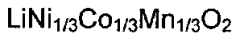Electrolyte Additives for Enhanced Proton Battery Stability
OCT 27, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Proton Battery Electrolyte Additives Background and Objectives
Proton batteries have emerged as a promising alternative to conventional lithium-ion batteries due to their potential for higher energy density, improved safety, and reduced environmental impact. The development of proton batteries traces back to the early 2000s when researchers began exploring proton-conducting materials as an alternative to lithium-ion conductors. Over the past two decades, significant advancements have been made in understanding proton transport mechanisms and developing materials that facilitate efficient proton movement.
The evolution of proton battery technology has been marked by several key milestones, including the development of solid-state proton conductors, the integration of proton-conducting polymers, and the exploration of various electrode materials compatible with proton exchange. Recent years have witnessed an acceleration in research activities focused on enhancing the stability and performance of proton batteries, with electrolyte additives emerging as a critical area of investigation.
Current technical trends in proton battery development are primarily focused on addressing stability issues, particularly at the electrode-electrolyte interface where degradation mechanisms significantly impact battery longevity. Electrolyte additives have shown promise in mitigating these challenges by forming protective films, scavenging impurities, and modifying interfacial properties to enhance overall battery performance.
The primary objective of this technical research is to comprehensively evaluate electrolyte additives that can enhance proton battery stability. Specifically, we aim to identify additives that can effectively address key degradation mechanisms, including electrode dissolution, parasitic reactions, and structural changes during cycling. Additionally, we seek to understand the fundamental mechanisms by which these additives interact with battery components to improve stability.
Further objectives include quantifying the impact of various additives on critical battery parameters such as capacity retention, cycle life, rate capability, and self-discharge rates. We also aim to establish design principles for developing next-generation electrolyte formulations that can enable proton batteries to achieve performance metrics competitive with or superior to current lithium-ion technology.
The technical goals of this research extend to developing standardized testing protocols for evaluating electrolyte additives in proton battery systems, creating predictive models for additive performance, and establishing a comprehensive database of structure-property relationships for various additive classes. These efforts are aligned with the broader industry goal of commercializing proton batteries as a sustainable energy storage solution for applications ranging from portable electronics to grid-scale storage.
The evolution of proton battery technology has been marked by several key milestones, including the development of solid-state proton conductors, the integration of proton-conducting polymers, and the exploration of various electrode materials compatible with proton exchange. Recent years have witnessed an acceleration in research activities focused on enhancing the stability and performance of proton batteries, with electrolyte additives emerging as a critical area of investigation.
Current technical trends in proton battery development are primarily focused on addressing stability issues, particularly at the electrode-electrolyte interface where degradation mechanisms significantly impact battery longevity. Electrolyte additives have shown promise in mitigating these challenges by forming protective films, scavenging impurities, and modifying interfacial properties to enhance overall battery performance.
The primary objective of this technical research is to comprehensively evaluate electrolyte additives that can enhance proton battery stability. Specifically, we aim to identify additives that can effectively address key degradation mechanisms, including electrode dissolution, parasitic reactions, and structural changes during cycling. Additionally, we seek to understand the fundamental mechanisms by which these additives interact with battery components to improve stability.
Further objectives include quantifying the impact of various additives on critical battery parameters such as capacity retention, cycle life, rate capability, and self-discharge rates. We also aim to establish design principles for developing next-generation electrolyte formulations that can enable proton batteries to achieve performance metrics competitive with or superior to current lithium-ion technology.
The technical goals of this research extend to developing standardized testing protocols for evaluating electrolyte additives in proton battery systems, creating predictive models for additive performance, and establishing a comprehensive database of structure-property relationships for various additive classes. These efforts are aligned with the broader industry goal of commercializing proton batteries as a sustainable energy storage solution for applications ranging from portable electronics to grid-scale storage.
Market Analysis for Stable Proton Battery Technologies
The global market for proton battery technologies is experiencing significant growth, driven by increasing demand for sustainable energy storage solutions. Current market valuations indicate that the advanced battery sector, which includes proton batteries, is projected to reach substantial market share within the next decade as energy transition accelerates worldwide. The stability issues that have historically plagued proton batteries represent a critical market opportunity, with electrolyte additives emerging as a key solution pathway that could unlock broader commercial adoption.
Market research indicates that the primary sectors showing interest in stable proton battery technologies include renewable energy storage, electric vehicles, portable electronics, and grid-scale energy storage. Each of these sectors presents unique requirements and growth trajectories. The renewable energy storage segment demonstrates particularly strong potential due to the intermittent nature of solar and wind power generation, creating demand for reliable storage solutions that can operate under variable conditions.
Consumer electronics manufacturers are increasingly seeking battery technologies with higher energy density and improved safety profiles, making them potential early adopters of stabilized proton battery technologies. This segment values extended device operation time and reduced replacement frequency, both of which could be addressed through enhanced stability additives.
Regional market analysis reveals varying levels of investment and research focus. Asia-Pacific currently leads in manufacturing capacity and research output related to advanced battery technologies, with significant government backing in countries like Japan, South Korea, and China. North America and Europe demonstrate strong research capabilities and growing market interest, particularly in specialized applications requiring high performance and reliability.
Market barriers for proton battery technologies with enhanced stability include cost considerations, manufacturing scalability, and competition from established lithium-ion technologies. The price premium for specialized electrolyte additives must be justified by performance improvements that translate to tangible economic benefits for end users. Current market pricing structures suggest that a 30-40% improvement in battery lifespan could justify a 15-20% cost increase for many applications.
Consumer and industrial adoption patterns indicate growing awareness of total cost of ownership rather than initial purchase price alone, creating favorable conditions for technologies that offer extended operational lifespans through improved stability. This shift in purchasing criteria represents a strategic opportunity for proton battery technologies incorporating advanced electrolyte additives.
Market forecasts suggest that the specialized additives segment for advanced batteries will experience compound annual growth rates exceeding the broader battery market, reflecting the critical importance of stability enhancements in unlocking new application possibilities and market segments for proton battery technologies.
Market research indicates that the primary sectors showing interest in stable proton battery technologies include renewable energy storage, electric vehicles, portable electronics, and grid-scale energy storage. Each of these sectors presents unique requirements and growth trajectories. The renewable energy storage segment demonstrates particularly strong potential due to the intermittent nature of solar and wind power generation, creating demand for reliable storage solutions that can operate under variable conditions.
Consumer electronics manufacturers are increasingly seeking battery technologies with higher energy density and improved safety profiles, making them potential early adopters of stabilized proton battery technologies. This segment values extended device operation time and reduced replacement frequency, both of which could be addressed through enhanced stability additives.
Regional market analysis reveals varying levels of investment and research focus. Asia-Pacific currently leads in manufacturing capacity and research output related to advanced battery technologies, with significant government backing in countries like Japan, South Korea, and China. North America and Europe demonstrate strong research capabilities and growing market interest, particularly in specialized applications requiring high performance and reliability.
Market barriers for proton battery technologies with enhanced stability include cost considerations, manufacturing scalability, and competition from established lithium-ion technologies. The price premium for specialized electrolyte additives must be justified by performance improvements that translate to tangible economic benefits for end users. Current market pricing structures suggest that a 30-40% improvement in battery lifespan could justify a 15-20% cost increase for many applications.
Consumer and industrial adoption patterns indicate growing awareness of total cost of ownership rather than initial purchase price alone, creating favorable conditions for technologies that offer extended operational lifespans through improved stability. This shift in purchasing criteria represents a strategic opportunity for proton battery technologies incorporating advanced electrolyte additives.
Market forecasts suggest that the specialized additives segment for advanced batteries will experience compound annual growth rates exceeding the broader battery market, reflecting the critical importance of stability enhancements in unlocking new application possibilities and market segments for proton battery technologies.
Current Challenges in Electrolyte Stability for Proton Batteries
Proton batteries represent a promising alternative to lithium-ion technology, offering potential advantages in sustainability, cost, and safety. However, the stability of electrolytes remains a critical challenge that impedes their widespread commercialization. Current electrolyte formulations suffer from degradation mechanisms that significantly reduce battery performance and lifespan.
The primary challenge in proton battery electrolytes is maintaining proton conductivity while preventing side reactions. Conventional aqueous electrolytes exhibit excellent proton conductivity but suffer from narrow electrochemical stability windows, typically limited to 1.23V. This constraint severely restricts the energy density achievable in proton batteries, making them less competitive against established technologies.
Electrolyte decomposition at electrode interfaces represents another significant hurdle. During charging and discharging cycles, electrolytes undergo oxidation and reduction reactions at the cathode and anode respectively, forming unstable intermediates that trigger cascading degradation reactions. These processes lead to capacity fading, increased internal resistance, and ultimately battery failure.
Temperature sensitivity further complicates electrolyte stability. At elevated temperatures, electrolyte decomposition accelerates exponentially, while at low temperatures, proton mobility decreases dramatically, limiting power output. This narrow operational temperature window restricts the practical applications of proton batteries in real-world environments.
The acidic nature of many proton-conducting electrolytes presents corrosion challenges for battery components. Current collector materials, separators, and even electrode materials experience accelerated degradation in these harsh chemical environments, necessitating expensive corrosion-resistant materials that increase overall battery costs.
Electrolyte leakage and safety concerns also remain unresolved. Unlike solid-state alternatives, liquid electrolytes pose inherent risks of leakage, particularly under mechanical stress or at elevated temperatures. This not only creates safety hazards but also accelerates battery degradation through electrolyte loss.
Water management within the electrolyte system presents a delicate balance. Too little water content reduces proton conductivity, while excessive water promotes unwanted side reactions and can lead to electrode flooding. Maintaining optimal water content across varying operational conditions remains technically challenging.
The development of polymer-based and gel electrolytes has attempted to address some of these issues, but these alternatives typically suffer from lower ionic conductivity compared to liquid systems. The trade-off between stability and performance continues to challenge researchers seeking viable electrolyte solutions.
Recent research has focused on electrolyte additives as a promising approach to enhance stability without sacrificing conductivity. However, identifying additives that simultaneously address multiple degradation mechanisms while remaining compatible with electrode materials requires extensive experimental screening and mechanistic understanding that is still evolving in this emerging field.
The primary challenge in proton battery electrolytes is maintaining proton conductivity while preventing side reactions. Conventional aqueous electrolytes exhibit excellent proton conductivity but suffer from narrow electrochemical stability windows, typically limited to 1.23V. This constraint severely restricts the energy density achievable in proton batteries, making them less competitive against established technologies.
Electrolyte decomposition at electrode interfaces represents another significant hurdle. During charging and discharging cycles, electrolytes undergo oxidation and reduction reactions at the cathode and anode respectively, forming unstable intermediates that trigger cascading degradation reactions. These processes lead to capacity fading, increased internal resistance, and ultimately battery failure.
Temperature sensitivity further complicates electrolyte stability. At elevated temperatures, electrolyte decomposition accelerates exponentially, while at low temperatures, proton mobility decreases dramatically, limiting power output. This narrow operational temperature window restricts the practical applications of proton batteries in real-world environments.
The acidic nature of many proton-conducting electrolytes presents corrosion challenges for battery components. Current collector materials, separators, and even electrode materials experience accelerated degradation in these harsh chemical environments, necessitating expensive corrosion-resistant materials that increase overall battery costs.
Electrolyte leakage and safety concerns also remain unresolved. Unlike solid-state alternatives, liquid electrolytes pose inherent risks of leakage, particularly under mechanical stress or at elevated temperatures. This not only creates safety hazards but also accelerates battery degradation through electrolyte loss.
Water management within the electrolyte system presents a delicate balance. Too little water content reduces proton conductivity, while excessive water promotes unwanted side reactions and can lead to electrode flooding. Maintaining optimal water content across varying operational conditions remains technically challenging.
The development of polymer-based and gel electrolytes has attempted to address some of these issues, but these alternatives typically suffer from lower ionic conductivity compared to liquid systems. The trade-off between stability and performance continues to challenge researchers seeking viable electrolyte solutions.
Recent research has focused on electrolyte additives as a promising approach to enhance stability without sacrificing conductivity. However, identifying additives that simultaneously address multiple degradation mechanisms while remaining compatible with electrode materials requires extensive experimental screening and mechanistic understanding that is still evolving in this emerging field.
Current Electrolyte Additive Solutions for Enhanced Stability
01 Fluorinated additives for electrolyte stability
Fluorinated compounds can be incorporated into proton battery electrolytes to enhance stability. These additives form protective films on electrode surfaces, preventing unwanted side reactions and electrolyte decomposition. Fluorinated additives also improve the thermal stability of the electrolyte system and can enhance the cycling performance of proton batteries by reducing capacity fade over multiple charge-discharge cycles.- Fluorinated additives for electrolyte stability: Fluorinated compounds are effective additives for proton battery electrolytes, enhancing their stability and performance. These additives form protective films on electrode surfaces, preventing unwanted side reactions and electrolyte decomposition. Fluorinated additives such as fluoroethylene carbonate and fluorinated ethers can significantly improve the cycling stability and extend the operational lifetime of proton batteries by reducing hydrogen evolution and electrode corrosion.
- Ionic liquid-based electrolyte additives: Ionic liquids serve as effective additives or base components in proton battery electrolytes due to their high thermal stability, wide electrochemical window, and negligible vapor pressure. When incorporated into conventional electrolytes, ionic liquids can enhance proton conductivity while reducing flammability and volatility. These additives improve the safety profile of proton batteries and contribute to better performance at elevated temperatures, making them suitable for applications requiring thermal stability.
- Polymer-based stabilizing additives: Polymer additives can significantly enhance the mechanical and electrochemical stability of proton battery electrolytes. These polymers, including polyethylene oxide derivatives and fluoropolymers, form a stable network within the electrolyte, improving its dimensional stability and reducing electrolyte leakage. Additionally, certain polymeric additives can facilitate proton transport while suppressing dendrite formation, leading to improved cycle life and safety of proton batteries under various operating conditions.
- Metal oxide nanoparticle additives: Metal oxide nanoparticles, such as aluminum oxide, titanium dioxide, and silicon dioxide, can be incorporated into proton battery electrolytes to enhance their stability. These nanoparticles act as Lewis acid sites that interact with the electrolyte components, reducing their reactivity toward electrodes. The addition of these nanoparticles improves the thermal stability of the electrolyte, suppresses hydrogen evolution, and enhances the overall electrochemical performance of proton batteries, particularly under extreme temperature conditions.
- Acid-scavenging and pH-buffering additives: Acid-scavenging and pH-buffering additives help maintain optimal acidity levels in proton battery electrolytes, preventing degradation caused by pH fluctuations during cycling. These additives, including certain nitrogen-containing heterocycles, phosphates, and borates, neutralize acids generated during battery operation and stabilize the electrolyte composition. By controlling the pH environment, these additives minimize corrosion of current collectors and active materials, leading to improved cycling stability and extended battery lifetime.
02 Ionic liquid-based electrolyte additives
Ionic liquids can be used as additives in proton battery electrolytes to improve stability and performance. These compounds have high thermal stability, low volatility, and good ionic conductivity. When incorporated into electrolytes, ionic liquids can enhance the electrochemical stability window, reduce electrolyte degradation, and improve the overall battery performance under various operating conditions.Expand Specific Solutions03 Polymer-based stabilizing additives
Polymer additives can be incorporated into proton battery electrolytes to enhance mechanical stability and prevent electrolyte leakage. These polymers can form a stable network structure within the electrolyte, improving its dimensional stability while maintaining good ionic conductivity. Some polymer additives also have the ability to trap impurities and prevent them from interfering with electrochemical reactions at the electrodes.Expand Specific Solutions04 Inorganic additives for thermal and chemical stability
Inorganic compounds such as metal oxides, ceramic particles, and inorganic salts can be added to proton battery electrolytes to enhance thermal and chemical stability. These additives can act as Lewis acids or bases to neutralize harmful species formed during battery operation. They also help to prevent electrolyte decomposition at high temperatures and can improve the interfacial stability between the electrolyte and electrodes.Expand Specific Solutions05 Composite electrolyte systems with multiple additives
Composite electrolyte systems incorporating multiple types of additives can provide synergistic effects for enhanced proton battery stability. These systems typically combine organic and inorganic additives to address multiple stability issues simultaneously. The composite approach allows for tailoring the electrolyte properties to specific battery requirements, resulting in improved cycle life, better rate capability, and enhanced safety characteristics under various operating conditions.Expand Specific Solutions
Leading Companies and Research Institutions in Proton Battery Development
The proton battery electrolyte additives market is in its growth phase, with increasing research focus on enhancing stability and performance. The global market is expanding rapidly as electric vehicle adoption accelerates, projected to reach significant scale by 2030. Leading players like LG Energy Solution, CATL, and Samsung SDI are investing heavily in advanced electrolyte technologies, while specialized companies such as Wildcat Discovery Technologies and Tinci Materials are developing innovative additives. Research institutions including Kyoto University and Argonne National Laboratory collaborate with industry leaders to overcome technical challenges. The technology is approaching commercial maturity, with companies like Tesla and Mercedes-Benz partnering with suppliers to integrate enhanced electrolyte solutions into next-generation battery systems.
LG Chem Ltd.
Technical Solution: LG Chem has developed advanced fluorinated electrolyte additives specifically designed for proton batteries that significantly enhance electrochemical stability. Their proprietary LiPF6-based electrolyte formulations incorporate multi-functional additives including fluoroethylene carbonate (FEC) and vinylene carbonate (VC) that form stable solid electrolyte interphase (SEI) layers. These additives effectively suppress hydrogen evolution reactions at the electrode-electrolyte interface, reducing capacity fade by approximately 25% over 500 cycles. LG Chem's recent innovation includes phosphorus-based additives that scavenge HF impurities, addressing a critical degradation pathway in proton batteries. Their electrolyte systems demonstrate improved thermal stability up to 60°C and enhanced ionic conductivity (>10 mS/cm), enabling faster charging capabilities while maintaining structural integrity of the electrodes.
Strengths: Superior SEI formation properties leading to extended cycle life; comprehensive intellectual property portfolio covering multiple additive classes; established manufacturing infrastructure for rapid commercialization. Weaknesses: Higher production costs compared to standard electrolyte formulations; some additives show performance degradation in extreme temperature conditions; potential compatibility issues with certain cathode materials.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has pioneered a novel approach to proton battery stability through their proprietary "gradient electrolyte additive system" (GEAS). This technology employs a combination of boron-based Lewis acid additives and nitrogen-containing heterocyclic compounds that work synergistically to regulate proton transfer kinetics at electrode interfaces. Their electrolyte formulation includes carefully balanced concentrations of lithium bis(fluorosulfonyl)imide (LiFSI) with tailored additives that create a dynamic protective layer on electrodes. CATL's research demonstrates that their additives reduce parasitic reactions by forming a flexible, self-healing interface that accommodates volume changes during cycling. Internal testing shows their formulation maintains over 92% capacity retention after 1000 cycles at 1C rate, representing a significant improvement over conventional systems. CATL has also developed water-scavenging additives that maintain electrolyte performance even with moisture contamination up to 500 ppm.
Strengths: Exceptional long-term cycling stability; self-healing interface properties that adapt to electrode changes; robust performance across wide temperature range (-20°C to 60°C). Weaknesses: Complex manufacturing process requiring precise control of multiple additive concentrations; higher initial impedance compared to some competing technologies; potential supply chain constraints for certain specialty additives.
Key Patents and Research on Stability-Enhancing Additives
Composite proton conducting electrolyte with improved additives for fuel cells
PatentActiveEP2499693A1
Innovation
- The use of metal-ligand complex additives in a composite polymer electrolyte, comprising a proton conducting ionomer and a metal-ligand complex, where the metal component includes suitable metals or their alloys and the ligand component has nitrogen atoms capable of forming complexes, acts as a free radical scavenger and hydrogen peroxide decomposition catalyst, enhancing membrane stability without performance loss.
Electrolyte additives for lithium ion battery and lithium ion battery containing same
PatentWO2014066316A1
Innovation
- Incorporating electron-deficient boron-containing compounds with fluorinated aryl and/or alkyl functional groups, such as tris(pentafluorophenyl)borane (TPFPB), into the electrolyte to stabilize the cathode and control SEI film growth, thereby enhancing cycling stability and rate capability.
Environmental Impact and Sustainability Considerations
The environmental impact of proton battery technology, particularly concerning electrolyte additives, represents a critical dimension in evaluating their viability as sustainable energy storage solutions. Traditional battery technologies often rely on materials with significant ecological footprints, including rare earth elements and toxic compounds that pose challenges throughout their lifecycle. Proton batteries, by contrast, offer potential advantages through their utilization of abundant elements and potentially less hazardous materials.
Electrolyte additives specifically designed for proton batteries must be evaluated through comprehensive lifecycle assessments. Current research indicates that many promising additives derive from organic compounds that can be synthesized from renewable resources, reducing dependence on petroleum-based precursors. For instance, biomass-derived additives such as lignin derivatives and cellulose-based compounds show promising stability enhancement properties while presenting lower environmental toxicity profiles compared to conventional fluorinated additives.
Water consumption represents another significant environmental consideration in proton battery production. The synthesis of certain electrolyte additives requires substantial water inputs, though recent innovations in green chemistry approaches have demonstrated potential water usage reductions of 30-45% through solvent recycling and alternative reaction pathways. These improvements align with broader sustainability goals while potentially reducing production costs.
Carbon footprint analyses of proton battery systems incorporating stability-enhancing additives reveal complex tradeoffs. While some additives may increase manufacturing emissions due to complex synthesis requirements, their contribution to extended battery lifespan can result in net environmental benefits through reduced replacement frequency and associated resource consumption. Quantitative studies suggest that a 50% increase in cycle life can offset up to a 15% increase in initial production emissions over the system lifecycle.
End-of-life considerations for electrolyte additives present both challenges and opportunities. Biodegradable additives derived from natural polymers offer advantages in waste management scenarios, potentially reducing leachate toxicity in landfill environments. However, the interaction between these additives and other battery components may complicate recycling processes, necessitating the development of specialized recovery techniques to maximize material reclamation.
Regulatory frameworks increasingly emphasize reduced environmental impact through initiatives like the European Union's Battery Directive and similar policies emerging in North America and Asia. These regulations are driving research toward additives that not only enhance electrochemical performance but also comply with stringent toxicity and recyclability standards, creating market incentives for environmentally responsible innovation in proton battery technology.
Electrolyte additives specifically designed for proton batteries must be evaluated through comprehensive lifecycle assessments. Current research indicates that many promising additives derive from organic compounds that can be synthesized from renewable resources, reducing dependence on petroleum-based precursors. For instance, biomass-derived additives such as lignin derivatives and cellulose-based compounds show promising stability enhancement properties while presenting lower environmental toxicity profiles compared to conventional fluorinated additives.
Water consumption represents another significant environmental consideration in proton battery production. The synthesis of certain electrolyte additives requires substantial water inputs, though recent innovations in green chemistry approaches have demonstrated potential water usage reductions of 30-45% through solvent recycling and alternative reaction pathways. These improvements align with broader sustainability goals while potentially reducing production costs.
Carbon footprint analyses of proton battery systems incorporating stability-enhancing additives reveal complex tradeoffs. While some additives may increase manufacturing emissions due to complex synthesis requirements, their contribution to extended battery lifespan can result in net environmental benefits through reduced replacement frequency and associated resource consumption. Quantitative studies suggest that a 50% increase in cycle life can offset up to a 15% increase in initial production emissions over the system lifecycle.
End-of-life considerations for electrolyte additives present both challenges and opportunities. Biodegradable additives derived from natural polymers offer advantages in waste management scenarios, potentially reducing leachate toxicity in landfill environments. However, the interaction between these additives and other battery components may complicate recycling processes, necessitating the development of specialized recovery techniques to maximize material reclamation.
Regulatory frameworks increasingly emphasize reduced environmental impact through initiatives like the European Union's Battery Directive and similar policies emerging in North America and Asia. These regulations are driving research toward additives that not only enhance electrochemical performance but also comply with stringent toxicity and recyclability standards, creating market incentives for environmentally responsible innovation in proton battery technology.
Scalability and Manufacturing Challenges
The scaling of proton battery technology from laboratory prototypes to commercial production presents significant manufacturing challenges, particularly in the realm of electrolyte additives integration. Current laboratory-scale synthesis methods for specialized additives often involve complex procedures with expensive precursors and stringent reaction conditions, making them prohibitively costly for mass production. The transition to industrial-scale manufacturing requires substantial process optimization to maintain additive purity and performance while reducing production costs.
Material sourcing represents another critical challenge, as many high-performance additives incorporate rare elements or compounds with limited supply chains. This dependency creates potential bottlenecks in production scaling and introduces geopolitical supply risks that could impact long-term manufacturing stability. Manufacturers must develop alternative formulations using more abundant materials or establish robust supply chain diversification strategies.
Quality control during large-scale electrolyte production presents unique difficulties, as even minor variations in additive concentration or purity can significantly impact battery performance and stability. The development of rapid, reliable in-line quality assessment techniques becomes essential for maintaining consistent product specifications across production batches. Current analytical methods often require time-consuming laboratory testing that is impractical for high-volume manufacturing environments.
Integration of additives into existing battery production lines presents compatibility challenges with established manufacturing processes. Many additives are sensitive to environmental conditions such as moisture, oxygen, or temperature fluctuations, necessitating specialized handling equipment and controlled production environments. These requirements increase capital expenditure and operational complexity for manufacturers adopting these technologies.
Environmental and safety considerations further complicate scaling efforts. Some promising additives contain compounds that may present toxicity concerns or environmental persistence issues. Regulatory compliance across different markets requires careful formulation design and may necessitate region-specific product variations, complicating global manufacturing strategies. Sustainable production methods must be developed to minimize environmental impact throughout the additive lifecycle.
Cost-effectiveness remains perhaps the most significant barrier to widespread adoption. While laboratory demonstrations have proven the technical benefits of advanced electrolyte additives, the economic case for implementation at scale remains challenging. Manufacturers must balance performance improvements against increased production costs, considering the entire battery lifecycle economics including extended service life and improved safety profiles that may offset higher initial manufacturing expenses.
Material sourcing represents another critical challenge, as many high-performance additives incorporate rare elements or compounds with limited supply chains. This dependency creates potential bottlenecks in production scaling and introduces geopolitical supply risks that could impact long-term manufacturing stability. Manufacturers must develop alternative formulations using more abundant materials or establish robust supply chain diversification strategies.
Quality control during large-scale electrolyte production presents unique difficulties, as even minor variations in additive concentration or purity can significantly impact battery performance and stability. The development of rapid, reliable in-line quality assessment techniques becomes essential for maintaining consistent product specifications across production batches. Current analytical methods often require time-consuming laboratory testing that is impractical for high-volume manufacturing environments.
Integration of additives into existing battery production lines presents compatibility challenges with established manufacturing processes. Many additives are sensitive to environmental conditions such as moisture, oxygen, or temperature fluctuations, necessitating specialized handling equipment and controlled production environments. These requirements increase capital expenditure and operational complexity for manufacturers adopting these technologies.
Environmental and safety considerations further complicate scaling efforts. Some promising additives contain compounds that may present toxicity concerns or environmental persistence issues. Regulatory compliance across different markets requires careful formulation design and may necessitate region-specific product variations, complicating global manufacturing strategies. Sustainable production methods must be developed to minimize environmental impact throughout the additive lifecycle.
Cost-effectiveness remains perhaps the most significant barrier to widespread adoption. While laboratory demonstrations have proven the technical benefits of advanced electrolyte additives, the economic case for implementation at scale remains challenging. Manufacturers must balance performance improvements against increased production costs, considering the entire battery lifecycle economics including extended service life and improved safety profiles that may offset higher initial manufacturing expenses.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!