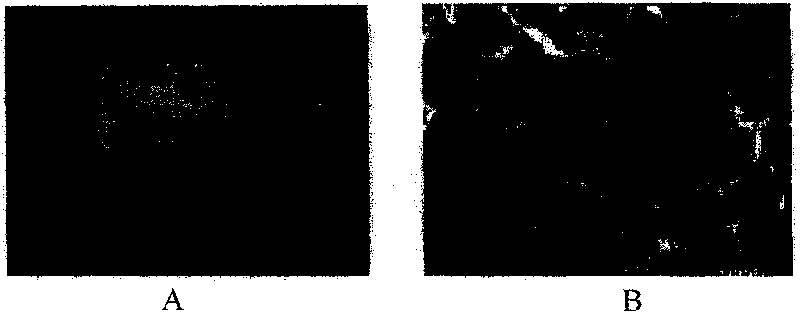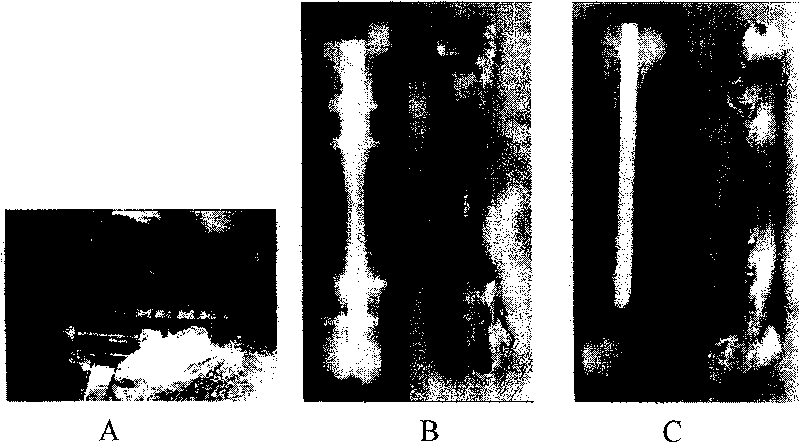Tissue engineering bone
A technology for tissue engineering bone and seed cells, applied in the field of biomedical tissue engineering, can solve problems such as the inability to achieve large-scale preparation and market promotion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Preparation process of tissue engineered bone constructed by allogeneic MSCs
[0036] 1. Obtain allogeneic red bone marrow: obtain red bone marrow by bone marrow puncture from a healthy donor, and anticoagulate with an equal amount of 1.0 U / ml heparin saline.
[0037] 2. Isolation and cultivation of primary bone marrow mesenchymal stem cells (MSCs): add Percoll separation medium with a density of 1.073g / mL to the diluted bone marrow sample, centrifuge at 400g for 20min, absorb the milky white cloudy nucleated cell layer in the middle, and add D-Hanks Liquid 20ml Gently blow and rinse with 2×10 5 Inoculate the cells at a cell density of / cm2 into plastic culture bottles, add 5ml of complete medium containing 15% fetal bovine serum to each bottle, and incubate in a 37°C, 5% CO2, saturated humidity incubator. Change the medium after 48 hours, and change the medium every other day thereafter. After most of the cell colonies are confluent (usually 10-12 days), su...
Embodiment 2
[0044] Example 2 Preparation process of tissue engineered bone constructed by allogeneic osteoblasts
[0045] 1. Obtain allogeneic periosteum: take out fresh periosteum tissue from a healthy donor through aseptic surgery.
[0046] 2. Isolation and culture of primary allogeneic osteoblasts: Cut the fresh periosteum into small pieces with a side length of about 1mm, digest with 0.1% collagenase for about 40 minutes at 37°C, and stop the reaction with an equal amount of serum-containing medium. After repeated pipetting, inoculate into plastic culture bottles at a cell density of 2×105 / cm2, add 5ml of complete medium containing 15% fetal bovine serum to each bottle, and incubate in an incubator at 37°C, 5% CO2, and saturated humidity. Change the medium after 48 hours, and change the medium every other day thereafter. After most of the cell colonies are confluent (usually 8-10 days), subculture is carried out immediately. Cells of passage 3-5 were collected for later use.
[004...
Embodiment 3
[0051] Example 3 In vitro detection of the above-mentioned demineralized bone matrix (DBM) raw materials:
[0052] See attached figure 1 , 2 , decalcified bone matrix tissue engineering scaffold materials are irregular porous solid particles, white or light yellow, no visible impurities, particle size of about 8mm; other scaffold materials, such as tricalcium phosphate (TCP), coral, polylactic acid (PLA) all conform to the three-dimensional shape and external dimensions of the respective specifications. Histological observation: The cancellous bone part of each material is observed under a microscope as a mesh-like structure, with clean pores, smooth walls, and no obvious content. The pore size is 20-300um.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com