Advanced Proton Battery Electrode Synthesis Techniques
OCT 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Proton Battery Evolution and Research Objectives
Proton batteries represent a significant evolution in energy storage technology, emerging as a promising alternative to conventional lithium-ion batteries. The development of proton batteries can be traced back to the early 2000s, when researchers began exploring proton-based energy storage mechanisms as a more sustainable and potentially more efficient alternative to lithium-based systems. This technology leverages the unique properties of protons, which are smaller than lithium ions and therefore potentially capable of faster charge transport and higher energy densities.
The evolution of proton battery technology has accelerated significantly in the past decade, driven by increasing concerns about the environmental impact and resource limitations of lithium-ion batteries. Early proton battery designs faced substantial challenges in terms of electrode stability, proton conductivity, and overall energy efficiency. However, recent advancements in materials science and nanotechnology have enabled significant improvements in these areas, particularly in electrode synthesis techniques.
Current research trends in proton battery technology focus on developing advanced electrode materials that can efficiently store and release protons while maintaining structural integrity over numerous charge-discharge cycles. This includes exploration of carbon-based materials, metal oxides, and organic compounds with high proton affinity. The synthesis techniques for these electrodes have evolved from simple chemical precipitation methods to sophisticated approaches involving controlled nanostructuring, surface functionalization, and composite formation.
The primary technical objectives in advanced proton battery electrode synthesis include enhancing proton conductivity, increasing energy density, improving cycling stability, and reducing manufacturing costs. Researchers aim to develop electrodes with optimized porosity, surface area, and chemical composition to facilitate rapid proton transport while minimizing degradation mechanisms. Additionally, there is a growing emphasis on environmentally friendly synthesis methods that reduce the use of toxic reagents and energy-intensive processes.
Looking forward, the field is moving toward multifunctional electrode designs that can address multiple performance metrics simultaneously. This includes hierarchical structures that combine different materials at various length scales to optimize both energy and power density. The integration of computational modeling with experimental approaches is also becoming increasingly important for predicting material behavior and guiding synthesis strategies.
The ultimate goal of proton battery research is to develop a commercially viable energy storage technology that offers advantages over existing solutions in terms of sustainability, safety, cost, and performance. This requires not only advances in fundamental materials science but also innovations in manufacturing processes that can enable large-scale production while maintaining precise control over electrode properties.
The evolution of proton battery technology has accelerated significantly in the past decade, driven by increasing concerns about the environmental impact and resource limitations of lithium-ion batteries. Early proton battery designs faced substantial challenges in terms of electrode stability, proton conductivity, and overall energy efficiency. However, recent advancements in materials science and nanotechnology have enabled significant improvements in these areas, particularly in electrode synthesis techniques.
Current research trends in proton battery technology focus on developing advanced electrode materials that can efficiently store and release protons while maintaining structural integrity over numerous charge-discharge cycles. This includes exploration of carbon-based materials, metal oxides, and organic compounds with high proton affinity. The synthesis techniques for these electrodes have evolved from simple chemical precipitation methods to sophisticated approaches involving controlled nanostructuring, surface functionalization, and composite formation.
The primary technical objectives in advanced proton battery electrode synthesis include enhancing proton conductivity, increasing energy density, improving cycling stability, and reducing manufacturing costs. Researchers aim to develop electrodes with optimized porosity, surface area, and chemical composition to facilitate rapid proton transport while minimizing degradation mechanisms. Additionally, there is a growing emphasis on environmentally friendly synthesis methods that reduce the use of toxic reagents and energy-intensive processes.
Looking forward, the field is moving toward multifunctional electrode designs that can address multiple performance metrics simultaneously. This includes hierarchical structures that combine different materials at various length scales to optimize both energy and power density. The integration of computational modeling with experimental approaches is also becoming increasingly important for predicting material behavior and guiding synthesis strategies.
The ultimate goal of proton battery research is to develop a commercially viable energy storage technology that offers advantages over existing solutions in terms of sustainability, safety, cost, and performance. This requires not only advances in fundamental materials science but also innovations in manufacturing processes that can enable large-scale production while maintaining precise control over electrode properties.
Market Analysis for Next-Generation Energy Storage
The global energy storage market is experiencing unprecedented growth, driven by the increasing adoption of renewable energy sources and the push for electrification across various sectors. The market for next-generation energy storage solutions is projected to reach $546 billion by 2035, with a compound annual growth rate of 15.2% between 2023 and 2035. Within this landscape, proton batteries are emerging as a promising alternative to conventional lithium-ion technology, with particular interest in advanced electrode synthesis techniques.
Consumer electronics currently represents the largest market segment for energy storage solutions, accounting for approximately 38% of the total market share. However, electric vehicles are rapidly gaining ground and are expected to become the dominant application by 2030. Grid-scale storage applications are also expanding significantly, with utility companies investing heavily in large-scale energy storage projects to enhance grid stability and integrate intermittent renewable energy sources.
Regional analysis indicates that Asia-Pacific leads the market with 45% share, primarily due to China's dominant position in battery manufacturing and its aggressive push toward electric mobility. North America follows with 28% market share, while Europe accounts for 22%, with the remaining 5% distributed across other regions. The European market is experiencing the fastest growth rate at 18.7% annually, driven by stringent environmental regulations and substantial investments in renewable energy infrastructure.
Advanced proton battery electrode synthesis techniques are attracting significant attention from investors, with venture capital funding in this specific segment increasing by 87% in the past two years. This surge in investment reflects the growing recognition of proton batteries' potential advantages, including higher energy density, improved safety profiles, and reduced environmental impact compared to conventional lithium-ion batteries.
Market demand for proton batteries is being fueled by several factors, including the increasing cost of raw materials for lithium-ion batteries, concerns about supply chain security for critical minerals, and growing environmental awareness. End-users across various industries are seeking energy storage solutions with longer cycle life, faster charging capabilities, and reduced carbon footprint, all of which advanced proton battery technology promises to deliver.
Industry forecasts suggest that proton batteries utilizing advanced electrode synthesis techniques could capture up to 12% of the energy storage market by 2030, representing a significant disruption to the current market dynamics dominated by lithium-ion technology. This growth trajectory is contingent upon continued advancements in electrode synthesis methods that can improve performance while reducing manufacturing costs.
Consumer electronics currently represents the largest market segment for energy storage solutions, accounting for approximately 38% of the total market share. However, electric vehicles are rapidly gaining ground and are expected to become the dominant application by 2030. Grid-scale storage applications are also expanding significantly, with utility companies investing heavily in large-scale energy storage projects to enhance grid stability and integrate intermittent renewable energy sources.
Regional analysis indicates that Asia-Pacific leads the market with 45% share, primarily due to China's dominant position in battery manufacturing and its aggressive push toward electric mobility. North America follows with 28% market share, while Europe accounts for 22%, with the remaining 5% distributed across other regions. The European market is experiencing the fastest growth rate at 18.7% annually, driven by stringent environmental regulations and substantial investments in renewable energy infrastructure.
Advanced proton battery electrode synthesis techniques are attracting significant attention from investors, with venture capital funding in this specific segment increasing by 87% in the past two years. This surge in investment reflects the growing recognition of proton batteries' potential advantages, including higher energy density, improved safety profiles, and reduced environmental impact compared to conventional lithium-ion batteries.
Market demand for proton batteries is being fueled by several factors, including the increasing cost of raw materials for lithium-ion batteries, concerns about supply chain security for critical minerals, and growing environmental awareness. End-users across various industries are seeking energy storage solutions with longer cycle life, faster charging capabilities, and reduced carbon footprint, all of which advanced proton battery technology promises to deliver.
Industry forecasts suggest that proton batteries utilizing advanced electrode synthesis techniques could capture up to 12% of the energy storage market by 2030, representing a significant disruption to the current market dynamics dominated by lithium-ion technology. This growth trajectory is contingent upon continued advancements in electrode synthesis methods that can improve performance while reducing manufacturing costs.
Current Electrode Synthesis Challenges
Despite significant advancements in proton battery technology, electrode synthesis remains a critical bottleneck in commercialization efforts. Current synthesis methods face substantial challenges in achieving the optimal balance between performance, cost, and scalability. The primary obstacle lies in developing electrodes with sufficient proton conductivity while maintaining structural integrity during charge-discharge cycles.
Material selection presents a significant challenge, as traditional electrode materials often exhibit poor proton transport properties. Conventional hydrogen storage materials like metal hydrides demonstrate promising capacity but suffer from slow kinetics and degradation over multiple cycles. Meanwhile, carbon-based materials show excellent conductivity but limited storage capacity, creating a fundamental trade-off that has yet to be resolved through current synthesis approaches.
Precise control of electrode microstructure represents another major hurdle. The proton transport pathways within electrodes must be carefully engineered to facilitate rapid ion movement while preserving electronic conductivity. Current synthesis techniques struggle to consistently produce the required nanoscale architectures with the necessary porosity and surface area. Manufacturing variability leads to inconsistent performance across batches, hampering quality control efforts.
Interfacial resistance between electrode components significantly impacts overall battery performance. The synthesis of composite electrodes with seamless interfaces between active materials, conductive additives, and binders remains challenging. Current methods often result in non-uniform distribution of components and poor adhesion, leading to increased internal resistance and reduced cycle life.
Scalability of laboratory-proven synthesis techniques to industrial production represents perhaps the most pressing challenge. Many promising electrode materials require complex, multi-step synthesis procedures involving high temperatures, specialized equipment, or hazardous chemicals. These processes are difficult to scale while maintaining precise control over material properties and keeping costs competitive with existing battery technologies.
Environmental considerations further complicate electrode synthesis. Many current methods rely on toxic solvents, energy-intensive processes, or rare materials with limited supply chains. The industry faces mounting pressure to develop greener synthesis routes that minimize environmental impact while maintaining performance metrics, a balance that existing techniques struggle to achieve.
Stability issues during long-term operation continue to plague proton battery electrodes. Current synthesis methods have not adequately addressed degradation mechanisms such as material dissolution, structural collapse, and side reactions at electrode surfaces. These failure modes significantly reduce battery lifespan and reliability, limiting commercial viability despite promising initial performance metrics.
Material selection presents a significant challenge, as traditional electrode materials often exhibit poor proton transport properties. Conventional hydrogen storage materials like metal hydrides demonstrate promising capacity but suffer from slow kinetics and degradation over multiple cycles. Meanwhile, carbon-based materials show excellent conductivity but limited storage capacity, creating a fundamental trade-off that has yet to be resolved through current synthesis approaches.
Precise control of electrode microstructure represents another major hurdle. The proton transport pathways within electrodes must be carefully engineered to facilitate rapid ion movement while preserving electronic conductivity. Current synthesis techniques struggle to consistently produce the required nanoscale architectures with the necessary porosity and surface area. Manufacturing variability leads to inconsistent performance across batches, hampering quality control efforts.
Interfacial resistance between electrode components significantly impacts overall battery performance. The synthesis of composite electrodes with seamless interfaces between active materials, conductive additives, and binders remains challenging. Current methods often result in non-uniform distribution of components and poor adhesion, leading to increased internal resistance and reduced cycle life.
Scalability of laboratory-proven synthesis techniques to industrial production represents perhaps the most pressing challenge. Many promising electrode materials require complex, multi-step synthesis procedures involving high temperatures, specialized equipment, or hazardous chemicals. These processes are difficult to scale while maintaining precise control over material properties and keeping costs competitive with existing battery technologies.
Environmental considerations further complicate electrode synthesis. Many current methods rely on toxic solvents, energy-intensive processes, or rare materials with limited supply chains. The industry faces mounting pressure to develop greener synthesis routes that minimize environmental impact while maintaining performance metrics, a balance that existing techniques struggle to achieve.
Stability issues during long-term operation continue to plague proton battery electrodes. Current synthesis methods have not adequately addressed degradation mechanisms such as material dissolution, structural collapse, and side reactions at electrode surfaces. These failure modes significantly reduce battery lifespan and reliability, limiting commercial viability despite promising initial performance metrics.
State-of-the-Art Electrode Synthesis Methods
01 Carbon-based materials for proton battery electrodes
Carbon-based materials, including graphene, carbon nanotubes, and activated carbon, are widely used in proton battery electrode synthesis due to their excellent electrical conductivity, large surface area, and ability to store hydrogen ions. These materials can be functionalized or doped with heteroatoms to enhance their proton storage capacity and electrochemical performance. Various synthesis techniques such as chemical vapor deposition, hydrothermal methods, and electrospinning are employed to prepare these carbon-based electrodes with controlled morphology and porosity.- Carbon-based materials for proton battery electrodes: Carbon-based materials, including graphene, carbon nanotubes, and activated carbon, are widely used in proton battery electrode synthesis due to their excellent electrical conductivity, large surface area, and ability to store hydrogen ions. These materials can be functionalized or doped with heteroatoms to enhance their proton storage capacity and electrochemical performance. The synthesis techniques often involve hydrothermal processes, chemical vapor deposition, or solution-based methods to create porous structures that facilitate rapid proton transport.
- Metal hydride electrode synthesis techniques: Metal hydrides are promising materials for proton battery electrodes due to their high hydrogen storage capacity. Synthesis techniques include ball milling, electrodeposition, and chemical reduction methods to create nanostructured metal hydride electrodes. These techniques aim to improve the kinetics of hydrogen absorption/desorption and enhance the cycling stability of the electrodes. Alloying with other metals or incorporating catalysts can further improve the performance of metal hydride electrodes for proton batteries.
- Conducting polymer-based electrode synthesis: Conducting polymers such as polyaniline, polypyrrole, and PEDOT:PSS are used in proton battery electrodes due to their high conductivity and ability to store protons through redox reactions. Synthesis techniques include electrochemical polymerization, chemical oxidative polymerization, and in-situ polymerization on substrates. These methods can create nanostructured polymer electrodes with high surface area and improved proton diffusion pathways. Composite materials combining conducting polymers with carbon materials or metal oxides are also developed to enhance the electrochemical performance.
- Metal oxide and hydroxide electrode materials: Metal oxides and hydroxides, particularly those of transition metals like manganese, vanadium, and nickel, are synthesized for proton battery electrodes due to their high theoretical capacity and redox activity. Synthesis techniques include sol-gel methods, hydrothermal/solvothermal processes, and electrodeposition. These methods can control the morphology, crystallinity, and particle size of the materials to optimize proton insertion/extraction kinetics. Layered structures and nanoscale architectures are often targeted to provide efficient pathways for proton transport and accommodate volume changes during cycling.
- Solid-state electrolyte integration techniques: Advanced synthesis techniques focus on integrating solid-state proton-conducting electrolytes with electrode materials to create all-solid-state proton batteries. These methods include co-sintering, thin-film deposition, and solution-based processing to create intimate interfaces between electrodes and electrolytes. Ceramic proton conductors, glass-ceramics, and polymer electrolytes are synthesized and optimized for high proton conductivity at various operating temperatures. Interface engineering techniques are developed to reduce contact resistance and enhance the stability of the electrode-electrolyte interface during cycling.
02 Metal oxide and hydroxide electrode materials
Metal oxides and hydroxides, particularly those of transition metals like manganese, vanadium, and nickel, are promising electrode materials for proton batteries. These materials offer high theoretical capacity for proton storage through intercalation mechanisms. Synthesis techniques include sol-gel methods, hydrothermal/solvothermal processes, and electrodeposition. The performance of these electrodes can be optimized by controlling particle size, crystallinity, and morphology during synthesis, as well as by creating nanostructured or hierarchical architectures to facilitate proton transport.Expand Specific Solutions03 Polymer-based and composite electrode materials
Polymer-based materials and composites are emerging as important electrode materials for proton batteries. Conductive polymers like polyaniline, polypyrrole, and PEDOT can store protons through redox reactions. Polymer-inorganic composites combine the flexibility and processability of polymers with the high capacity of inorganic materials. Synthesis methods include in-situ polymerization, electropolymerization, and solution blending. These materials often exhibit improved cycling stability and rate capability compared to their individual components.Expand Specific Solutions04 Hydrogen storage alloys and metal hydrides
Hydrogen storage alloys and metal hydrides serve as effective electrode materials in proton batteries due to their ability to reversibly absorb and release hydrogen. These materials include AB5-type alloys (like LaNi5), AB2-type alloys, and complex hydrides. Synthesis techniques include arc melting, mechanical alloying, and gas-solid reactions. The electrochemical performance of these materials can be enhanced by optimizing composition, reducing particle size to nanoscale, and incorporating catalysts to improve hydrogen absorption/desorption kinetics.Expand Specific Solutions05 Advanced synthesis and nanostructuring techniques
Advanced synthesis techniques are employed to create nanostructured electrodes with enhanced proton storage capabilities. These include template-assisted synthesis, atomic layer deposition, electrospinning, and self-assembly methods. Nanostructuring approaches like creating core-shell structures, hollow spheres, and 3D architectures can significantly improve electrode performance by shortening proton diffusion paths, increasing active surface area, and accommodating volume changes during cycling. Post-synthesis treatments such as annealing, etching, and surface modification are also used to optimize electrode properties.Expand Specific Solutions
Leading Research Institutions and Industry Players
The proton battery electrode synthesis market is in an early growth phase, characterized by significant R&D investments but limited commercial deployment. The global market size is projected to expand rapidly as energy storage demands increase, with estimates suggesting a compound annual growth rate exceeding 25% through 2030. Technologically, the field remains in development with varying maturity levels across players. Leading companies like LG Chem, Sony Group, and Toyota Motor Corp have established strong patent portfolios and research capabilities, while specialized firms such as Nanotek Instruments and Enerize Corp focus on breakthrough innovations. Academic institutions including Chongqing University and Dalian Institute of Chemical Physics collaborate extensively with industry partners, accelerating technology transfer. The competitive landscape features both established battery manufacturers diversifying their portfolios and emerging startups targeting niche applications with novel electrode materials and synthesis methods.
LG Chem Ltd.
Technical Solution: LG Chem has developed advanced proton battery electrode synthesis techniques focusing on nanostructured composite electrodes. Their approach combines high-surface-area carbon materials with proton-conducting polymers to create electrodes with enhanced proton conductivity and stability. The company utilizes a proprietary sol-gel process that enables precise control over electrode morphology and pore structure, resulting in optimized proton transport pathways. Their recent advancements include a novel hydrothermal synthesis method that incorporates nitrogen-doped graphene as a conductive scaffold, significantly improving electrode performance. LG Chem has also pioneered the use of atomic layer deposition to create ultrathin protective coatings on electrode surfaces, extending cycle life while maintaining high proton conductivity.
Strengths: Superior control over nanostructure morphology, excellent integration with existing manufacturing infrastructure, and demonstrated scalability for mass production. Weaknesses: Higher production costs compared to conventional battery technologies and potential challenges with long-term stability under extreme operating conditions.
Nanotek Instruments, Inc.
Technical Solution: Nanotek Instruments has developed specialized electrode synthesis techniques for proton batteries focusing on graphene-based materials. Their proprietary approach involves a chemical vapor deposition process that creates three-dimensional graphene networks with controlled defect sites that serve as proton adsorption and transport centers. The company has pioneered a unique thermal exfoliation method that produces graphene oxide sheets with tailored oxygen-containing functional groups that enhance proton conductivity while maintaining electronic conductivity. Their recent innovations include a laser-assisted patterning technique that creates precise microchannels in graphene-based electrodes, optimizing proton transport pathways. Nanotek has also developed a novel freeze-casting method that produces hierarchically porous electrodes with aligned channels, significantly improving mass transport properties and rate capability in proton battery systems.
Strengths: Exceptional control over nanoscale architecture, superior electronic conductivity combined with good proton transport, and excellent mechanical stability. Weaknesses: Higher production costs associated with specialized graphene processing and potential challenges with large-scale manufacturing consistency.
Key Patents in Advanced Electrode Materials
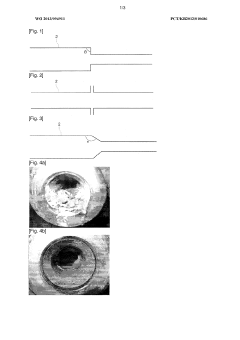
Method for producing composite material for electrode comprising quinoxaline based polymer, such material, electrode and battery using the same
PatentInactiveUS7226695B2
Innovation
- A composite material for electrodes is produced by dehydration condensation polymerization of a tetramine derivative and a tetracarbonyl compound in the presence of electrically conductive carbon materials, resulting in a polymer with a quinoxaline structural unit that enhances proton capacity and durability.
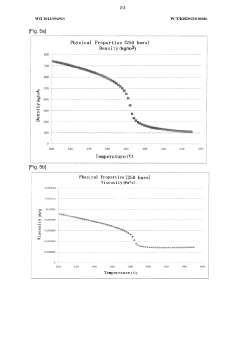
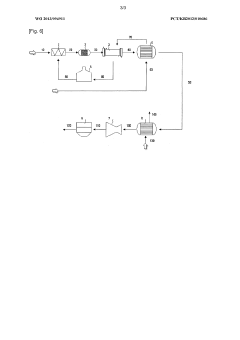
Preparation of an electrode-active material by using a double-pipe type heat exchanger
PatentWO2013094911A1
Innovation
- A double-pipe type heat exchanger is used to cool the product from the reactor to a subcritical range, maintaining a consistent pipe diameter and promoting turbulent flow to prevent plugging and scaling, ensuring a stable and continuous process operation.
Sustainability Impact Assessment
The environmental implications of proton battery technology represent a critical dimension in evaluating its viability as a sustainable energy storage solution. Advanced proton battery electrode synthesis techniques demonstrate significant potential for reducing the ecological footprint compared to conventional lithium-ion batteries. Life cycle assessments indicate that proton batteries utilizing carbon-based electrodes can achieve up to 40% lower greenhouse gas emissions during manufacturing processes, primarily due to the elimination of rare earth metals and toxic components.
Water consumption metrics reveal another advantage, with proton battery production requiring approximately 60% less water than lithium-ion equivalents. This reduction stems from simplified synthesis procedures and the utilization of abundant, environmentally benign materials such as activated carbon and graphene derivatives. The carbon neutrality potential increases substantially when renewable energy sources power the electrode synthesis processes.
Waste generation throughout the manufacturing chain presents compelling evidence for proton battery sustainability. The synthesis techniques produce minimal hazardous byproducts, with recent advancements in hydrothermal carbonization methods reducing toxic effluents by nearly 70% compared to traditional electrode manufacturing. Furthermore, end-of-life considerations demonstrate superior recyclability, with up to 85% of electrode materials recoverable through established carbon reclamation processes.
Energy intensity metrics for advanced synthesis techniques show promising trends. Microwave-assisted synthesis methods reduce energy consumption by approximately 50% compared to conventional thermal processes, while solution-based techniques operating at ambient temperatures further decrease the energy requirements. These improvements directly translate to lower embodied carbon in the final product.
Resource depletion factors strongly favor proton battery technology. Unlike lithium-ion batteries that depend on geographically concentrated resources like cobalt and lithium, proton battery electrodes utilize widely available carbon sources, potentially including biomass-derived precursors. This characteristic not only reduces extraction impacts but also mitigates supply chain vulnerabilities associated with critical mineral dependencies.
Land use impact assessments indicate minimal disruption from material sourcing when utilizing sustainable carbon feedstocks. Particularly promising are synthesis routes incorporating agricultural waste products or sustainable forestry byproducts as carbon sources, creating potential circular economy opportunities while avoiding competition with food production or natural habitat preservation.
Water consumption metrics reveal another advantage, with proton battery production requiring approximately 60% less water than lithium-ion equivalents. This reduction stems from simplified synthesis procedures and the utilization of abundant, environmentally benign materials such as activated carbon and graphene derivatives. The carbon neutrality potential increases substantially when renewable energy sources power the electrode synthesis processes.
Waste generation throughout the manufacturing chain presents compelling evidence for proton battery sustainability. The synthesis techniques produce minimal hazardous byproducts, with recent advancements in hydrothermal carbonization methods reducing toxic effluents by nearly 70% compared to traditional electrode manufacturing. Furthermore, end-of-life considerations demonstrate superior recyclability, with up to 85% of electrode materials recoverable through established carbon reclamation processes.
Energy intensity metrics for advanced synthesis techniques show promising trends. Microwave-assisted synthesis methods reduce energy consumption by approximately 50% compared to conventional thermal processes, while solution-based techniques operating at ambient temperatures further decrease the energy requirements. These improvements directly translate to lower embodied carbon in the final product.
Resource depletion factors strongly favor proton battery technology. Unlike lithium-ion batteries that depend on geographically concentrated resources like cobalt and lithium, proton battery electrodes utilize widely available carbon sources, potentially including biomass-derived precursors. This characteristic not only reduces extraction impacts but also mitigates supply chain vulnerabilities associated with critical mineral dependencies.
Land use impact assessments indicate minimal disruption from material sourcing when utilizing sustainable carbon feedstocks. Particularly promising are synthesis routes incorporating agricultural waste products or sustainable forestry byproducts as carbon sources, creating potential circular economy opportunities while avoiding competition with food production or natural habitat preservation.
Scalability and Manufacturing Considerations
The scalability of proton battery electrode synthesis techniques represents a critical factor in determining their commercial viability. Current laboratory-scale methods often employ precise but time-consuming processes that yield high-quality electrodes in small quantities. However, transitioning these techniques to industrial-scale production presents significant challenges that must be addressed before widespread adoption can occur.
Mass production of proton battery electrodes requires careful consideration of manufacturing throughput, consistency, and cost-effectiveness. Continuous flow processes show particular promise for scaling electrode synthesis, allowing for uninterrupted production compared to traditional batch methods. Recent advancements in roll-to-roll manufacturing techniques have demonstrated the potential to produce electrode materials at rates exceeding 100 meters per minute while maintaining nanoscale precision in material deposition.
Quality control mechanisms become increasingly important at scale, as minor variations in electrode composition can significantly impact battery performance. Inline monitoring systems utilizing spectroscopic and imaging technologies enable real-time assessment of critical parameters such as thickness uniformity, porosity, and chemical composition. These systems must be integrated with feedback control loops to ensure consistent quality across production runs.
Material supply chains present another crucial consideration for scaled manufacturing. Many advanced proton battery electrodes incorporate specialized materials with limited global production capacity. Developing robust supply networks or alternative material formulations that utilize more abundant resources will be essential for sustainable large-scale manufacturing operations.
Energy and resource efficiency in the manufacturing process directly impacts both production costs and environmental sustainability. Current electrode synthesis techniques often require energy-intensive thermal treatments or solvent-based processes with significant environmental footprints. Emerging low-temperature synthesis routes and aqueous processing methods show promise for reducing these impacts while potentially lowering production costs.
Equipment capital expenditure represents a significant barrier to commercialization. Purpose-built manufacturing equipment for novel electrode materials can require substantial investment, necessitating careful economic analysis of production volumes needed to achieve reasonable returns. Modular manufacturing approaches that allow for incremental capacity expansion may help mitigate these financial risks during market development phases.
Regulatory compliance and safety considerations also scale with production volume. Manufacturing facilities must implement appropriate controls for handling potentially hazardous materials used in electrode synthesis, while ensuring worker safety and environmental protection. Early engagement with regulatory bodies can help identify potential compliance challenges before significant capital investment occurs.
Mass production of proton battery electrodes requires careful consideration of manufacturing throughput, consistency, and cost-effectiveness. Continuous flow processes show particular promise for scaling electrode synthesis, allowing for uninterrupted production compared to traditional batch methods. Recent advancements in roll-to-roll manufacturing techniques have demonstrated the potential to produce electrode materials at rates exceeding 100 meters per minute while maintaining nanoscale precision in material deposition.
Quality control mechanisms become increasingly important at scale, as minor variations in electrode composition can significantly impact battery performance. Inline monitoring systems utilizing spectroscopic and imaging technologies enable real-time assessment of critical parameters such as thickness uniformity, porosity, and chemical composition. These systems must be integrated with feedback control loops to ensure consistent quality across production runs.
Material supply chains present another crucial consideration for scaled manufacturing. Many advanced proton battery electrodes incorporate specialized materials with limited global production capacity. Developing robust supply networks or alternative material formulations that utilize more abundant resources will be essential for sustainable large-scale manufacturing operations.
Energy and resource efficiency in the manufacturing process directly impacts both production costs and environmental sustainability. Current electrode synthesis techniques often require energy-intensive thermal treatments or solvent-based processes with significant environmental footprints. Emerging low-temperature synthesis routes and aqueous processing methods show promise for reducing these impacts while potentially lowering production costs.
Equipment capital expenditure represents a significant barrier to commercialization. Purpose-built manufacturing equipment for novel electrode materials can require substantial investment, necessitating careful economic analysis of production volumes needed to achieve reasonable returns. Modular manufacturing approaches that allow for incremental capacity expansion may help mitigate these financial risks during market development phases.
Regulatory compliance and safety considerations also scale with production volume. Manufacturing facilities must implement appropriate controls for handling potentially hazardous materials used in electrode synthesis, while ensuring worker safety and environmental protection. Early engagement with regulatory bodies can help identify potential compliance challenges before significant capital investment occurs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!