Vacuum Pump Applications in Biomedical Device Sterilization
JUL 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Vacuum Sterilization Evolution and Objectives
Vacuum sterilization has evolved significantly since its inception in the early 20th century. Initially developed as an alternative to heat-based methods, vacuum sterilization has become a cornerstone in biomedical device sterilization due to its effectiveness and versatility. The technology's evolution has been driven by the increasing complexity of medical devices and the need for more efficient, reliable sterilization processes.
The primary objective of vacuum sterilization in biomedical applications is to achieve complete elimination of microorganisms while preserving the integrity and functionality of sensitive medical devices. This goal has led to continuous improvements in vacuum pump technology, chamber design, and process control systems over the decades.
Early vacuum sterilization systems relied on simple mechanical pumps and basic pressure control. However, as the biomedical industry advanced, so did the demands on sterilization technology. The introduction of electronic controls and more sophisticated vacuum pumps in the 1970s and 1980s marked a significant leap forward, enabling more precise control over the sterilization environment.
The 1990s saw the integration of computer-controlled systems, which allowed for better monitoring and documentation of the sterilization process. This advancement was crucial in meeting increasingly stringent regulatory requirements and ensuring the consistency and reliability of sterilization outcomes.
In recent years, the focus has shifted towards developing more energy-efficient and environmentally friendly vacuum sterilization systems. This trend aligns with broader sustainability goals in the healthcare industry and has led to innovations in pump design and process optimization.
Another key objective in the evolution of vacuum sterilization has been to reduce cycle times without compromising sterilization efficacy. This has been achieved through advancements in vacuum pump technology, including the development of high-performance oil-free pumps and the implementation of multi-stage vacuum systems.
The current frontier in vacuum sterilization technology involves the integration of smart sensors and IoT capabilities. These innovations aim to provide real-time monitoring, predictive maintenance, and data-driven optimization of sterilization processes. The ultimate goal is to create fully automated, self-adjusting systems that can adapt to varying load conditions and maintain optimal performance.
As biomedical devices continue to become more sophisticated and diverse, the objectives for vacuum sterilization technology are expanding. Future developments will likely focus on customizable sterilization cycles for specific device types, enhanced material compatibility, and further reductions in environmental impact. The ongoing evolution of vacuum sterilization technology remains crucial in supporting the advancement of medical device innovation and ensuring patient safety.
The primary objective of vacuum sterilization in biomedical applications is to achieve complete elimination of microorganisms while preserving the integrity and functionality of sensitive medical devices. This goal has led to continuous improvements in vacuum pump technology, chamber design, and process control systems over the decades.
Early vacuum sterilization systems relied on simple mechanical pumps and basic pressure control. However, as the biomedical industry advanced, so did the demands on sterilization technology. The introduction of electronic controls and more sophisticated vacuum pumps in the 1970s and 1980s marked a significant leap forward, enabling more precise control over the sterilization environment.
The 1990s saw the integration of computer-controlled systems, which allowed for better monitoring and documentation of the sterilization process. This advancement was crucial in meeting increasingly stringent regulatory requirements and ensuring the consistency and reliability of sterilization outcomes.
In recent years, the focus has shifted towards developing more energy-efficient and environmentally friendly vacuum sterilization systems. This trend aligns with broader sustainability goals in the healthcare industry and has led to innovations in pump design and process optimization.
Another key objective in the evolution of vacuum sterilization has been to reduce cycle times without compromising sterilization efficacy. This has been achieved through advancements in vacuum pump technology, including the development of high-performance oil-free pumps and the implementation of multi-stage vacuum systems.
The current frontier in vacuum sterilization technology involves the integration of smart sensors and IoT capabilities. These innovations aim to provide real-time monitoring, predictive maintenance, and data-driven optimization of sterilization processes. The ultimate goal is to create fully automated, self-adjusting systems that can adapt to varying load conditions and maintain optimal performance.
As biomedical devices continue to become more sophisticated and diverse, the objectives for vacuum sterilization technology are expanding. Future developments will likely focus on customizable sterilization cycles for specific device types, enhanced material compatibility, and further reductions in environmental impact. The ongoing evolution of vacuum sterilization technology remains crucial in supporting the advancement of medical device innovation and ensuring patient safety.
Biomedical Device Sterilization Market Analysis
The biomedical device sterilization market has experienced significant growth in recent years, driven by the increasing demand for sterile medical equipment and devices across healthcare facilities worldwide. This market segment is closely tied to the broader healthcare industry, with sterilization being a critical process to ensure patient safety and prevent healthcare-associated infections.
The global biomedical device sterilization market was valued at approximately $5.3 billion in 2020 and is projected to reach $7.8 billion by 2025, growing at a compound annual growth rate (CAGR) of 8.2% during the forecast period. This growth is primarily attributed to the rising number of surgical procedures, the growing prevalence of hospital-acquired infections, and the increasing focus on infection control and prevention measures.
North America currently holds the largest share of the biomedical device sterilization market, followed by Europe and Asia-Pacific. The United States, in particular, dominates the market due to its advanced healthcare infrastructure, stringent regulatory standards, and high adoption rate of innovative sterilization technologies. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the fastest growth in the coming years, driven by improving healthcare infrastructure and increasing healthcare expenditure.
The market is segmented based on sterilization method, including ethylene oxide sterilization, gamma sterilization, electron beam sterilization, and steam sterilization. Among these, ethylene oxide sterilization currently holds the largest market share due to its effectiveness in sterilizing heat-sensitive medical devices. However, concerns over the environmental impact and potential health risks associated with ethylene oxide have led to increased interest in alternative sterilization methods, including vacuum-based technologies.
Vacuum pump applications in biomedical device sterilization are gaining traction as they offer several advantages, including shorter cycle times, lower temperature requirements, and reduced chemical residues. The vacuum sterilization segment is expected to grow at a CAGR of 9.5% from 2020 to 2025, outpacing the overall market growth rate.
Key market trends include the shift towards eco-friendly sterilization methods, the adoption of single-use medical devices, and the increasing demand for contract sterilization services. Additionally, the COVID-19 pandemic has further emphasized the importance of effective sterilization practices, leading to increased investments in sterilization technologies and equipment across healthcare facilities.
The global biomedical device sterilization market was valued at approximately $5.3 billion in 2020 and is projected to reach $7.8 billion by 2025, growing at a compound annual growth rate (CAGR) of 8.2% during the forecast period. This growth is primarily attributed to the rising number of surgical procedures, the growing prevalence of hospital-acquired infections, and the increasing focus on infection control and prevention measures.
North America currently holds the largest share of the biomedical device sterilization market, followed by Europe and Asia-Pacific. The United States, in particular, dominates the market due to its advanced healthcare infrastructure, stringent regulatory standards, and high adoption rate of innovative sterilization technologies. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the fastest growth in the coming years, driven by improving healthcare infrastructure and increasing healthcare expenditure.
The market is segmented based on sterilization method, including ethylene oxide sterilization, gamma sterilization, electron beam sterilization, and steam sterilization. Among these, ethylene oxide sterilization currently holds the largest market share due to its effectiveness in sterilizing heat-sensitive medical devices. However, concerns over the environmental impact and potential health risks associated with ethylene oxide have led to increased interest in alternative sterilization methods, including vacuum-based technologies.
Vacuum pump applications in biomedical device sterilization are gaining traction as they offer several advantages, including shorter cycle times, lower temperature requirements, and reduced chemical residues. The vacuum sterilization segment is expected to grow at a CAGR of 9.5% from 2020 to 2025, outpacing the overall market growth rate.
Key market trends include the shift towards eco-friendly sterilization methods, the adoption of single-use medical devices, and the increasing demand for contract sterilization services. Additionally, the COVID-19 pandemic has further emphasized the importance of effective sterilization practices, leading to increased investments in sterilization technologies and equipment across healthcare facilities.
Vacuum Pump Technology: Current State and Challenges
Vacuum pump technology has made significant strides in recent years, particularly in its applications for biomedical device sterilization. However, the current state of this technology still faces several challenges that need to be addressed to enhance its effectiveness and efficiency in the medical field.
One of the primary advancements in vacuum pump technology is the development of oil-free pumps, which have become increasingly popular in biomedical applications. These pumps eliminate the risk of oil contamination, a critical factor in maintaining sterile environments. Despite this progress, oil-free pumps often struggle with achieving the deep vacuum levels required for some sterilization processes, presenting a significant technical hurdle.
Another notable improvement is the integration of smart control systems in vacuum pumps. These systems allow for precise pressure control and real-time monitoring, enhancing the reliability and reproducibility of sterilization processes. However, the complexity of these control systems can lead to increased maintenance requirements and potential points of failure, posing challenges for long-term operation in medical settings.
Energy efficiency remains a key focus area for vacuum pump technology. While newer models have shown improvements in power consumption, there is still a considerable demand for pumps that can maintain high performance while reducing energy usage. This challenge is particularly relevant in healthcare facilities where operational costs are a significant concern.
Noise reduction is another aspect where current vacuum pump technology faces obstacles. The noise generated by these pumps can be disruptive in medical environments, necessitating the development of quieter operation methods without compromising performance.
Scalability presents an additional challenge, especially in larger medical facilities. Current vacuum pump systems often struggle to efficiently scale up to meet the demands of high-volume sterilization processes without significant increases in cost and complexity.
Lastly, the materials used in vacuum pump construction for biomedical applications must withstand frequent sterilization cycles and harsh cleaning agents. While progress has been made in developing corrosion-resistant materials, finding the optimal balance between durability, cost-effectiveness, and performance remains an ongoing challenge in the field.
One of the primary advancements in vacuum pump technology is the development of oil-free pumps, which have become increasingly popular in biomedical applications. These pumps eliminate the risk of oil contamination, a critical factor in maintaining sterile environments. Despite this progress, oil-free pumps often struggle with achieving the deep vacuum levels required for some sterilization processes, presenting a significant technical hurdle.
Another notable improvement is the integration of smart control systems in vacuum pumps. These systems allow for precise pressure control and real-time monitoring, enhancing the reliability and reproducibility of sterilization processes. However, the complexity of these control systems can lead to increased maintenance requirements and potential points of failure, posing challenges for long-term operation in medical settings.
Energy efficiency remains a key focus area for vacuum pump technology. While newer models have shown improvements in power consumption, there is still a considerable demand for pumps that can maintain high performance while reducing energy usage. This challenge is particularly relevant in healthcare facilities where operational costs are a significant concern.
Noise reduction is another aspect where current vacuum pump technology faces obstacles. The noise generated by these pumps can be disruptive in medical environments, necessitating the development of quieter operation methods without compromising performance.
Scalability presents an additional challenge, especially in larger medical facilities. Current vacuum pump systems often struggle to efficiently scale up to meet the demands of high-volume sterilization processes without significant increases in cost and complexity.
Lastly, the materials used in vacuum pump construction for biomedical applications must withstand frequent sterilization cycles and harsh cleaning agents. While progress has been made in developing corrosion-resistant materials, finding the optimal balance between durability, cost-effectiveness, and performance remains an ongoing challenge in the field.
Vacuum Pump Solutions for Biomedical Sterilization
01 Improved vacuum pump designs
Various innovations in vacuum pump designs aim to enhance efficiency, reduce noise, and improve overall performance. These designs may include modifications to rotor configurations, sealing mechanisms, or the integration of advanced materials to optimize pump operation.- Improved pump design for enhanced efficiency: Vacuum pumps with innovative designs to increase efficiency and performance. These improvements may include optimized rotor configurations, advanced sealing mechanisms, or novel compression techniques to achieve better vacuum levels with reduced energy consumption.
- Multi-stage vacuum pump systems: Development of multi-stage vacuum pump systems that combine different pump types or stages to achieve higher vacuum levels and improved pumping speeds. These systems may integrate various technologies such as rotary vane, scroll, or turbomolecular pumps to optimize performance across different pressure ranges.
- Vacuum pump control and monitoring systems: Integration of advanced control and monitoring systems in vacuum pumps to optimize operation, detect faults, and improve maintenance schedules. These systems may include sensors, data analytics, and remote monitoring capabilities to enhance pump performance and reliability.
- Specialized vacuum pumps for specific applications: Development of vacuum pumps tailored for specific industrial or scientific applications, such as semiconductor manufacturing, food packaging, or scientific research. These pumps may have unique features or materials to meet the requirements of particular processes or environments.
- Energy-efficient and environmentally friendly vacuum pumps: Design of vacuum pumps with a focus on energy efficiency and environmental sustainability. These pumps may incorporate features such as variable speed drives, heat recovery systems, or eco-friendly materials to reduce energy consumption and environmental impact.
02 Energy-efficient vacuum pump systems
Development of energy-efficient vacuum pump systems focuses on reducing power consumption while maintaining high performance. These systems may incorporate advanced control algorithms, variable speed drives, or heat recovery mechanisms to optimize energy usage.Expand Specific Solutions03 Vacuum pump cooling and lubrication
Innovations in cooling and lubrication systems for vacuum pumps aim to improve reliability and extend operational life. These may include advanced heat dissipation methods, self-lubricating materials, or intelligent lubrication control systems.Expand Specific Solutions04 Specialized vacuum pumps for specific applications
Development of vacuum pumps tailored for specific industries or applications, such as semiconductor manufacturing, medical devices, or aerospace. These pumps may have unique features or operating parameters optimized for their intended use.Expand Specific Solutions05 Smart vacuum pump control and monitoring
Integration of advanced control and monitoring systems in vacuum pumps, incorporating sensors, IoT connectivity, and predictive maintenance capabilities. These features allow for real-time performance optimization and early detection of potential issues.Expand Specific Solutions
Key Manufacturers in Biomedical Vacuum Pump Industry
The vacuum pump applications in biomedical device sterilization market is in a growth phase, driven by increasing demand for sterilization in healthcare settings. The global market size is expanding, with projections indicating significant growth in the coming years. Technologically, the field is advancing rapidly, with companies like Shinva Medical Instrument, Edwards Japan, and Laoken Medical Technology leading innovation. These firms are developing more efficient and specialized vacuum pumps for sterilization processes. Established players such as Johnson & Johnson and Abbott Laboratories are also investing in this technology, indicating its growing importance in the medical device industry. The market is characterized by a mix of specialized vacuum pump manufacturers and large medical device companies, suggesting a competitive and dynamic landscape.
Shinva Medical Instrument Co., Ltd.
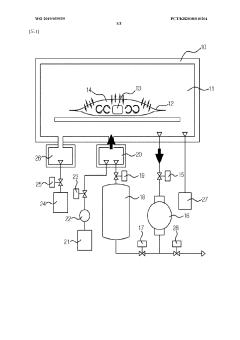
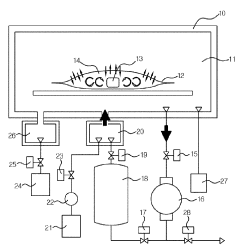
Technical Solution: Shinva Medical Instrument Co., Ltd. has developed a range of vacuum-based sterilization equipment for biomedical devices, including their LOWTEM series of low-temperature plasma sterilizers. These systems utilize a combination of vacuum technology and hydrogen peroxide plasma to achieve effective sterilization at temperatures below 50°C. Shinva's vacuum pumps are designed to reach pressures as low as 0.3 mbar, ensuring thorough removal of air and moisture before the introduction of sterilant [10]. The company has also implemented pulsed vacuum technology in their systems, which enhances penetration of sterilant into complex device lumens and crevices. Shinva's sterilizers feature programmable cycle parameters and automatic leak testing to ensure consistent and reliable sterilization results across various load types [11].
Strengths: Versatility in handling different medical devices, low-temperature operation, and user-friendly interface. Weaknesses: May have limitations in processing certain materials and requires careful management of hydrogen peroxide consumption.
Ateliers Busch SA
Technical Solution: Ateliers Busch SA has pioneered vacuum technology for medical sterilization with their COBRA and MINK series vacuum pumps. These dry screw vacuum pumps are specifically engineered for steam sterilization processes in hospitals and medical device manufacturing. The COBRA NX series, for instance, can achieve ultimate pressures below 0.1 hPa and features a variable speed drive for energy efficiency [4]. Busch's vacuum systems are designed to handle the challenges of steam and moisture in sterilization cycles, incorporating corrosion-resistant materials and advanced sealing technologies. Their pumps also include intelligent control systems that optimize performance based on sterilization cycle requirements, reducing energy consumption by up to 50% compared to conventional systems [5].
Strengths: High reliability, energy efficiency, and adaptability to various sterilization processes. Weaknesses: May require higher initial investment and specialized technical support.
Innovative Vacuum Pump Technologies for Sterilization
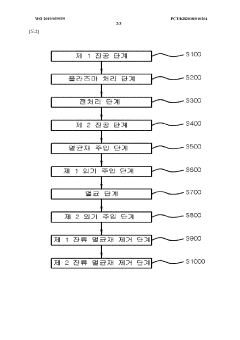
Sterilization method using low-temperature sterilizing device
PatentWO2019059559A1
Innovation
- A low-temperature sterilization device with an air circulation unit, comprising a vacuum pump and tank, that repeatedly varies pressure in the sterilization space to facilitate the penetration of sterilizing materials into sealed pouches, while maintaining a suitable temperature for sterilization through heated air injection.
Vacuum packaging paper plasma sterilization apparatus
PatentWO2017105000A1
Innovation
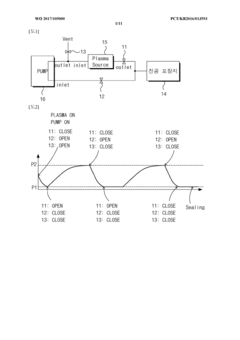
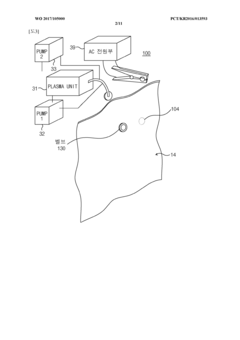
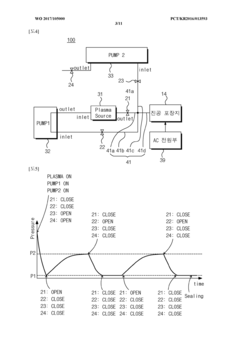
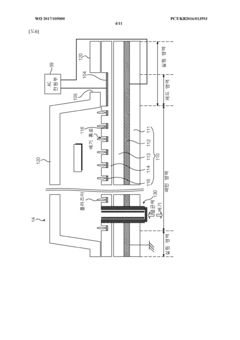
- A vacuum packaging plasma sterilization device utilizing two vacuum pumps and an external plasma source to create a vacuum and introduce sterilizing agents like hydrogen peroxide or reactive oxygen species directly into the packaging, allowing for efficient sterilization within the packaging without the need for large sterilization chambers.
Regulatory Framework for Medical Device Sterilization
The regulatory framework for medical device sterilization is a critical aspect of ensuring patient safety and product efficacy in the biomedical industry. Vacuum pump applications in biomedical device sterilization must adhere to stringent regulations set forth by various governing bodies worldwide.
In the United States, the Food and Drug Administration (FDA) oversees the regulatory requirements for medical device sterilization. The FDA's Center for Devices and Radiological Health (CDRH) is responsible for enforcing these regulations, which are outlined in the Code of Federal Regulations (CFR) Title 21, Part 820 - Quality System Regulation. This regulation mandates that manufacturers establish and maintain procedures for the validation of sterilization processes, including the use of vacuum pumps.
The European Union (EU) has its own set of regulations for medical device sterilization, governed by the Medical Device Regulation (MDR) 2017/745. This regulation emphasizes the importance of risk management and requires manufacturers to implement appropriate sterilization processes that ensure the safety and performance of medical devices throughout their lifecycle.
Internationally, the International Organization for Standardization (ISO) provides guidelines for sterilization of medical devices through ISO 11135 for ethylene oxide sterilization and ISO 17665 for moist heat sterilization. These standards often incorporate vacuum processes and are widely recognized and adopted by regulatory bodies worldwide.
Manufacturers must demonstrate compliance with these regulations through extensive documentation and validation processes. This includes providing evidence of the effectiveness of their sterilization methods, including those utilizing vacuum pump technology. Validation protocols typically involve three main stages: installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ).
The regulatory framework also addresses the environmental impact of sterilization processes. For instance, the use of ethylene oxide in sterilization is subject to strict emission controls due to its potential environmental and health hazards. Vacuum pump applications in this context must not only ensure effective sterilization but also comply with environmental regulations.
Continuous monitoring and periodic revalidation of sterilization processes are required to maintain regulatory compliance. This includes regular calibration and maintenance of vacuum pumps used in sterilization cycles. Manufacturers must also establish procedures for handling non-conformities and implementing corrective actions when deviations from validated processes occur.
As technology advances, regulatory bodies continually update their guidelines to address new sterilization methods and equipment. For example, the emergence of low-temperature sterilization techniques using hydrogen peroxide plasma, which often involve vacuum systems, has led to the development of specific regulatory guidance for these processes.
In the United States, the Food and Drug Administration (FDA) oversees the regulatory requirements for medical device sterilization. The FDA's Center for Devices and Radiological Health (CDRH) is responsible for enforcing these regulations, which are outlined in the Code of Federal Regulations (CFR) Title 21, Part 820 - Quality System Regulation. This regulation mandates that manufacturers establish and maintain procedures for the validation of sterilization processes, including the use of vacuum pumps.
The European Union (EU) has its own set of regulations for medical device sterilization, governed by the Medical Device Regulation (MDR) 2017/745. This regulation emphasizes the importance of risk management and requires manufacturers to implement appropriate sterilization processes that ensure the safety and performance of medical devices throughout their lifecycle.
Internationally, the International Organization for Standardization (ISO) provides guidelines for sterilization of medical devices through ISO 11135 for ethylene oxide sterilization and ISO 17665 for moist heat sterilization. These standards often incorporate vacuum processes and are widely recognized and adopted by regulatory bodies worldwide.
Manufacturers must demonstrate compliance with these regulations through extensive documentation and validation processes. This includes providing evidence of the effectiveness of their sterilization methods, including those utilizing vacuum pump technology. Validation protocols typically involve three main stages: installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ).
The regulatory framework also addresses the environmental impact of sterilization processes. For instance, the use of ethylene oxide in sterilization is subject to strict emission controls due to its potential environmental and health hazards. Vacuum pump applications in this context must not only ensure effective sterilization but also comply with environmental regulations.
Continuous monitoring and periodic revalidation of sterilization processes are required to maintain regulatory compliance. This includes regular calibration and maintenance of vacuum pumps used in sterilization cycles. Manufacturers must also establish procedures for handling non-conformities and implementing corrective actions when deviations from validated processes occur.
As technology advances, regulatory bodies continually update their guidelines to address new sterilization methods and equipment. For example, the emergence of low-temperature sterilization techniques using hydrogen peroxide plasma, which often involve vacuum systems, has led to the development of specific regulatory guidance for these processes.
Environmental Impact of Vacuum Sterilization Methods
Vacuum sterilization methods, while effective in biomedical device sterilization, have significant environmental implications that warrant careful consideration. The primary environmental concern associated with these methods is the use of ethylene oxide (EtO), a commonly employed sterilant gas. EtO is a known carcinogen and air pollutant, posing risks to both human health and the environment when released into the atmosphere.
The production and disposal of EtO contribute to greenhouse gas emissions, exacerbating climate change concerns. Moreover, the process of vacuum sterilization often requires substantial energy consumption, particularly in maintaining the necessary vacuum conditions and temperature control. This energy demand typically relies on fossil fuel-based power sources, further increasing the carbon footprint of sterilization processes.
Water usage is another environmental factor to consider. Some vacuum sterilization methods involve steam or water-based processes, which can lead to increased water consumption and potential contamination of water sources if not properly managed. The disposal of contaminated water from these processes requires additional treatment, adding to the overall environmental impact.
The materials used in vacuum sterilization equipment and packaging also contribute to environmental concerns. Many components are made from non-biodegradable materials, leading to long-term waste management issues. Additionally, the frequent replacement of filters and other consumables in vacuum sterilization systems generates ongoing waste streams that require proper disposal.
However, it is important to note that vacuum sterilization methods often have lower environmental impacts compared to alternative sterilization techniques, such as radiation or chemical treatments. The controlled environment of vacuum sterilization can reduce the overall use of harmful chemicals and minimize waste generation.
Efforts to mitigate the environmental impact of vacuum sterilization are ongoing. These include the development of more energy-efficient vacuum pumps, the exploration of alternative sterilant gases with lower environmental impacts, and the implementation of closed-loop systems to capture and recycle sterilant gases. Additionally, advancements in materials science are leading to more environmentally friendly packaging and equipment components.
As the biomedical industry continues to grow, balancing the need for effective sterilization with environmental sustainability remains a critical challenge. Future developments in vacuum sterilization technology will likely focus on further reducing energy consumption, minimizing chemical usage, and improving overall process efficiency to mitigate environmental impacts while maintaining the high standards of sterility required for biomedical devices.
The production and disposal of EtO contribute to greenhouse gas emissions, exacerbating climate change concerns. Moreover, the process of vacuum sterilization often requires substantial energy consumption, particularly in maintaining the necessary vacuum conditions and temperature control. This energy demand typically relies on fossil fuel-based power sources, further increasing the carbon footprint of sterilization processes.
Water usage is another environmental factor to consider. Some vacuum sterilization methods involve steam or water-based processes, which can lead to increased water consumption and potential contamination of water sources if not properly managed. The disposal of contaminated water from these processes requires additional treatment, adding to the overall environmental impact.
The materials used in vacuum sterilization equipment and packaging also contribute to environmental concerns. Many components are made from non-biodegradable materials, leading to long-term waste management issues. Additionally, the frequent replacement of filters and other consumables in vacuum sterilization systems generates ongoing waste streams that require proper disposal.
However, it is important to note that vacuum sterilization methods often have lower environmental impacts compared to alternative sterilization techniques, such as radiation or chemical treatments. The controlled environment of vacuum sterilization can reduce the overall use of harmful chemicals and minimize waste generation.
Efforts to mitigate the environmental impact of vacuum sterilization are ongoing. These include the development of more energy-efficient vacuum pumps, the exploration of alternative sterilant gases with lower environmental impacts, and the implementation of closed-loop systems to capture and recycle sterilant gases. Additionally, advancements in materials science are leading to more environmentally friendly packaging and equipment components.
As the biomedical industry continues to grow, balancing the need for effective sterilization with environmental sustainability remains a critical challenge. Future developments in vacuum sterilization technology will likely focus on further reducing energy consumption, minimizing chemical usage, and improving overall process efficiency to mitigate environmental impacts while maintaining the high standards of sterility required for biomedical devices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!