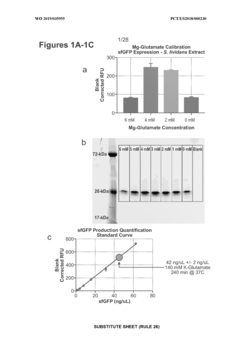
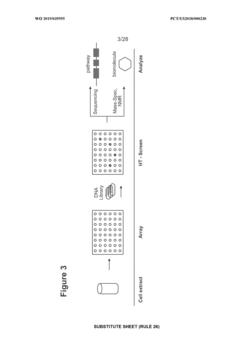
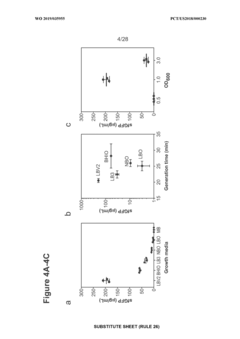
Utilizing cell-free systems for rapid prototyping of biosynthetic pathways.
SEP 5, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-free Systems Background and Objectives
Cell-free systems represent a revolutionary approach in synthetic biology, offering a simplified and controllable environment for studying biological processes outside the constraints of living cells. The concept dates back to the 1950s when Nirenberg and Matthaei utilized cell extracts to decipher the genetic code. However, recent advancements in biochemical techniques and analytical methods have transformed these systems from purely analytical tools into powerful platforms for biosynthetic pathway engineering.
The evolution of cell-free systems has been marked by significant milestones, including the development of the PURE (Protein synthesis Using Recombinant Elements) system in the early 2000s, which provided a defined environment composed of purified components necessary for transcription and translation. This advancement enabled unprecedented control over reaction conditions and eliminated interference from cellular processes irrelevant to the pathway of interest.
Current cell-free systems can be broadly categorized into crude extracts derived from various organisms (E. coli, yeast, insect cells, etc.) and reconstituted systems assembled from purified components. Each variant offers distinct advantages depending on the specific application requirements, from high-yield protein production to complex metabolic pathway prototyping.
The primary objective of utilizing cell-free systems for biosynthetic pathway prototyping is to accelerate the design-build-test cycle in metabolic engineering. Traditional in vivo approaches require time-consuming transformation, selection, and cultivation steps for each design iteration. In contrast, cell-free systems enable rapid testing of multiple pathway variants within hours rather than days or weeks, dramatically reducing development timelines.
Additionally, these systems aim to overcome several limitations inherent to whole-cell systems, including toxicity issues, growth inhibition by intermediates or products, and competing metabolic pathways. By providing an open and customizable environment, cell-free systems allow direct manipulation of reaction conditions, precise control of component concentrations, and real-time monitoring of pathway performance.
Looking forward, the technical goals for cell-free biosynthetic pathway prototyping include enhancing system longevity to support extended reaction times, improving energy regeneration systems to sustain ATP-dependent pathways, developing standardized protocols for extract preparation to ensure reproducibility, and creating computational models that accurately predict pathway behavior in cell-free environments.
The ultimate vision is to establish cell-free systems as a standard platform in the synthetic biology toolkit, enabling researchers to rapidly prototype and optimize complex biosynthetic pathways before implementation in living cells, thereby accelerating the development of sustainable biomanufacturing processes for chemicals, pharmaceuticals, and materials.
The evolution of cell-free systems has been marked by significant milestones, including the development of the PURE (Protein synthesis Using Recombinant Elements) system in the early 2000s, which provided a defined environment composed of purified components necessary for transcription and translation. This advancement enabled unprecedented control over reaction conditions and eliminated interference from cellular processes irrelevant to the pathway of interest.
Current cell-free systems can be broadly categorized into crude extracts derived from various organisms (E. coli, yeast, insect cells, etc.) and reconstituted systems assembled from purified components. Each variant offers distinct advantages depending on the specific application requirements, from high-yield protein production to complex metabolic pathway prototyping.
The primary objective of utilizing cell-free systems for biosynthetic pathway prototyping is to accelerate the design-build-test cycle in metabolic engineering. Traditional in vivo approaches require time-consuming transformation, selection, and cultivation steps for each design iteration. In contrast, cell-free systems enable rapid testing of multiple pathway variants within hours rather than days or weeks, dramatically reducing development timelines.
Additionally, these systems aim to overcome several limitations inherent to whole-cell systems, including toxicity issues, growth inhibition by intermediates or products, and competing metabolic pathways. By providing an open and customizable environment, cell-free systems allow direct manipulation of reaction conditions, precise control of component concentrations, and real-time monitoring of pathway performance.
Looking forward, the technical goals for cell-free biosynthetic pathway prototyping include enhancing system longevity to support extended reaction times, improving energy regeneration systems to sustain ATP-dependent pathways, developing standardized protocols for extract preparation to ensure reproducibility, and creating computational models that accurately predict pathway behavior in cell-free environments.
The ultimate vision is to establish cell-free systems as a standard platform in the synthetic biology toolkit, enabling researchers to rapidly prototype and optimize complex biosynthetic pathways before implementation in living cells, thereby accelerating the development of sustainable biomanufacturing processes for chemicals, pharmaceuticals, and materials.
Market Analysis for Cell-free Biosynthesis Applications
The cell-free biosynthesis market is experiencing significant growth, driven by increasing demand for sustainable production methods across pharmaceutical, chemical, and agricultural sectors. Current market estimates value the global cell-free protein synthesis market at approximately $250 million, with projections indicating a compound annual growth rate of 8-10% through 2028. This growth trajectory is supported by the technology's ability to accelerate product development cycles while reducing costs associated with traditional cell-based manufacturing processes.
Pharmaceutical applications currently dominate the market landscape, accounting for roughly 60% of cell-free biosynthesis implementations. This sector utilizes the technology primarily for rapid prototyping of novel therapeutics, including peptides, proteins, and small molecule drugs. The ability to quickly iterate designs without cellular constraints has proven particularly valuable for companies developing personalized medicine solutions and addressing emerging infectious diseases.
Industrial biotechnology represents the fastest-growing application segment, with annual growth rates exceeding 12%. Companies in this space are leveraging cell-free systems to develop novel enzymes, biofuels, and specialty chemicals. The market appeal stems from the technology's capacity to bypass cellular toxicity issues that often hamper traditional metabolic engineering approaches.
Consumer demand for sustainable products is creating new market opportunities, particularly in the food and cosmetics industries. Cell-free systems enable the production of natural flavors, fragrances, and cosmetic ingredients without agricultural land use or extraction from endangered species. This segment is projected to expand at 15% annually as consumers increasingly prioritize environmentally responsible manufacturing methods.
Regional analysis reveals North America currently leads the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is expected to demonstrate the highest growth rate over the next five years due to increasing biotechnology investments in China, Japan, and Singapore.
Market challenges include high initial setup costs, technical expertise requirements, and regulatory uncertainties surrounding novel production methods. Despite these barriers, venture capital investment in cell-free biosynthesis startups has tripled over the past three years, reaching approximately $800 million in 2022, indicating strong investor confidence in the technology's commercial potential.
Customer surveys indicate that reducing time-to-market is the primary motivation for adopting cell-free systems, followed by access to previously unattainable molecules and improved sustainability metrics. As the technology matures and economies of scale are achieved, market penetration is expected to accelerate across multiple industry verticals.
Pharmaceutical applications currently dominate the market landscape, accounting for roughly 60% of cell-free biosynthesis implementations. This sector utilizes the technology primarily for rapid prototyping of novel therapeutics, including peptides, proteins, and small molecule drugs. The ability to quickly iterate designs without cellular constraints has proven particularly valuable for companies developing personalized medicine solutions and addressing emerging infectious diseases.
Industrial biotechnology represents the fastest-growing application segment, with annual growth rates exceeding 12%. Companies in this space are leveraging cell-free systems to develop novel enzymes, biofuels, and specialty chemicals. The market appeal stems from the technology's capacity to bypass cellular toxicity issues that often hamper traditional metabolic engineering approaches.
Consumer demand for sustainable products is creating new market opportunities, particularly in the food and cosmetics industries. Cell-free systems enable the production of natural flavors, fragrances, and cosmetic ingredients without agricultural land use or extraction from endangered species. This segment is projected to expand at 15% annually as consumers increasingly prioritize environmentally responsible manufacturing methods.
Regional analysis reveals North America currently leads the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is expected to demonstrate the highest growth rate over the next five years due to increasing biotechnology investments in China, Japan, and Singapore.
Market challenges include high initial setup costs, technical expertise requirements, and regulatory uncertainties surrounding novel production methods. Despite these barriers, venture capital investment in cell-free biosynthesis startups has tripled over the past three years, reaching approximately $800 million in 2022, indicating strong investor confidence in the technology's commercial potential.
Customer surveys indicate that reducing time-to-market is the primary motivation for adopting cell-free systems, followed by access to previously unattainable molecules and improved sustainability metrics. As the technology matures and economies of scale are achieved, market penetration is expected to accelerate across multiple industry verticals.
Technical Challenges in Cell-free Pathway Engineering
Despite the promising potential of cell-free systems for biosynthetic pathway prototyping, several significant technical challenges persist in this rapidly evolving field. One primary obstacle involves the stability of cell-free reaction components over time. Enzymes and cofactors in cell-free environments often exhibit reduced stability compared to their in vivo counterparts, limiting reaction duration and yield. This instability stems from the absence of cellular regeneration mechanisms that typically maintain enzyme functionality in living cells.
Energy supply represents another critical challenge. Cell-free systems lack the continuous metabolic processes that regenerate essential cofactors like ATP, NAD(P)H, and CoA in living cells. Current approaches employ auxiliary energy regeneration systems, but these often become rate-limiting as reactions progress, resulting in diminished pathway efficiency over extended periods.
The reconstitution of complex multi-enzyme pathways presents significant difficulties in maintaining optimal stoichiometric ratios between pathway components. Unlike cellular systems where evolution has fine-tuned enzyme expression levels, cell-free systems require careful optimization of each enzyme's concentration to prevent bottlenecks or accumulation of potentially toxic intermediates. This challenge intensifies with pathway complexity, as more components must be balanced simultaneously.
Transport limitations also impede cell-free pathway engineering. In cellular systems, compartmentalization and membrane transport proteins regulate metabolite flow between different cellular regions. Cell-free systems typically lack these sophisticated transport mechanisms, potentially leading to inefficient substrate channeling and product inhibition effects that reduce overall pathway performance.
Scale-up considerations present formidable barriers to industrial application. While cell-free systems excel at small-scale prototyping, transitioning to production scales introduces challenges in maintaining reaction homogeneity, component stability, and cost-effectiveness. The relatively high cost of enzyme production and cofactor supplementation becomes particularly problematic at larger scales.
Analytical limitations further complicate cell-free pathway engineering. Real-time monitoring of complex reaction networks remains challenging, with current techniques often requiring sampling that may disrupt the reaction environment. This hampers the ability to track intermediate formation and identify rate-limiting steps during pathway optimization.
Finally, standardization across different cell-free platforms remains underdeveloped. Various extract preparation methods and reaction conditions create significant variability between laboratories, complicating the reproducibility of results and the establishment of universal design principles for cell-free pathway engineering. Addressing these technical challenges will be crucial for realizing the full potential of cell-free systems in biosynthetic pathway development.
Energy supply represents another critical challenge. Cell-free systems lack the continuous metabolic processes that regenerate essential cofactors like ATP, NAD(P)H, and CoA in living cells. Current approaches employ auxiliary energy regeneration systems, but these often become rate-limiting as reactions progress, resulting in diminished pathway efficiency over extended periods.
The reconstitution of complex multi-enzyme pathways presents significant difficulties in maintaining optimal stoichiometric ratios between pathway components. Unlike cellular systems where evolution has fine-tuned enzyme expression levels, cell-free systems require careful optimization of each enzyme's concentration to prevent bottlenecks or accumulation of potentially toxic intermediates. This challenge intensifies with pathway complexity, as more components must be balanced simultaneously.
Transport limitations also impede cell-free pathway engineering. In cellular systems, compartmentalization and membrane transport proteins regulate metabolite flow between different cellular regions. Cell-free systems typically lack these sophisticated transport mechanisms, potentially leading to inefficient substrate channeling and product inhibition effects that reduce overall pathway performance.
Scale-up considerations present formidable barriers to industrial application. While cell-free systems excel at small-scale prototyping, transitioning to production scales introduces challenges in maintaining reaction homogeneity, component stability, and cost-effectiveness. The relatively high cost of enzyme production and cofactor supplementation becomes particularly problematic at larger scales.
Analytical limitations further complicate cell-free pathway engineering. Real-time monitoring of complex reaction networks remains challenging, with current techniques often requiring sampling that may disrupt the reaction environment. This hampers the ability to track intermediate formation and identify rate-limiting steps during pathway optimization.
Finally, standardization across different cell-free platforms remains underdeveloped. Various extract preparation methods and reaction conditions create significant variability between laboratories, complicating the reproducibility of results and the establishment of universal design principles for cell-free pathway engineering. Addressing these technical challenges will be crucial for realizing the full potential of cell-free systems in biosynthetic pathway development.
Current Methodologies for Cell-free Pathway Prototyping
01 Cell-free protein synthesis systems for rapid prototyping
Cell-free protein synthesis systems enable rapid prototyping of proteins without the constraints of living cells. These systems utilize cellular extracts containing transcription and translation machinery to produce proteins directly from DNA templates. This approach allows for faster iteration cycles in protein engineering, rapid testing of genetic constructs, and efficient production of difficult-to-express proteins. The technology supports high-throughput screening and optimization of protein variants for various applications in biotechnology and pharmaceutical development.- Cell-free protein synthesis systems for rapid prototyping: Cell-free protein synthesis systems enable rapid prototyping of proteins without the constraints of living cells. These systems utilize extracted cellular components to produce proteins directly from DNA templates, allowing for faster iteration cycles in protein engineering and design. The technology facilitates quick testing of protein variants and can be integrated with automated platforms for high-throughput screening, significantly accelerating the development process of biopharmaceuticals and enzymes.
- 3D printing technologies for rapid prototyping: Advanced 3D printing technologies enable rapid prototyping of complex structures and functional components. These systems utilize various materials including polymers, metals, and composites to create physical prototypes directly from digital designs. The technology allows for iterative design improvements, reduced development time, and cost-effective production of customized parts without traditional manufacturing constraints, making it valuable across industries from medical devices to aerospace components.
- Automated design and manufacturing systems: Integrated automated systems combine design software with manufacturing capabilities for streamlined rapid prototyping. These systems feature computer-aided design interfaces connected to production equipment, enabling direct translation of digital models to physical prototypes. The automation reduces human intervention, minimizes errors, and accelerates the development cycle from concept to prototype, allowing for faster validation and refinement of designs across various industrial applications.
- Microfluidic platforms for cell-free applications: Microfluidic technologies provide miniaturized platforms for cell-free rapid prototyping applications. These systems utilize microscale channels and chambers to manipulate small volumes of reagents, enabling parallel testing of multiple conditions with minimal material consumption. The integration of sensors and control systems allows for precise manipulation of reaction conditions, making these platforms particularly valuable for developing diagnostics, screening drug candidates, and optimizing biochemical processes.
- Rapid assembly and fabrication techniques: Advanced assembly and fabrication techniques enable quick construction of prototypes from modular components. These methods include snap-fit designs, adhesive bonding systems, and reconfigurable manufacturing platforms that allow for rapid assembly, modification, and testing of physical prototypes. The techniques reduce tooling requirements and setup times, facilitating iterative design improvements and enabling manufacturers to quickly respond to changing requirements or explore multiple design variants simultaneously.
02 3D printing and additive manufacturing for rapid prototyping
Additive manufacturing technologies, particularly 3D printing, have revolutionized rapid prototyping by enabling the direct fabrication of complex three-dimensional objects from digital models. These systems allow for quick iteration of design concepts, reduced development time, and cost-effective production of prototypes. Various materials can be used, including polymers, metals, and composites, to create functional prototypes with specific mechanical properties. The technology supports applications across industries including medical devices, aerospace, and consumer products.Expand Specific Solutions03 Computer-aided design systems for rapid prototyping
Computer-aided design (CAD) systems form the foundation of modern rapid prototyping workflows by enabling digital modeling and simulation before physical production. These systems allow engineers to create, modify, analyze, and optimize designs virtually, significantly reducing development cycles. Advanced CAD tools incorporate features like parametric modeling, simulation capabilities, and integration with manufacturing systems to streamline the transition from concept to prototype. The technology supports collaborative design processes and enables rapid iteration based on testing feedback.Expand Specific Solutions04 Automated fabrication systems for rapid prototyping
Automated fabrication systems integrate various manufacturing technologies to accelerate the prototyping process. These systems combine robotics, computer numerical control (CNC), and specialized tooling to produce prototypes with minimal human intervention. The automation extends to material handling, assembly operations, and quality control, enabling consistent production of complex prototypes. Advanced systems incorporate feedback mechanisms to adjust fabrication parameters in real-time, further improving efficiency and quality in rapid prototyping applications.Expand Specific Solutions05 Rapid prototyping materials and processes for specialized applications
Specialized materials and processes have been developed to address specific requirements in rapid prototyping applications. These include biocompatible materials for medical prototypes, high-performance composites for aerospace applications, and food-grade materials for culinary innovations. Novel processing techniques such as selective laser sintering, stereolithography, and material jetting enable the creation of prototypes with specific properties like flexibility, transparency, or heat resistance. These advancements allow for functional testing of prototypes under conditions that closely mimic real-world applications.Expand Specific Solutions
Leading Organizations in Cell-free Systems Research
Cell-free biosynthetic pathway prototyping is evolving rapidly, with the market transitioning from early research to commercial applications. The global market is expanding, projected to reach significant scale as industrial biotechnology embraces sustainable manufacturing solutions. Technologically, academic institutions like MIT, Harvard, and Cornell lead fundamental research, while companies demonstrate varying maturity levels. Kangma Biological Technology and Debut Biotechnology have developed proprietary cell-free platforms for commercial applications, while GreenLight Biosciences and Cellfree Sciences offer established cell-free protein synthesis technologies. Emerging players like Progeneer and Touchlight IP are advancing novel approaches to rapid prototyping systems. This competitive landscape reflects a technology approaching inflection point between academic innovation and industrial adoption.
President & Fellows of Harvard College
Technical Solution: Harvard has developed an advanced cell-free platform called "BioBricks-CF" specifically designed for rapid prototyping of biosynthetic pathways. Their system utilizes highly purified components rather than crude cell extracts, allowing for precise control over reaction conditions and eliminating interference from unwanted cellular activities. Harvard researchers have pioneered a technique called "Protein Fusion Technology" that links sequential pathway enzymes together into multi-enzyme complexes, enhancing substrate channeling and reaction efficiency[6]. Their platform incorporates specialized liposome and nanodisc technologies that enable the functional incorporation of membrane-associated enzymes into cell-free systems, expanding the range of pathways that can be prototyped. Harvard has developed a machine learning framework that analyzes pathway performance data to guide iterative optimization, significantly accelerating the development process. The university has demonstrated successful implementation of their technology for producing various natural products, pharmaceuticals, and biomaterials through rapid prototyping of complex biosynthetic pathways involving up to 30 enzymatic steps[7].
Strengths: Exceptional purity and control using defined components; advanced capabilities for incorporating membrane enzymes; sophisticated computational optimization tools; demonstrated success with extremely complex pathways. Weaknesses: Higher cost compared to crude extract systems; requires specialized expertise in protein purification and handling; more time-intensive initial setup compared to commercial kits.
Massachusetts Institute of Technology
Technical Solution: MIT has developed a comprehensive cell-free platform called CFPU (Cell-Free Pathway Utilities) for rapid prototyping of biosynthetic pathways. Their system utilizes highly optimized E. coli extracts supplemented with energy regeneration components and molecular crowding agents that mimic the intracellular environment. MIT researchers have pioneered a technique called "TX-TL" (Transcription-Translation) that allows for the simultaneous synthesis of pathway enzymes directly from DNA templates within the cell-free reaction, eliminating the need for separate protein purification steps[4]. Their platform incorporates a microfluidic "pathway-on-a-chip" technology that enables testing of thousands of pathway variants in parallel using nanoliter-scale reactions, dramatically accelerating the optimization process. MIT has developed computational tools that integrate kinetic modeling with machine learning algorithms to predict optimal pathway configurations based on limited experimental data. The institute has demonstrated successful implementation of their technology for producing various compounds including biofuels, pharmaceuticals, and specialty chemicals through rapid prototyping of novel biosynthetic pathways[5].
Strengths: Highly integrated approach combining DNA-to-product in single reactions; sophisticated microfluidic implementation for high-throughput testing; advanced computational modeling capabilities; demonstrated success with diverse compound classes. Weaknesses: Complex setup requiring specialized expertise; potential challenges with scaling up from microfluidic systems; higher resource requirements for implementation.
Key Innovations in Cell-free Expression Technologies
High-throughput system using a cell-free expression system and in SITU sequencing
PatentWO2019035955A1
Innovation
- A high-throughput system utilizing a cell-free expression system combined with in situ sequencing for rapid screening of nucleic acid templates, allowing for the detection and characterization of small molecules produced in multiple reaction volumes, enabling the identification of biosynthetic pathways and optimization of conditions for biomolecule production.
Scalability and Industrial Translation Considerations
The scalability of cell-free systems represents a critical challenge in transitioning from laboratory-scale prototyping to industrial implementation. Current cell-free reaction volumes typically range from microliters to milliliters, which are sufficient for proof-of-concept studies but inadequate for commercial production. Scaling up these systems requires addressing several key engineering challenges, including maintaining reaction homogeneity, ensuring consistent enzyme activity, and managing heat transfer in larger reaction vessels.
Cost considerations present another significant barrier to industrial adoption. The production of cell extracts and purified enzymes remains expensive, with estimates suggesting that enzyme costs alone can account for 30-50% of total production expenses in cell-free systems. Additionally, the substrates and cofactors required for these reactions often come with high price tags, further impacting economic viability. Developing more cost-effective methods for enzyme production and cofactor regeneration will be essential for industrial feasibility.
Stability issues also emerge prominently when considering industrial translation. Cell-free systems typically maintain optimal activity for hours rather than days, limiting their practical application in continuous manufacturing processes. Research into enzyme stabilization techniques, such as immobilization strategies and the development of thermostable enzyme variants, shows promise for extending reaction lifetimes but requires further optimization for industrial settings.
Regulatory frameworks present another dimension of complexity. As relatively new biotechnology platforms, cell-free systems face evolving regulatory landscapes. Companies must navigate uncertain approval pathways, particularly for applications in pharmaceuticals or food production. Establishing standardized quality control metrics and validation protocols specific to cell-free manufacturing will be crucial for regulatory acceptance.
Integration with existing industrial infrastructure represents a practical consideration often overlooked in academic research. Most current bioprocessing facilities are designed for microbial fermentation or mammalian cell culture, not cell-free reactions. Adapting cell-free systems to work within established manufacturing paradigms—or developing new dedicated facilities—will require significant capital investment and engineering expertise.
Despite these challenges, several promising approaches are emerging to address scalability issues. Continuous-flow cell-free systems offer one potential solution, allowing for longer reaction times and easier product separation. Additionally, the development of freeze-dried cell-free reaction components has improved stability and simplified storage requirements, potentially enabling distributed manufacturing models that could revolutionize supply chains for bioproducts.
Cost considerations present another significant barrier to industrial adoption. The production of cell extracts and purified enzymes remains expensive, with estimates suggesting that enzyme costs alone can account for 30-50% of total production expenses in cell-free systems. Additionally, the substrates and cofactors required for these reactions often come with high price tags, further impacting economic viability. Developing more cost-effective methods for enzyme production and cofactor regeneration will be essential for industrial feasibility.
Stability issues also emerge prominently when considering industrial translation. Cell-free systems typically maintain optimal activity for hours rather than days, limiting their practical application in continuous manufacturing processes. Research into enzyme stabilization techniques, such as immobilization strategies and the development of thermostable enzyme variants, shows promise for extending reaction lifetimes but requires further optimization for industrial settings.
Regulatory frameworks present another dimension of complexity. As relatively new biotechnology platforms, cell-free systems face evolving regulatory landscapes. Companies must navigate uncertain approval pathways, particularly for applications in pharmaceuticals or food production. Establishing standardized quality control metrics and validation protocols specific to cell-free manufacturing will be crucial for regulatory acceptance.
Integration with existing industrial infrastructure represents a practical consideration often overlooked in academic research. Most current bioprocessing facilities are designed for microbial fermentation or mammalian cell culture, not cell-free reactions. Adapting cell-free systems to work within established manufacturing paradigms—or developing new dedicated facilities—will require significant capital investment and engineering expertise.
Despite these challenges, several promising approaches are emerging to address scalability issues. Continuous-flow cell-free systems offer one potential solution, allowing for longer reaction times and easier product separation. Additionally, the development of freeze-dried cell-free reaction components has improved stability and simplified storage requirements, potentially enabling distributed manufacturing models that could revolutionize supply chains for bioproducts.
Intellectual Property Landscape in Cell-free Biotechnology
The cell-free biotechnology intellectual property landscape has evolved significantly over the past two decades, reflecting the growing interest in utilizing cell-free systems for rapid prototyping of biosynthetic pathways. Patent filings in this domain have increased exponentially since 2010, with major clusters focusing on cell-free protein synthesis (CFPS) technologies, extract preparation methods, and pathway optimization techniques.
Key patent holders include established biotechnology companies such as Sutro Biopharma, which maintains a substantial portfolio covering cell-free protein synthesis for therapeutic applications. Greenlight Biosciences has secured critical patents for cell-free metabolic engineering platforms specifically designed for rapid prototyping of biosynthetic pathways. Academic institutions like Stanford University and MIT have also established strong patent positions in fundamental cell-free technologies.
The patent landscape reveals several strategic trends. First, there is increasing protection around specialized cell lysate preparations optimized for specific biosynthetic pathways. Second, patents covering modular reaction components that facilitate rapid assembly and testing of pathway variants have gained prominence. Third, there is growing intellectual property around computational tools that integrate with cell-free systems to predict and optimize pathway performance.
Geographical analysis shows the United States leading in patent filings (approximately 45%), followed by China (25%), Europe (15%), and Japan (10%). This distribution reflects the global centers of excellence in synthetic biology research and commercialization efforts. Notably, Chinese patent applications have shown the steepest growth curve over the past five years, indicating increasing investment in this technology area.
Freedom-to-operate challenges exist particularly around foundational technologies. Several key patents covering basic cell-free expression systems will expire within the next 3-5 years, potentially opening new opportunities for commercial applications. However, newer patents focusing on specific pathway optimization techniques and high-throughput screening methods using cell-free systems may present barriers to commercialization.
Licensing trends indicate a move toward more collaborative intellectual property models. Cross-licensing agreements between major players have increased by approximately 30% since 2018, suggesting recognition that pathway prototyping requires access to diverse technological components. Several patent pools have emerged specifically for cell-free biosynthetic applications, allowing smaller companies and academic institutions to participate in the innovation ecosystem.
Emerging white space opportunities exist in patents covering the integration of cell-free systems with advanced analytical technologies, particularly real-time monitoring of complex biosynthetic pathways. Additionally, intellectual property covering the scale-up of cell-free prototyped pathways to industrial production represents an underdeveloped area with significant commercial potential.
Key patent holders include established biotechnology companies such as Sutro Biopharma, which maintains a substantial portfolio covering cell-free protein synthesis for therapeutic applications. Greenlight Biosciences has secured critical patents for cell-free metabolic engineering platforms specifically designed for rapid prototyping of biosynthetic pathways. Academic institutions like Stanford University and MIT have also established strong patent positions in fundamental cell-free technologies.
The patent landscape reveals several strategic trends. First, there is increasing protection around specialized cell lysate preparations optimized for specific biosynthetic pathways. Second, patents covering modular reaction components that facilitate rapid assembly and testing of pathway variants have gained prominence. Third, there is growing intellectual property around computational tools that integrate with cell-free systems to predict and optimize pathway performance.
Geographical analysis shows the United States leading in patent filings (approximately 45%), followed by China (25%), Europe (15%), and Japan (10%). This distribution reflects the global centers of excellence in synthetic biology research and commercialization efforts. Notably, Chinese patent applications have shown the steepest growth curve over the past five years, indicating increasing investment in this technology area.
Freedom-to-operate challenges exist particularly around foundational technologies. Several key patents covering basic cell-free expression systems will expire within the next 3-5 years, potentially opening new opportunities for commercial applications. However, newer patents focusing on specific pathway optimization techniques and high-throughput screening methods using cell-free systems may present barriers to commercialization.
Licensing trends indicate a move toward more collaborative intellectual property models. Cross-licensing agreements between major players have increased by approximately 30% since 2018, suggesting recognition that pathway prototyping requires access to diverse technological components. Several patent pools have emerged specifically for cell-free biosynthetic applications, allowing smaller companies and academic institutions to participate in the innovation ecosystem.
Emerging white space opportunities exist in patents covering the integration of cell-free systems with advanced analytical technologies, particularly real-time monitoring of complex biosynthetic pathways. Additionally, intellectual property covering the scale-up of cell-free prototyped pathways to industrial production represents an underdeveloped area with significant commercial potential.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!