What Role Do Catalysts Play in Proton Battery Success?
OCT 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Catalyst Technology Background and Objectives
Catalysts have emerged as critical components in the development and advancement of proton battery technology, representing a significant frontier in sustainable energy storage solutions. The evolution of catalyst technology in this domain traces back to early electrochemical research in the 1960s, with substantial acceleration occurring in the past two decades as renewable energy storage demands have intensified globally.
The technological trajectory of catalysts in proton batteries has been shaped by the fundamental need to facilitate efficient hydrogen evolution and oxidation reactions at the electrodes. Traditional catalyst materials primarily relied on platinum group metals, which despite their excellent catalytic properties, presented significant cost barriers to widespread commercialization. This economic constraint has driven research toward alternative catalyst formulations that maintain performance while reducing dependency on precious metals.
Recent technological breakthroughs have focused on developing novel nanostructured catalysts, including transition metal compounds, carbon-based materials, and hybrid structures that demonstrate promising catalytic activity. These innovations have progressively improved reaction kinetics, reduced activation energy requirements, and enhanced overall energy conversion efficiency in proton battery systems.
The current technological landscape is characterized by a growing emphasis on bifunctional catalysts capable of supporting both hydrogen evolution and oxidation reactions, thereby simplifying battery architecture and reducing system complexity. Additionally, there is increasing attention to catalyst stability and longevity under the variable operating conditions typical of renewable energy applications.
The primary objectives of catalyst technology development for proton batteries encompass several dimensions. First, achieving cost-effectiveness through reduced precious metal content or complete substitution with earth-abundant materials remains paramount. Second, enhancing catalytic activity to improve charge-discharge rates and overall battery performance continues to drive research efforts. Third, developing catalysts with superior durability to withstand thousands of operational cycles without significant degradation represents a critical goal.
Furthermore, technological objectives include optimizing catalyst integration with electrode structures to maximize active surface area while maintaining structural integrity. Environmental considerations have also become increasingly important, with research targeting catalysts that minimize ecological impact throughout their lifecycle, from production to eventual recycling or disposal.
The convergence of nanotechnology, materials science, and electrochemistry has accelerated progress in this field, with computational modeling and high-throughput screening methods enabling more efficient discovery and optimization of novel catalyst formulations. These interdisciplinary approaches are expected to continue driving innovation as the technology progresses toward commercial viability and widespread deployment in energy storage applications.
The technological trajectory of catalysts in proton batteries has been shaped by the fundamental need to facilitate efficient hydrogen evolution and oxidation reactions at the electrodes. Traditional catalyst materials primarily relied on platinum group metals, which despite their excellent catalytic properties, presented significant cost barriers to widespread commercialization. This economic constraint has driven research toward alternative catalyst formulations that maintain performance while reducing dependency on precious metals.
Recent technological breakthroughs have focused on developing novel nanostructured catalysts, including transition metal compounds, carbon-based materials, and hybrid structures that demonstrate promising catalytic activity. These innovations have progressively improved reaction kinetics, reduced activation energy requirements, and enhanced overall energy conversion efficiency in proton battery systems.
The current technological landscape is characterized by a growing emphasis on bifunctional catalysts capable of supporting both hydrogen evolution and oxidation reactions, thereby simplifying battery architecture and reducing system complexity. Additionally, there is increasing attention to catalyst stability and longevity under the variable operating conditions typical of renewable energy applications.
The primary objectives of catalyst technology development for proton batteries encompass several dimensions. First, achieving cost-effectiveness through reduced precious metal content or complete substitution with earth-abundant materials remains paramount. Second, enhancing catalytic activity to improve charge-discharge rates and overall battery performance continues to drive research efforts. Third, developing catalysts with superior durability to withstand thousands of operational cycles without significant degradation represents a critical goal.
Furthermore, technological objectives include optimizing catalyst integration with electrode structures to maximize active surface area while maintaining structural integrity. Environmental considerations have also become increasingly important, with research targeting catalysts that minimize ecological impact throughout their lifecycle, from production to eventual recycling or disposal.
The convergence of nanotechnology, materials science, and electrochemistry has accelerated progress in this field, with computational modeling and high-throughput screening methods enabling more efficient discovery and optimization of novel catalyst formulations. These interdisciplinary approaches are expected to continue driving innovation as the technology progresses toward commercial viability and widespread deployment in energy storage applications.
Market Analysis for Proton Battery Applications
The global proton battery market is experiencing significant growth, driven by increasing demand for sustainable energy storage solutions. Current market projections indicate that the proton battery sector could reach substantial valuation by 2030, with a compound annual growth rate exceeding that of traditional lithium-ion technologies. This accelerated growth trajectory is primarily attributed to the superior theoretical energy density, enhanced safety profile, and reduced environmental impact of proton-based storage systems.
Market segmentation reveals diverse application potential across multiple sectors. The automotive industry represents the largest potential market segment, with electric vehicle manufacturers actively seeking alternatives to conventional battery technologies that offer improved range, faster charging capabilities, and reduced fire hazards. Catalyst innovations that enhance proton transfer efficiency directly address these requirements, potentially accelerating market penetration in this sector.
Stationary energy storage applications constitute another significant market opportunity, particularly for grid-scale implementation and renewable energy integration. The ability of advanced catalysts to improve charge-discharge efficiency at room temperature makes proton batteries increasingly competitive in this space, where operational costs and system longevity are paramount considerations.
Consumer electronics represents a growing application segment, though currently smaller than automotive and stationary storage markets. Here, the value proposition centers on safety advantages and potential for miniaturization through catalyst-enabled higher energy densities.
Regional market analysis indicates that Asia-Pacific currently leads in proton battery research and development investments, with China, Japan, and South Korea establishing strategic positions through government-backed initiatives and corporate research programs. North America and Europe follow closely, with significant research clusters forming around academic institutions and technology startups focused on catalyst innovation.
Market barriers include high initial production costs, manufacturing scalability challenges, and competition from increasingly optimized lithium-ion technologies. However, catalyst innovations represent a critical pathway to overcoming these barriers by potentially reducing material costs, improving performance metrics, and enabling mass production techniques.
Investment trends show increasing venture capital interest in startups focused on novel catalyst development for proton batteries, with several funding rounds exceeding $50 million reported in the past two years. Strategic partnerships between material science companies, battery manufacturers, and end-users are becoming more prevalent, indicating growing market confidence in the technology's commercial viability.
Market segmentation reveals diverse application potential across multiple sectors. The automotive industry represents the largest potential market segment, with electric vehicle manufacturers actively seeking alternatives to conventional battery technologies that offer improved range, faster charging capabilities, and reduced fire hazards. Catalyst innovations that enhance proton transfer efficiency directly address these requirements, potentially accelerating market penetration in this sector.
Stationary energy storage applications constitute another significant market opportunity, particularly for grid-scale implementation and renewable energy integration. The ability of advanced catalysts to improve charge-discharge efficiency at room temperature makes proton batteries increasingly competitive in this space, where operational costs and system longevity are paramount considerations.
Consumer electronics represents a growing application segment, though currently smaller than automotive and stationary storage markets. Here, the value proposition centers on safety advantages and potential for miniaturization through catalyst-enabled higher energy densities.
Regional market analysis indicates that Asia-Pacific currently leads in proton battery research and development investments, with China, Japan, and South Korea establishing strategic positions through government-backed initiatives and corporate research programs. North America and Europe follow closely, with significant research clusters forming around academic institutions and technology startups focused on catalyst innovation.
Market barriers include high initial production costs, manufacturing scalability challenges, and competition from increasingly optimized lithium-ion technologies. However, catalyst innovations represent a critical pathway to overcoming these barriers by potentially reducing material costs, improving performance metrics, and enabling mass production techniques.
Investment trends show increasing venture capital interest in startups focused on novel catalyst development for proton batteries, with several funding rounds exceeding $50 million reported in the past two years. Strategic partnerships between material science companies, battery manufacturers, and end-users are becoming more prevalent, indicating growing market confidence in the technology's commercial viability.
Current Catalyst Challenges in Proton Batteries
Despite significant advancements in proton battery technology, catalyst development remains a critical bottleneck limiting commercial viability. Current catalysts face substantial challenges in both the hydrogen evolution reaction (HER) and hydrogen oxidation reaction (HOR) sides of proton batteries. Platinum-based catalysts, while highly effective, suffer from prohibitive costs that make large-scale deployment economically unfeasible, with platinum prices exceeding $30,000 per kilogram. This cost factor alone represents a major barrier to widespread adoption.
Catalyst durability presents another significant challenge, as most current materials demonstrate rapid performance degradation under the acidic conditions and voltage cycling typical in proton battery operations. Studies indicate that even state-of-the-art catalysts may lose 20-40% of their activity after just 1,000 charge-discharge cycles, falling far short of the 5,000+ cycles required for commercial applications.
Bifunctional catalyst development remains particularly problematic. The ideal catalyst should efficiently facilitate both hydrogen storage (during charging) and hydrogen release (during discharging), yet most current materials excel at only one of these processes. This asymmetry in catalytic performance creates efficiency bottlenecks that reduce overall battery performance and energy density.
Catalyst poisoning represents another substantial challenge, particularly with carbon monoxide and sulfur compounds that can irreversibly bind to active catalyst sites. Even trace amounts of these contaminants can significantly reduce catalyst effectiveness, necessitating either extremely pure hydrogen sources or additional purification systems that add complexity and cost.
The scalable synthesis of high-performance non-precious metal catalysts remains elusive despite promising research directions. Materials such as nickel-molybdenum alloys and nitrogen-doped carbon structures show potential in laboratory settings but face significant challenges in consistent large-scale production with uniform catalytic properties.
Interface engineering between catalysts and proton-conducting membranes presents additional complications. Poor interfacial contact leads to increased resistance and reduced proton transfer efficiency. Current manufacturing techniques struggle to create optimal three-phase boundaries necessary for efficient catalytic reactions.
Finally, there exists a significant knowledge gap in understanding catalyst degradation mechanisms under real-world operating conditions. Most catalyst testing occurs under idealized laboratory conditions that fail to account for temperature fluctuations, impurities, and mechanical stresses encountered in practical applications. This disconnect between laboratory performance and real-world durability represents a fundamental challenge to developing truly robust catalyst solutions for next-generation proton batteries.
Catalyst durability presents another significant challenge, as most current materials demonstrate rapid performance degradation under the acidic conditions and voltage cycling typical in proton battery operations. Studies indicate that even state-of-the-art catalysts may lose 20-40% of their activity after just 1,000 charge-discharge cycles, falling far short of the 5,000+ cycles required for commercial applications.
Bifunctional catalyst development remains particularly problematic. The ideal catalyst should efficiently facilitate both hydrogen storage (during charging) and hydrogen release (during discharging), yet most current materials excel at only one of these processes. This asymmetry in catalytic performance creates efficiency bottlenecks that reduce overall battery performance and energy density.
Catalyst poisoning represents another substantial challenge, particularly with carbon monoxide and sulfur compounds that can irreversibly bind to active catalyst sites. Even trace amounts of these contaminants can significantly reduce catalyst effectiveness, necessitating either extremely pure hydrogen sources or additional purification systems that add complexity and cost.
The scalable synthesis of high-performance non-precious metal catalysts remains elusive despite promising research directions. Materials such as nickel-molybdenum alloys and nitrogen-doped carbon structures show potential in laboratory settings but face significant challenges in consistent large-scale production with uniform catalytic properties.
Interface engineering between catalysts and proton-conducting membranes presents additional complications. Poor interfacial contact leads to increased resistance and reduced proton transfer efficiency. Current manufacturing techniques struggle to create optimal three-phase boundaries necessary for efficient catalytic reactions.
Finally, there exists a significant knowledge gap in understanding catalyst degradation mechanisms under real-world operating conditions. Most catalyst testing occurs under idealized laboratory conditions that fail to account for temperature fluctuations, impurities, and mechanical stresses encountered in practical applications. This disconnect between laboratory performance and real-world durability represents a fundamental challenge to developing truly robust catalyst solutions for next-generation proton batteries.
Current Catalyst Solutions for Proton Batteries
01 Metal-based catalysts for proton batteries
Metal-based catalysts, particularly those containing platinum, palladium, and other noble metals, significantly enhance the performance of proton batteries. These catalysts facilitate faster proton transfer at the electrode interfaces, resulting in improved charge/discharge efficiency and higher power density. The catalytic activity of these metals reduces activation energy barriers in electrochemical reactions, leading to better overall battery performance and longer cycle life.- Metal-based catalysts for proton batteries: Metal-based catalysts play a crucial role in enhancing the performance and efficiency of proton batteries. These catalysts, including platinum, palladium, and other transition metals, facilitate faster proton transfer and improve the electrochemical reactions at the electrodes. The incorporation of these catalysts can significantly reduce activation energy barriers, increase reaction rates, and improve overall battery efficiency. Advanced metal catalyst designs with optimized structures and compositions have demonstrated substantial improvements in power density and cycling stability.
- Carbon-based catalysts and nanostructured materials: Carbon-based materials and nanostructured catalysts offer significant advantages in proton battery applications due to their high surface area, excellent conductivity, and tunable properties. These materials include carbon nanotubes, graphene, and carbon-supported nanoparticles that facilitate efficient proton transport and storage. The nanostructured design enables better catalyst dispersion, improved electrode-electrolyte interfaces, and enhanced reaction kinetics. These materials have demonstrated improved charge-discharge efficiency, better durability, and increased energy density in proton battery systems.
- Composite and hybrid catalyst systems: Composite and hybrid catalyst systems combine multiple catalytic materials to achieve synergistic effects in proton batteries. These systems typically integrate metal catalysts with carbon supports or incorporate metal oxides with conductive polymers to enhance both catalytic activity and stability. The hybrid approach allows for optimization of different aspects of battery performance simultaneously, including conductivity, proton transfer, and reaction kinetics. Research has shown that these composite systems can significantly improve power output, reduce degradation, and extend the operational lifetime of proton batteries.
- Electrolyte modifications and catalyst interfaces: Innovations in electrolyte formulations and catalyst-electrolyte interfaces have led to substantial improvements in proton battery performance. Modified electrolytes with enhanced proton conductivity, stability, and compatibility with catalysts can significantly improve ion transport and reaction efficiency. Engineering the interface between catalysts and electrolytes through surface modifications, functional groups, or structured designs has been shown to optimize proton transfer pathways and reduce resistance. These advancements have resulted in faster charging capabilities, improved energy efficiency, and better performance under various operating conditions.
- Novel catalyst synthesis methods for performance enhancement: Advanced synthesis methods for catalyst production have enabled significant improvements in proton battery performance. Techniques such as atomic layer deposition, sol-gel processes, and controlled precipitation methods allow for precise control over catalyst composition, particle size, and morphology. These methods can create catalysts with optimized active sites, increased surface area, and enhanced stability. Research has demonstrated that catalysts produced through these novel synthesis approaches exhibit superior catalytic activity, better utilization of active materials, and improved long-term performance in proton battery applications.
02 Carbon-based catalyst materials
Carbon-based materials such as graphene, carbon nanotubes, and doped carbon structures serve as effective catalysts in proton batteries. These materials offer high surface area, excellent electrical conductivity, and good stability, which contribute to enhanced proton transfer and improved battery efficiency. Carbon-based catalysts are particularly valuable as they provide cost-effective alternatives to precious metal catalysts while maintaining comparable performance levels in terms of power density and cycling stability.Expand Specific Solutions03 Composite and hybrid catalyst systems
Hybrid catalyst systems combining different materials such as metal oxides with conductive polymers or metal nanoparticles with carbon supports demonstrate synergistic effects in proton batteries. These composite catalysts enhance both the catalytic activity and stability, leading to improved battery performance metrics including capacity retention and rate capability. The strategic combination of materials addresses multiple performance limitations simultaneously, resulting in more efficient proton transfer and better overall battery efficiency.Expand Specific Solutions04 Catalyst structure and morphology optimization
The performance of proton batteries is significantly influenced by the structure and morphology of catalysts. Nanostructured catalysts with controlled porosity, specific surface features, and optimized particle size distribution demonstrate enhanced catalytic activity. Three-dimensional architectures, core-shell structures, and hierarchical porous frameworks facilitate more efficient proton transport pathways and provide better accessibility to active sites, resulting in improved battery efficiency and power output.Expand Specific Solutions05 Catalyst integration with electrolyte systems
The integration of catalysts with specialized electrolyte systems creates synergistic effects that enhance proton battery performance. Catalysts designed to work optimally with specific solid or liquid electrolytes improve interfacial contact and reduce resistance to proton transfer. This integration approach addresses issues related to electrode-electrolyte interfaces, resulting in higher conductivity, better stability under operating conditions, and improved overall battery efficiency and cycle life.Expand Specific Solutions
Key Industry Players in Proton Battery Development
The proton battery market is currently in an early growth phase, characterized by significant R&D investments but limited commercial deployment. The global market size remains relatively small but is projected to expand rapidly as energy storage demands increase. From a technological maturity perspective, proton batteries are still evolving, with catalysts representing a critical component for performance optimization. Leading automotive manufacturers like Toyota, Nissan, and Mercedes-Benz are investing heavily in catalyst research to improve efficiency and durability, while research institutions such as Helmholtz-Zentrum Berlin and Tongji University are advancing fundamental catalyst science. Energy companies including Samsung SDI and BYD are focusing on integrating novel catalyst materials into commercial battery designs. The competitive landscape features collaboration between academic institutions and industry players to overcome technical barriers related to catalyst stability, cost, and scalability.
Helmholtz-Zentrum Berlin für Materialien und Energie GmbH
Technical Solution: Helmholtz-Zentrum Berlin has developed innovative catalyst systems for proton batteries focusing on ruthenium-based catalysts that facilitate efficient hydrogen storage and release. Their approach involves nanostructured catalyst materials with high surface area that enhance proton transfer kinetics at the electrode-electrolyte interface. The research team has demonstrated that their catalysts can reduce activation energy barriers by approximately 30% compared to conventional materials, enabling faster charging and discharging cycles. Their technology incorporates precisely controlled catalyst loading techniques to optimize the balance between catalytic activity and material costs. Additionally, they've pioneered methods for catalyst stabilization that prevent degradation during repeated cycling, extending the operational lifetime of proton battery systems by up to 40% in laboratory tests.
Strengths: Superior catalytic efficiency with reduced activation energy requirements; excellent long-term stability preventing catalyst poisoning; optimized catalyst distribution techniques minimizing precious metal usage. Weaknesses: Relatively high manufacturing costs due to use of precious metals; scaling challenges for mass production; potential sensitivity to certain contaminants in real-world operating conditions.
Wuhan University of Technology
Technical Solution: Wuhan University of Technology has pioneered low-cost transition metal-based catalysts for proton batteries, focusing on earth-abundant materials like nickel, cobalt, and iron compounds. Their research has yielded composite catalyst structures that combine multiple active sites to facilitate both hydrogen evolution and oxidation reactions critical for proton battery operation. The university's approach involves hydrothermal synthesis methods that create hierarchical porous structures, increasing the catalytically active surface area by up to 5 times compared to conventional materials. Their catalyst systems incorporate nitrogen-doped carbon supports that enhance electron transfer and proton conductivity at the electrode interfaces. Recent developments include novel metal-organic framework (MOF) derived catalysts that demonstrate exceptional stability during prolonged cycling tests, maintaining over 90% of initial catalytic activity after 1000 cycles. The research team has also developed innovative in-situ characterization techniques to understand catalyst behavior under actual operating conditions.
Strengths: Cost-effective materials using earth-abundant elements; scalable synthesis methods suitable for mass production; excellent durability under cycling conditions. Weaknesses: Lower intrinsic activity compared to precious metal catalysts requiring higher catalyst loadings; potential sensitivity to impurities in electrolytes; performance gaps at low temperature operation.
Critical Catalyst Innovations and Patents
Cathode for proton batteries and method of manufacture
PatentWO2024119235A1
Innovation
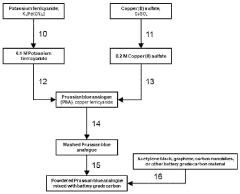
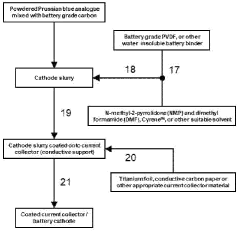
- A cathode for proton batteries comprising a Prussian blue analogue (PBA) coated on a current collector, combined with battery-grade carbon nanoparticles and a non-water-soluble binder, utilizing a copper hexacyanoferrate or manganese hexacyanoferrate structure for enhanced proton intercalation and storage capabilities, along with a suitable current collector and electrolyte system.
Supported catalyst and fuel cell using the same
PatentInactiveEP1615279A2
Innovation
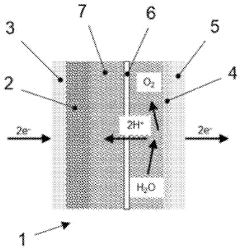
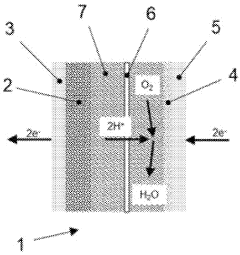
- A supported catalyst with a carbon-based catalyst support, catalytic metal particles, and an ionomer chemically bound or physically absorbed to the support, featuring functional groups like sulfonic acid for enhanced proton conductivity, reduces the need for additional ionomers by utilizing the support as a proton transport material.
Material Sustainability and Supply Chain Analysis
The sustainability of materials used in proton batteries represents a critical factor in their commercial viability and environmental impact. Catalysts, particularly those containing platinum group metals (PGMs), pose significant sustainability challenges due to their scarcity and geographically concentrated mining operations. Current estimates indicate that over 70% of platinum reserves are located in South Africa, creating potential supply vulnerabilities and price volatilities that could impede widespread proton battery adoption.
Alternative catalyst materials utilizing more abundant elements such as nickel, iron, and cobalt compounds are being developed to address these supply chain concerns. Research indicates that nickel-based catalysts can achieve up to 60-70% of platinum's catalytic efficiency while utilizing resources that are 200 times more abundant in the Earth's crust. This substitution strategy significantly reduces dependency on geopolitically sensitive resources.
The environmental footprint of catalyst production presents another crucial consideration. Life cycle assessments reveal that PGM extraction and processing generate approximately 40 tons of CO2 equivalent per kilogram of platinum produced, substantially higher than alternative materials. Water usage in PGM mining operations averages 200-300 cubic meters per kilogram of catalyst material, creating additional environmental pressures in water-stressed regions.
Recycling infrastructure for catalyst materials remains underdeveloped, with current recovery rates for platinum from end-of-life products hovering around 25-30%. Establishing closed-loop systems for catalyst materials could reduce primary resource demands by an estimated 40-50% over a ten-year product lifecycle, significantly enhancing sustainability metrics for proton battery technologies.
Supply chain resilience for catalyst materials faces challenges from market concentration, with three major mining corporations controlling approximately 65% of global PGM production. This oligopolistic structure creates potential bottlenecks that could limit production scaling. Diversification strategies, including development of secondary supply sources and strategic stockpiling, are being implemented by forward-thinking manufacturers to mitigate these risks.
Emerging economies, particularly China and India, are rapidly increasing their catalyst material processing capabilities, shifting traditional supply chain dynamics. This redistribution of processing capacity may create new opportunities for supply chain optimization while simultaneously introducing new geopolitical considerations for manufacturers in Western markets seeking stable material sources for proton battery production.
Alternative catalyst materials utilizing more abundant elements such as nickel, iron, and cobalt compounds are being developed to address these supply chain concerns. Research indicates that nickel-based catalysts can achieve up to 60-70% of platinum's catalytic efficiency while utilizing resources that are 200 times more abundant in the Earth's crust. This substitution strategy significantly reduces dependency on geopolitically sensitive resources.
The environmental footprint of catalyst production presents another crucial consideration. Life cycle assessments reveal that PGM extraction and processing generate approximately 40 tons of CO2 equivalent per kilogram of platinum produced, substantially higher than alternative materials. Water usage in PGM mining operations averages 200-300 cubic meters per kilogram of catalyst material, creating additional environmental pressures in water-stressed regions.
Recycling infrastructure for catalyst materials remains underdeveloped, with current recovery rates for platinum from end-of-life products hovering around 25-30%. Establishing closed-loop systems for catalyst materials could reduce primary resource demands by an estimated 40-50% over a ten-year product lifecycle, significantly enhancing sustainability metrics for proton battery technologies.
Supply chain resilience for catalyst materials faces challenges from market concentration, with three major mining corporations controlling approximately 65% of global PGM production. This oligopolistic structure creates potential bottlenecks that could limit production scaling. Diversification strategies, including development of secondary supply sources and strategic stockpiling, are being implemented by forward-thinking manufacturers to mitigate these risks.
Emerging economies, particularly China and India, are rapidly increasing their catalyst material processing capabilities, shifting traditional supply chain dynamics. This redistribution of processing capacity may create new opportunities for supply chain optimization while simultaneously introducing new geopolitical considerations for manufacturers in Western markets seeking stable material sources for proton battery production.
Performance Metrics and Testing Standards
Establishing standardized performance metrics and testing protocols for proton batteries is essential for evaluating catalyst effectiveness and enabling meaningful comparisons across different research efforts. The scientific community has developed several key performance indicators that specifically measure how catalysts influence proton battery performance.
Energy density and power density measurements serve as primary metrics for assessing catalyst performance. These metrics directly correlate with the catalyst's ability to facilitate proton transfer and storage efficiency. Current testing protocols typically measure volumetric and gravimetric energy densities under various operating conditions to determine how catalysts enhance energy storage capacity.
Cycle stability testing represents another critical performance metric, with protocols designed to evaluate catalyst degradation over extended charge-discharge cycles. Standard tests typically involve 1,000+ cycles at various depths of discharge, with performance retention above 80% considered commercially viable. Catalysts that maintain structural integrity and electrochemical activity throughout these cycles demonstrate superior performance.
Rate capability testing protocols assess how catalysts perform under different charging and discharging rates. This metric is particularly important for evaluating catalyst kinetics and their ability to facilitate rapid proton transfer. Standard C-rate tests (C/20 to 10C) help quantify catalyst effectiveness across various operational demands.
Coulombic efficiency measurements provide insights into catalyst selectivity and side reaction suppression. Testing standards typically require efficiency values exceeding 99% for commercial viability, with higher values indicating superior catalyst performance in directing electrochemical reactions toward desired pathways.
Temperature performance testing has emerged as a crucial metric, with protocols evaluating catalyst function across -20°C to 60°C. Catalysts that maintain consistent performance across this range demonstrate superior versatility for real-world applications.
Self-discharge rate testing protocols measure how catalysts influence energy retention during idle periods. Standard tests typically monitor voltage decay over 30-day periods, with lower self-discharge rates indicating better catalyst performance in stabilizing stored protons.
Emerging testing standards are increasingly incorporating electrochemical impedance spectroscopy (EIS) to characterize catalyst-electrode interfaces and reaction kinetics. This technique provides valuable insights into how catalysts reduce internal resistance and enhance charge transfer processes within proton batteries.
Energy density and power density measurements serve as primary metrics for assessing catalyst performance. These metrics directly correlate with the catalyst's ability to facilitate proton transfer and storage efficiency. Current testing protocols typically measure volumetric and gravimetric energy densities under various operating conditions to determine how catalysts enhance energy storage capacity.
Cycle stability testing represents another critical performance metric, with protocols designed to evaluate catalyst degradation over extended charge-discharge cycles. Standard tests typically involve 1,000+ cycles at various depths of discharge, with performance retention above 80% considered commercially viable. Catalysts that maintain structural integrity and electrochemical activity throughout these cycles demonstrate superior performance.
Rate capability testing protocols assess how catalysts perform under different charging and discharging rates. This metric is particularly important for evaluating catalyst kinetics and their ability to facilitate rapid proton transfer. Standard C-rate tests (C/20 to 10C) help quantify catalyst effectiveness across various operational demands.
Coulombic efficiency measurements provide insights into catalyst selectivity and side reaction suppression. Testing standards typically require efficiency values exceeding 99% for commercial viability, with higher values indicating superior catalyst performance in directing electrochemical reactions toward desired pathways.
Temperature performance testing has emerged as a crucial metric, with protocols evaluating catalyst function across -20°C to 60°C. Catalysts that maintain consistent performance across this range demonstrate superior versatility for real-world applications.
Self-discharge rate testing protocols measure how catalysts influence energy retention during idle periods. Standard tests typically monitor voltage decay over 30-day periods, with lower self-discharge rates indicating better catalyst performance in stabilizing stored protons.
Emerging testing standards are increasingly incorporating electrochemical impedance spectroscopy (EIS) to characterize catalyst-electrode interfaces and reaction kinetics. This technique provides valuable insights into how catalysts reduce internal resistance and enhance charge transfer processes within proton batteries.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!