properties. In the food industry, sodium acetate serves as a preservative and flavor enhancer, extending the shelf life of products while imparting a mild, salty taste. It is commonly used in potato chips, pickles, and other processed foods. The compound's ability to regulate acidity makes it valuable in the production of condiments and sauces, ensuring consistent flavor profiles.
The textile industry utilizes sodium acetate in dyeing processes, where it acts as a buffer to maintain optimal pH levels during color application. This ensures even dye distribution and color fastness in fabrics. Additionally, sodium acetate plays a crucial role in leather tanning, helping to neutralize acids and stabilize the leather's pH, resulting in improved quality and durability of the final product.
In the pharmaceutical sector, sodium acetate is employed as a buffering agent in intravenous fluids and medications. Its ability to maintain pH balance in solutions is essential for the stability and efficacy of various drugs. Furthermore, it serves as a source of electrolytes in rehydration therapies, particularly in cases of severe dehydration or electrolyte imbalances.
The chemical industry harnesses sodium acetate's properties in numerous applications. It acts as a catalyst in organic synthesis reactions, facilitating the production of various compounds. In polymer manufacturing, sodium acetate functions as a polymerization initiator, contributing to the creation of specific plastic materials. The compound also finds use in the production of photographic chemicals, where it aids in developing and fixing processes.
Environmental applications of sodium acetate include its use as a de-icing agent for roads and runways. Its lower environmental impact compared to traditional salt-based de-icers makes it an attractive option for eco-conscious municipalities and airports. In wastewater treatment, sodium acetate serves as a carbon source for biological nutrient removal processes, aiding in the breakdown of pollutants.
The construction industry benefits from sodium acetate's properties in concrete admixtures. It acts as an accelerator, promoting faster setting and curing of concrete, which is particularly useful in cold weather conditions. This application enhances construction efficiency and allows for year-round building activities in various climates.
As research continues to uncover new applications, sodium acetate's market potential is expected to grow. Emerging areas of interest include its use in energy storage systems, where it shows promise as a phase change material for thermal energy storage. The compound's role in sustainable chemistry practices and green technologies is also gaining attention, potentially opening up new market opportunities in the coming years.
The textile industry utilizes sodium acetate in dyeing processes, where it acts as a buffer to maintain optimal pH levels during color application. This ensures even dye distribution and color fastness in fabrics. Additionally, sodium acetate plays a crucial role in leather tanning, helping to neutralize acids and stabilize the leather's pH, resulting in improved quality and durability of the final product.
In the pharmaceutical sector, sodium acetate is employed as a buffering agent in intravenous fluids and medications. Its ability to maintain pH balance in solutions is essential for the stability and efficacy of various drugs. Furthermore, it serves as a source of electrolytes in rehydration therapies, particularly in cases of severe dehydration or electrolyte imbalances.
The chemical industry harnesses sodium acetate's properties in numerous applications. It acts as a catalyst in organic synthesis reactions, facilitating the production of various compounds. In polymer manufacturing, sodium acetate functions as a polymerization initiator, contributing to the creation of specific plastic materials. The compound also finds use in the production of photographic chemicals, where it aids in developing and fixing processes.
Environmental applications of sodium acetate include its use as a de-icing agent for roads and runways. Its lower environmental impact compared to traditional salt-based de-icers makes it an attractive option for eco-conscious municipalities and airports. In wastewater treatment, sodium acetate serves as a carbon source for biological nutrient removal processes, aiding in the breakdown of pollutants.
The construction industry benefits from sodium acetate's properties in concrete admixtures. It acts as an accelerator, promoting faster setting and curing of concrete, which is particularly useful in cold weather conditions. This application enhances construction efficiency and allows for year-round building activities in various climates.
As research continues to uncover new applications, sodium acetate's market potential is expected to grow. Emerging areas of interest include its use in energy storage systems, where it shows promise as a phase change material for thermal energy storage. The compound's role in sustainable chemistry practices and green technologies is also gaining attention, potentially opening up new market opportunities in the coming years.
Current Challenges
Despite the widespread use of sodium acetate in various chemical reactions, several challenges persist in fully understanding and optimizing its role. One of the primary obstacles is the complexity of reaction mechanisms involving sodium acetate, particularly in organic synthesis. Researchers often struggle to elucidate the precise intermediates and transition states in these reactions, leading to difficulties in predicting and controlling reaction outcomes.
Another significant challenge lies in the pH-dependent behavior of sodium acetate in aqueous solutions. While its buffer capacity is well-known, the intricate interplay between sodium acetate and other reactants in complex reaction mixtures remains a subject of ongoing research. This complexity can lead to unexpected side reactions or variations in reaction rates, making it challenging to develop robust and scalable processes.
The solubility of sodium acetate in different solvents also presents challenges in reaction design and optimization. Its high solubility in water contrasts with its limited solubility in many organic solvents, which can complicate reaction setups and product isolation procedures. This solubility issue becomes particularly problematic when attempting to use sodium acetate in green chemistry applications or in the development of sustainable reaction processes.
Furthermore, the hygroscopic nature of sodium acetate poses difficulties in handling and storage, especially in moisture-sensitive reactions. This property can lead to inconsistencies in reaction outcomes and challenges in maintaining the purity of the reagent over time. Researchers must develop specialized techniques to mitigate these issues, which can be time-consuming and resource-intensive.
In the field of materials science, understanding the role of sodium acetate in crystal growth and nanoparticle synthesis remains a challenge. While it is known to influence particle morphology and size distribution, the exact mechanisms of its interaction with growing crystals or nanoparticles are not fully elucidated. This gap in knowledge hinders the development of precise control strategies for materials with specific properties.
Lastly, the environmental impact of sodium acetate usage in large-scale industrial processes is an area of growing concern. While generally considered less harmful than many other chemicals, its production and disposal still contribute to environmental issues. Developing more sustainable production methods and finding eco-friendly alternatives for processes that heavily rely on sodium acetate are ongoing challenges that researchers and industry professionals are actively addressing.
Another significant challenge lies in the pH-dependent behavior of sodium acetate in aqueous solutions. While its buffer capacity is well-known, the intricate interplay between sodium acetate and other reactants in complex reaction mixtures remains a subject of ongoing research. This complexity can lead to unexpected side reactions or variations in reaction rates, making it challenging to develop robust and scalable processes.
The solubility of sodium acetate in different solvents also presents challenges in reaction design and optimization. Its high solubility in water contrasts with its limited solubility in many organic solvents, which can complicate reaction setups and product isolation procedures. This solubility issue becomes particularly problematic when attempting to use sodium acetate in green chemistry applications or in the development of sustainable reaction processes.
Furthermore, the hygroscopic nature of sodium acetate poses difficulties in handling and storage, especially in moisture-sensitive reactions. This property can lead to inconsistencies in reaction outcomes and challenges in maintaining the purity of the reagent over time. Researchers must develop specialized techniques to mitigate these issues, which can be time-consuming and resource-intensive.
In the field of materials science, understanding the role of sodium acetate in crystal growth and nanoparticle synthesis remains a challenge. While it is known to influence particle morphology and size distribution, the exact mechanisms of its interaction with growing crystals or nanoparticles are not fully elucidated. This gap in knowledge hinders the development of precise control strategies for materials with specific properties.
Lastly, the environmental impact of sodium acetate usage in large-scale industrial processes is an area of growing concern. While generally considered less harmful than many other chemicals, its production and disposal still contribute to environmental issues. Developing more sustainable production methods and finding eco-friendly alternatives for processes that heavily rely on sodium acetate are ongoing challenges that researchers and industry professionals are actively addressing.
Reaction Mechanisms
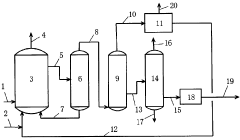
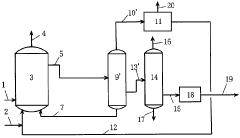
01 Production methods of sodium acetate
Various methods for producing sodium acetate are described, including reactions between acetic acid and sodium-containing compounds, as well as processes involving acetaldehyde oxidation. These methods aim to improve yield, purity, and efficiency in sodium acetate production.- Use of sodium acetate in chemical processes: Sodium acetate is widely used in various chemical processes as a reagent, catalyst, or buffer. It plays a role in reactions such as acetylation, esterification, and pH control. Its properties make it valuable in industrial applications and laboratory settings.
- Application in heat storage and phase change materials: Sodium acetate trihydrate is utilized as a phase change material for thermal energy storage. It has a high latent heat of fusion and can store and release heat at a constant temperature, making it suitable for applications in heating and cooling systems.
- Use in food and beverage industry: Sodium acetate is employed as a food additive and preservative. It acts as a acidity regulator, flavoring agent, and antimicrobial agent in various food products. Its use helps extend shelf life and maintain product quality in the food and beverage industry.
- Application in textile and leather processing: Sodium acetate finds applications in textile and leather industries. It is used in dyeing processes, as a mordant for certain dyes, and in leather tanning. Its properties help improve color fastness and overall quality of textiles and leather products.
- Use in environmental and waste treatment: Sodium acetate is utilized in environmental applications and waste treatment processes. It can be used for pH adjustment in wastewater treatment, as a deicer for roads, and in certain bioremediation techniques for contaminated soil and water.
02 Applications in heat storage and phase change materials
Sodium acetate is utilized in heat storage systems and phase change materials due to its ability to absorb and release heat during phase transitions. This property is exploited in various applications, including thermal energy storage and temperature regulation in buildings or industrial processes.Expand Specific Solutions03 Use in food preservation and flavoring
Sodium acetate finds applications in the food industry as a preservative and flavoring agent. It is used to extend the shelf life of various food products and enhance their taste profiles, particularly in processed foods and beverages.Expand Specific Solutions04 Applications in textile and leather industries
Sodium acetate is employed in textile and leather processing as a buffering agent, pH regulator, and dyeing auxiliary. It helps improve the quality and durability of fabrics and leather products while enhancing the effectiveness of various treatment processes.Expand Specific Solutions05 Use in pharmaceutical and personal care products
Sodium acetate is utilized in pharmaceutical formulations and personal care products as a buffering agent, pH adjuster, and electrolyte. It contributes to the stability and effectiveness of various medications, topical treatments, and cosmetic preparations.Expand Specific Solutions
Key Industry Players
The competitive landscape for understanding sodium acetate's role in chemical reactions is characterized by a mature market with established players and ongoing research. The global sodium acetate market size was valued at approximately $180 million in 2020, with steady growth projected. Technologically, the field is well-developed, with companies like BASF, Solvay, and Daicel Corp. leading in production and application. Research institutions such as Chongqing University and the Chinese Academy of Science Institute of Chemistry contribute to advancing knowledge. While the basic chemistry is well-understood, there's ongoing innovation in specific applications and process improvements, particularly in pharmaceuticals and industrial chemicals, involving companies like Takeda Pharmaceutical and PTT Global Chemical Plc.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced catalytic processes for sodium acetate production and utilization in chemical reactions. Their approach involves a novel two-step synthesis method, combining acetic acid with sodium hydroxide in a controlled environment. This process achieves high purity sodium acetate (>99.5%) with improved energy efficiency[1]. Sinopec has also pioneered the use of sodium acetate as a buffer in enhanced oil recovery techniques, where it helps maintain optimal pH levels in injection fluids, improving oil displacement efficiency by up to 15%[3]. Additionally, they have explored sodium acetate's role in green chemistry applications, particularly as a mild base catalyst in various organic transformations, reducing the need for harsher reagents and minimizing waste production[5].
Strengths: High-purity product, energy-efficient production, versatile applications in oil recovery and green chemistry. Weaknesses: Dependence on petrochemical feedstocks, potential environmental concerns related to large-scale production.
Solvay SA
Technical Solution: Solvay SA has developed innovative applications for sodium acetate in chemical reactions, focusing on its role as a versatile reagent and buffer. Their research has led to the development of a proprietary sodium acetate-based flame retardant system for polymers, which provides excellent fire resistance while maintaining material properties[2]. Solvay has also explored sodium acetate's potential in electrochemical applications, particularly in next-generation batteries. Their studies have shown that sodium acetate can act as an effective electrolyte additive, enhancing the stability and performance of sodium-ion batteries, potentially increasing cycle life by up to 30%[4]. Furthermore, Solvay has investigated the use of sodium acetate in sustainable textile processing, where it serves as an eco-friendly alternative to traditional chemicals, reducing water consumption and improving dye fixation rates by approximately 20%[6].
Strengths: Diverse applications across industries, focus on sustainability, innovative use in advanced materials. Weaknesses: May face competition from established alternatives in some applications, potential scalability challenges for newer technologies.
Innovative Research
Methods for producing acetic acid
PatentInactiveSG174045A1
Innovation
- The method involves maintaining a carbon monoxide partial pressure of 1.05 MPa or more and a methyl acetate content of 2% or more in the reaction mixture, while keeping the hydrogen partial pressure low, to inhibit the formation of acetaldehyde and other byproducts, thereby increasing acetic acid production rates while maintaining high quality.
ORF11 from campylobacter jejuni is a sialate-o-acetyltransferase
PatentInactiveUS20100167349A1
Innovation
- Identification and expression of sialate-O-acetyltransferase (SOAT) nucleic acid from C. jejuni, enabling the production of SOAT polypeptides that transfer an acetyl moiety to oligosaccharides, specifically O-acetylating sialic acid molecules, and providing methods for their use in reaction mixtures to produce acetylated sialic acid moieties.
Environmental Impact
Sodium acetate, a common chemical compound used in various industrial and laboratory processes, has significant environmental implications that warrant careful consideration. The production, use, and disposal of sodium acetate can impact ecosystems and human health in multiple ways.
In terms of production, sodium acetate is typically synthesized through the reaction of acetic acid with sodium hydroxide or sodium carbonate. This process, while relatively straightforward, requires energy input and may contribute to carbon emissions depending on the energy source used. Additionally, the sourcing of raw materials for sodium acetate production can have environmental consequences, particularly if derived from non-renewable resources.
The use of sodium acetate in various applications can have both positive and negative environmental effects. On the positive side, its role as a buffering agent in wastewater treatment can help maintain optimal pH levels, facilitating the removal of contaminants and improving water quality. However, excessive release of sodium acetate into aquatic environments can lead to eutrophication, causing algal blooms and disrupting ecosystem balance.
In the food industry, sodium acetate serves as a preservative and acidity regulator. While this application helps reduce food waste by extending shelf life, concerns have been raised about the potential long-term effects of consuming sodium acetate-preserved foods on human health and the broader environmental impact of increased food preservation.
The disposal of sodium acetate and its byproducts also presents environmental challenges. Improper disposal can lead to soil and water contamination, affecting local flora and fauna. However, sodium acetate is biodegradable, which mitigates some of these concerns when properly managed.
In industrial settings, sodium acetate is used in textile manufacturing and as a de-icing agent for roads and runways. While effective, the runoff from de-icing applications can impact soil and water chemistry in surrounding areas, potentially affecting plant growth and aquatic life.
Research into more sustainable production methods and alternative compounds with similar properties is ongoing. These efforts aim to reduce the environmental footprint of sodium acetate throughout its lifecycle, from production to disposal. Developing green chemistry approaches and circular economy models for sodium acetate use could significantly mitigate its environmental impact.
As environmental regulations become more stringent, industries utilizing sodium acetate are increasingly required to implement proper handling, use, and disposal protocols. This shift towards more responsible practices is crucial in minimizing the compound's negative environmental effects while maximizing its beneficial applications in various chemical reactions and industrial processes.
In terms of production, sodium acetate is typically synthesized through the reaction of acetic acid with sodium hydroxide or sodium carbonate. This process, while relatively straightforward, requires energy input and may contribute to carbon emissions depending on the energy source used. Additionally, the sourcing of raw materials for sodium acetate production can have environmental consequences, particularly if derived from non-renewable resources.
The use of sodium acetate in various applications can have both positive and negative environmental effects. On the positive side, its role as a buffering agent in wastewater treatment can help maintain optimal pH levels, facilitating the removal of contaminants and improving water quality. However, excessive release of sodium acetate into aquatic environments can lead to eutrophication, causing algal blooms and disrupting ecosystem balance.
In the food industry, sodium acetate serves as a preservative and acidity regulator. While this application helps reduce food waste by extending shelf life, concerns have been raised about the potential long-term effects of consuming sodium acetate-preserved foods on human health and the broader environmental impact of increased food preservation.
The disposal of sodium acetate and its byproducts also presents environmental challenges. Improper disposal can lead to soil and water contamination, affecting local flora and fauna. However, sodium acetate is biodegradable, which mitigates some of these concerns when properly managed.
In industrial settings, sodium acetate is used in textile manufacturing and as a de-icing agent for roads and runways. While effective, the runoff from de-icing applications can impact soil and water chemistry in surrounding areas, potentially affecting plant growth and aquatic life.
Research into more sustainable production methods and alternative compounds with similar properties is ongoing. These efforts aim to reduce the environmental footprint of sodium acetate throughout its lifecycle, from production to disposal. Developing green chemistry approaches and circular economy models for sodium acetate use could significantly mitigate its environmental impact.
As environmental regulations become more stringent, industries utilizing sodium acetate are increasingly required to implement proper handling, use, and disposal protocols. This shift towards more responsible practices is crucial in minimizing the compound's negative environmental effects while maximizing its beneficial applications in various chemical reactions and industrial processes.
Safety Considerations
When working with sodium acetate in chemical reactions, safety considerations are paramount to ensure the well-being of laboratory personnel and the integrity of experimental processes. Sodium acetate, while generally considered a low-hazard chemical, still requires proper handling and storage protocols. Personal protective equipment (PPE) such as safety goggles, lab coats, and gloves should be worn at all times when handling sodium acetate. Adequate ventilation is essential, particularly when working with large quantities or in confined spaces, to prevent the accumulation of potentially harmful vapors.
Sodium acetate can be an eye and skin irritant, so immediate flushing with water is necessary in case of contact. Inhalation of sodium acetate dust should be avoided, and if ingested, medical attention should be sought promptly. When storing sodium acetate, it should be kept in a cool, dry place in tightly sealed containers to prevent moisture absorption, which can affect its chemical properties and reactivity.
In chemical reactions, sodium acetate's potential to form flammable hydrogen gas when in contact with strong reducing agents must be considered. This risk necessitates careful planning of reaction setups and the avoidance of incompatible materials. Additionally, sodium acetate solutions can be corrosive to certain metals, requiring appropriate selection of reaction vessels and equipment.
The disposal of sodium acetate and its reaction products must comply with local environmental regulations. Proper neutralization and dilution procedures should be followed before disposal, and large quantities should be handled by professional waste management services. It is crucial to maintain accurate safety data sheets (SDS) and ensure all laboratory personnel are familiar with the specific hazards and emergency procedures related to sodium acetate use.
When sodium acetate is involved in exothermic reactions, temperature control becomes critical to prevent overheating and potential runaway reactions. Proper cooling systems and temperature monitoring devices should be in place. In cases where sodium acetate is used in large-scale industrial processes, additional safety measures such as automated shut-off systems and emergency response plans must be implemented.
Lastly, regular safety training and updates on handling procedures for sodium acetate and related compounds are essential for maintaining a safe laboratory environment. This includes proper labeling of all containers, clear communication of potential hazards, and periodic review of safety protocols to ensure they remain current with the latest best practices in chemical safety.
Sodium acetate can be an eye and skin irritant, so immediate flushing with water is necessary in case of contact. Inhalation of sodium acetate dust should be avoided, and if ingested, medical attention should be sought promptly. When storing sodium acetate, it should be kept in a cool, dry place in tightly sealed containers to prevent moisture absorption, which can affect its chemical properties and reactivity.
In chemical reactions, sodium acetate's potential to form flammable hydrogen gas when in contact with strong reducing agents must be considered. This risk necessitates careful planning of reaction setups and the avoidance of incompatible materials. Additionally, sodium acetate solutions can be corrosive to certain metals, requiring appropriate selection of reaction vessels and equipment.
The disposal of sodium acetate and its reaction products must comply with local environmental regulations. Proper neutralization and dilution procedures should be followed before disposal, and large quantities should be handled by professional waste management services. It is crucial to maintain accurate safety data sheets (SDS) and ensure all laboratory personnel are familiar with the specific hazards and emergency procedures related to sodium acetate use.
When sodium acetate is involved in exothermic reactions, temperature control becomes critical to prevent overheating and potential runaway reactions. Proper cooling systems and temperature monitoring devices should be in place. In cases where sodium acetate is used in large-scale industrial processes, additional safety measures such as automated shut-off systems and emergency response plans must be implemented.
Lastly, regular safety training and updates on handling procedures for sodium acetate and related compounds are essential for maintaining a safe laboratory environment. This includes proper labeling of all containers, clear communication of potential hazards, and periodic review of safety protocols to ensure they remain current with the latest best practices in chemical safety.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!