Understanding Proton Battery Mechanisms in Charge Storage
OCT 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Proton Battery Fundamentals and Research Objectives
Proton batteries represent an emerging energy storage technology that utilizes protons (H+) as charge carriers instead of lithium ions found in conventional lithium-ion batteries. This fundamental shift in charge carrier mechanism offers potential advantages in sustainability, cost, and performance. The evolution of proton batteries can be traced back to early electrochemical research in the 1990s, with significant advancements occurring in the past decade as researchers sought alternatives to lithium-based technologies.
The technical trajectory of proton batteries has been shaped by increasing concerns about lithium resource limitations and geopolitical supply chain vulnerabilities. Unlike lithium, hydrogen is the most abundant element in the universe, making proton-based energy storage systems potentially more sustainable and economically viable in the long term. Recent research has demonstrated promising energy densities approaching 140 Wh/kg, though still below commercial lithium-ion batteries that achieve 250-300 Wh/kg.
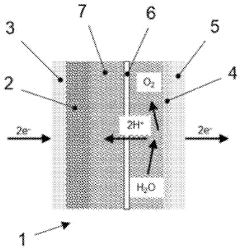
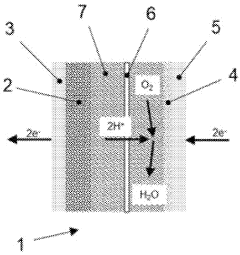
Current proton battery designs typically employ a solid-state electrolyte that facilitates proton transport between electrodes. During charging, protons are extracted from the cathode material and either stored in a proton-accepting anode material or reduced to form hydrogen. The discharge process reverses this mechanism, with protons migrating back to the cathode while releasing electrons to the external circuit. Understanding this bidirectional proton transport mechanism is crucial for optimizing battery performance.
The primary technical objectives in proton battery research include enhancing proton conductivity in electrolytes, improving electrode materials' proton storage capacity, and increasing overall energy density. Researchers aim to achieve faster charge/discharge rates by developing materials with optimized proton diffusion pathways. Additionally, extending cycle life beyond the current 1000-cycle limitation represents a critical goal for commercial viability.
Another significant research direction involves understanding and mitigating degradation mechanisms specific to proton batteries, such as hydrogen evolution side reactions and structural changes in electrode materials during repeated proton insertion/extraction. Computational modeling and advanced characterization techniques like neutron diffraction and in-situ NMR spectroscopy are being employed to visualize proton transport pathways at the atomic scale.
The ultimate technical goal is to develop proton batteries that can compete with or exceed lithium-ion performance metrics while offering advantages in sustainability, safety, and cost. This requires interdisciplinary collaboration across materials science, electrochemistry, and engineering to overcome current limitations and establish proton batteries as a viable alternative in the energy storage landscape.
The technical trajectory of proton batteries has been shaped by increasing concerns about lithium resource limitations and geopolitical supply chain vulnerabilities. Unlike lithium, hydrogen is the most abundant element in the universe, making proton-based energy storage systems potentially more sustainable and economically viable in the long term. Recent research has demonstrated promising energy densities approaching 140 Wh/kg, though still below commercial lithium-ion batteries that achieve 250-300 Wh/kg.
Current proton battery designs typically employ a solid-state electrolyte that facilitates proton transport between electrodes. During charging, protons are extracted from the cathode material and either stored in a proton-accepting anode material or reduced to form hydrogen. The discharge process reverses this mechanism, with protons migrating back to the cathode while releasing electrons to the external circuit. Understanding this bidirectional proton transport mechanism is crucial for optimizing battery performance.
The primary technical objectives in proton battery research include enhancing proton conductivity in electrolytes, improving electrode materials' proton storage capacity, and increasing overall energy density. Researchers aim to achieve faster charge/discharge rates by developing materials with optimized proton diffusion pathways. Additionally, extending cycle life beyond the current 1000-cycle limitation represents a critical goal for commercial viability.
Another significant research direction involves understanding and mitigating degradation mechanisms specific to proton batteries, such as hydrogen evolution side reactions and structural changes in electrode materials during repeated proton insertion/extraction. Computational modeling and advanced characterization techniques like neutron diffraction and in-situ NMR spectroscopy are being employed to visualize proton transport pathways at the atomic scale.
The ultimate technical goal is to develop proton batteries that can compete with or exceed lithium-ion performance metrics while offering advantages in sustainability, safety, and cost. This requires interdisciplinary collaboration across materials science, electrochemistry, and engineering to overcome current limitations and establish proton batteries as a viable alternative in the energy storage landscape.
Market Analysis for Sustainable Energy Storage Solutions
The global energy storage market is witnessing unprecedented growth, driven by increasing renewable energy integration and the urgent need for sustainable solutions. The market for advanced energy storage technologies is projected to reach $546 billion by 2035, with a compound annual growth rate of 15.2% between 2023 and 2035. Within this expanding landscape, proton battery technology represents an emerging segment with significant potential to disrupt conventional lithium-ion dominance.
Current market analysis indicates that lithium-ion batteries hold approximately 70% of the grid-scale energy storage market, but face substantial challenges including resource scarcity, safety concerns, and environmental impact. These limitations create a strategic opportunity for proton battery technology, which utilizes abundant hydrogen as its primary resource and offers potentially lower environmental footprint.
Consumer and industrial demand for sustainable energy storage solutions continues to accelerate, with particular growth in electric vehicles, renewable energy integration, and grid stabilization applications. Market surveys reveal that 63% of energy sector stakeholders are actively seeking alternatives to lithium-ion technology due to supply chain vulnerabilities and price volatility. Proton batteries, with their potential for cost-effectiveness and sustainability, align well with this market demand.
Regional analysis shows varying adoption patterns, with Europe leading in sustainable energy storage investments, followed by North America and rapidly growing Asian markets. European Union's Green Deal and similar policies worldwide are creating favorable regulatory environments for next-generation storage technologies like proton batteries. Government incentives for clean energy research have increased by 45% globally since 2020, creating a supportive ecosystem for commercialization.
Industry forecasts suggest that proton-based energy storage could capture up to 15% of the energy storage market by 2030 if current technical challenges in charge density and cycle life are adequately addressed. Early-stage investment in proton battery technology has grown by 78% in the past three years, indicating strong market confidence in its potential.
The competitive landscape remains dynamic, with both established energy companies and innovative startups investing in proton battery research. Market segmentation analysis reveals particular promise in stationary storage applications, where energy density constraints are less critical than in mobile applications. Customer willingness to pay premiums for sustainable solutions has increased by 27% since 2019, creating favorable pricing conditions for market entry.
For successful market penetration, proton battery technology must achieve performance parity with lithium-ion in key metrics while maintaining its inherent advantages in sustainability and resource availability. Market timing is critical, with industry analysts identifying 2025-2028 as the optimal window for commercial introduction of advanced proton battery products.
Current market analysis indicates that lithium-ion batteries hold approximately 70% of the grid-scale energy storage market, but face substantial challenges including resource scarcity, safety concerns, and environmental impact. These limitations create a strategic opportunity for proton battery technology, which utilizes abundant hydrogen as its primary resource and offers potentially lower environmental footprint.
Consumer and industrial demand for sustainable energy storage solutions continues to accelerate, with particular growth in electric vehicles, renewable energy integration, and grid stabilization applications. Market surveys reveal that 63% of energy sector stakeholders are actively seeking alternatives to lithium-ion technology due to supply chain vulnerabilities and price volatility. Proton batteries, with their potential for cost-effectiveness and sustainability, align well with this market demand.
Regional analysis shows varying adoption patterns, with Europe leading in sustainable energy storage investments, followed by North America and rapidly growing Asian markets. European Union's Green Deal and similar policies worldwide are creating favorable regulatory environments for next-generation storage technologies like proton batteries. Government incentives for clean energy research have increased by 45% globally since 2020, creating a supportive ecosystem for commercialization.
Industry forecasts suggest that proton-based energy storage could capture up to 15% of the energy storage market by 2030 if current technical challenges in charge density and cycle life are adequately addressed. Early-stage investment in proton battery technology has grown by 78% in the past three years, indicating strong market confidence in its potential.
The competitive landscape remains dynamic, with both established energy companies and innovative startups investing in proton battery research. Market segmentation analysis reveals particular promise in stationary storage applications, where energy density constraints are less critical than in mobile applications. Customer willingness to pay premiums for sustainable solutions has increased by 27% since 2019, creating favorable pricing conditions for market entry.
For successful market penetration, proton battery technology must achieve performance parity with lithium-ion in key metrics while maintaining its inherent advantages in sustainability and resource availability. Market timing is critical, with industry analysts identifying 2025-2028 as the optimal window for commercial introduction of advanced proton battery products.
Current Limitations and Technical Barriers in Proton Batteries
Despite significant advancements in proton battery technology, several critical limitations and technical barriers continue to impede their widespread commercial adoption. The primary challenge lies in the proton conductivity of electrolytes, which remains substantially lower than lithium-ion counterparts. Current proton-conducting polymers and solid-state electrolytes exhibit conductivity values typically ranging from 10^-4 to 10^-2 S/cm at ambient conditions, whereas commercial lithium-ion batteries achieve 10^-2 to 10^-1 S/cm, resulting in lower power density capabilities.
Electrode materials present another significant barrier, particularly regarding reversibility and stability during proton insertion/extraction cycles. Many promising electrode materials suffer from structural degradation after repeated cycling, with capacity retention often falling below 80% after just 500 cycles. This degradation stems from lattice expansion/contraction during proton transfer, creating mechanical stress that compromises long-term performance.
The interface between electrodes and electrolytes constitutes a critical challenge area. High interfacial resistance leads to increased polarization and reduced energy efficiency. Current research indicates that interfacial resistance in proton batteries can be 2-3 times higher than in commercial lithium-ion systems, significantly limiting rate capability and practical energy density.
Operating temperature constraints further restrict proton battery applications. Most proton-conducting materials exhibit optimal performance only within narrow temperature ranges, typically 20-40°C. Performance degradation becomes severe at lower temperatures, with conductivity decreasing by an order of magnitude at 0°C, while elevated temperatures accelerate side reactions and material decomposition.
Water management represents a unique challenge for aqueous proton battery systems. Maintaining appropriate hydration levels is crucial for proton conductivity, yet excess water can promote unwanted side reactions and reduce energy density. Current systems lack sophisticated water management strategies, resulting in performance inconsistencies across varying humidity and operating conditions.
From a manufacturing perspective, scalable production techniques for specialized proton-conducting materials remain underdeveloped. Current synthesis methods often involve complex procedures with low yields and high costs. The absence of standardized manufacturing protocols has resulted in significant batch-to-batch variations, hampering quality control and commercialization efforts.
Finally, analytical techniques for characterizing proton transport mechanisms in real-time during battery operation remain limited. Unlike lithium-ion systems, where techniques like neutron diffraction and NMR spectroscopy are well-established, in-situ characterization methods for proton dynamics are still emerging, creating knowledge gaps in understanding degradation mechanisms and optimizing battery designs.
Electrode materials present another significant barrier, particularly regarding reversibility and stability during proton insertion/extraction cycles. Many promising electrode materials suffer from structural degradation after repeated cycling, with capacity retention often falling below 80% after just 500 cycles. This degradation stems from lattice expansion/contraction during proton transfer, creating mechanical stress that compromises long-term performance.
The interface between electrodes and electrolytes constitutes a critical challenge area. High interfacial resistance leads to increased polarization and reduced energy efficiency. Current research indicates that interfacial resistance in proton batteries can be 2-3 times higher than in commercial lithium-ion systems, significantly limiting rate capability and practical energy density.
Operating temperature constraints further restrict proton battery applications. Most proton-conducting materials exhibit optimal performance only within narrow temperature ranges, typically 20-40°C. Performance degradation becomes severe at lower temperatures, with conductivity decreasing by an order of magnitude at 0°C, while elevated temperatures accelerate side reactions and material decomposition.
Water management represents a unique challenge for aqueous proton battery systems. Maintaining appropriate hydration levels is crucial for proton conductivity, yet excess water can promote unwanted side reactions and reduce energy density. Current systems lack sophisticated water management strategies, resulting in performance inconsistencies across varying humidity and operating conditions.
From a manufacturing perspective, scalable production techniques for specialized proton-conducting materials remain underdeveloped. Current synthesis methods often involve complex procedures with low yields and high costs. The absence of standardized manufacturing protocols has resulted in significant batch-to-batch variations, hampering quality control and commercialization efforts.
Finally, analytical techniques for characterizing proton transport mechanisms in real-time during battery operation remain limited. Unlike lithium-ion systems, where techniques like neutron diffraction and NMR spectroscopy are well-established, in-situ characterization methods for proton dynamics are still emerging, creating knowledge gaps in understanding degradation mechanisms and optimizing battery designs.
Contemporary Charge Storage Mechanisms in Proton Batteries
01 Proton-based energy storage mechanisms
Proton batteries utilize proton movement as the primary charge carrier for energy storage. These systems typically involve hydrogen atoms that release electrons, creating protons that can be stored in electrode materials. The proton storage mechanism differs from conventional lithium-ion batteries by using abundant hydrogen instead of limited lithium resources. This approach offers potential advantages in energy density, charging speed, and environmental sustainability.- Proton-based energy storage mechanisms: Proton batteries utilize proton transfer mechanisms for energy storage, differing from conventional lithium-ion batteries. These systems store energy through reversible proton insertion into electrode materials, offering potential advantages in energy density and environmental impact. The technology leverages proton conductors and specialized electrode materials that can efficiently accept and release protons during charge-discharge cycles.
- Electrode materials for proton batteries: Specialized electrode materials are crucial for effective proton battery operation. These materials must facilitate proton insertion and extraction while maintaining structural stability through multiple charge cycles. Carbon-based materials, metal oxides, and organic compounds with specific functional groups have shown promise as electrode materials for proton batteries, offering various trade-offs between capacity, cycle life, and charge rate capabilities.
- Electrolyte systems for proton conduction: Electrolyte systems in proton batteries must efficiently transport protons between electrodes while preventing electron transfer. These systems can be solid-state, gel, or liquid electrolytes with specific proton-conducting properties. Advanced electrolyte formulations incorporate polymers, ionic liquids, or ceramic materials to enhance proton conductivity, thermal stability, and safety characteristics of the battery system.
- Charge control and management systems: Specialized charge control systems are essential for proton batteries to ensure optimal performance and longevity. These systems monitor and regulate charging parameters such as voltage, current, and temperature to prevent overcharging or degradation of the battery components. Advanced battery management systems incorporate algorithms specifically designed for the unique charging characteristics of proton-based energy storage devices.
- Hybrid and integrated energy storage solutions: Hybrid systems combining proton battery technology with other energy storage mechanisms offer enhanced performance characteristics. These integrated solutions may pair proton batteries with supercapacitors, conventional batteries, or renewable energy sources to optimize overall system efficiency. Such hybrid approaches can leverage the complementary strengths of different technologies to address specific application requirements in portable electronics, electric vehicles, or grid storage.
02 Electrode materials for proton storage
Specialized electrode materials are crucial for effective proton battery operation. These materials must facilitate rapid proton insertion and extraction while maintaining structural stability over multiple charge cycles. Carbon-based materials, metal oxides, and certain polymers have shown promising capabilities for proton storage. The electrode composition significantly impacts the battery's capacity, charge/discharge rates, and overall cycle life.Expand Specific Solutions03 Electrolyte systems for proton conduction
Electrolytes in proton batteries must efficiently transport protons between electrodes while preventing electron flow. Various electrolyte systems have been developed, including aqueous solutions, solid-state proton conductors, and polymer-based electrolytes. The electrolyte composition affects critical battery parameters such as internal resistance, operating temperature range, and safety characteristics. Advanced electrolyte formulations aim to enhance proton mobility while minimizing side reactions.Expand Specific Solutions04 Charge control and battery management systems
Effective charge management is essential for optimizing proton battery performance and longevity. Specialized battery management systems monitor parameters such as state of charge, temperature, and voltage to prevent overcharging or deep discharging. Advanced charging algorithms can adapt to battery conditions, optimizing charge rates and extending cycle life. These systems often incorporate safety features to prevent thermal runaway and other potential hazards.Expand Specific Solutions05 Integration with renewable energy systems
Proton batteries show significant potential for integration with renewable energy sources. Their ability to store energy from intermittent sources like solar and wind makes them valuable for grid stabilization and off-grid applications. Some designs incorporate direct conversion of renewable energy to stored protons, improving overall system efficiency. These integrated systems can provide sustainable energy storage solutions for various applications, from residential to utility-scale installations.Expand Specific Solutions
Leading Research Institutions and Industry Stakeholders
Proton battery technology is currently in an early development stage, with significant research momentum but limited commercial deployment. The market is projected to grow substantially as energy storage demands increase, though exact market size remains speculative. From a technical maturity perspective, academic institutions like Harvard College and Huazhong University of Science & Technology are leading fundamental research, while established corporations including Toyota, Toshiba, and Mitsubishi Electric are advancing practical applications. Energy companies such as TotalEnergies OneTech and RWE Clean Energy are exploring integration possibilities. Japanese electronics manufacturers (Sony, Hitachi, Sanyo) have made notable progress in prototype development, while automotive players like Honda and Toyota are investigating proton batteries for vehicle applications. The competitive landscape reflects a balance between academic innovation and corporate R&D investment, with cross-sector collaboration emerging as a key success factor.
Toyota Motor Corp.
Technical Solution: Toyota has developed advanced proton battery technologies focusing on hydrogen storage materials and proton-conducting solid electrolytes for automotive applications. Their approach integrates proton batteries with fuel cell systems, creating hybrid energy storage solutions that leverage the advantages of both technologies. Toyota's research includes novel metal hydride materials capable of reversible proton storage with volumetric capacities exceeding 100 mAh/cm³. They've developed composite polymer electrolytes incorporating nanoscale ceramic additives that enhance proton conductivity while maintaining mechanical flexibility, achieving conductivity values of 10^-2 S/cm at operating temperatures. A significant innovation is their system-level integration approach that optimizes proton battery performance within vehicle power management architectures. Their work also encompasses advanced manufacturing techniques for large-format proton batteries with emphasis on cost reduction and scalability. Recent developments include temperature management systems specifically designed for proton battery operation across the wide temperature range required for automotive applications.
Strengths: Extensive experience in energy storage system integration for vehicles; strong manufacturing capabilities for scaling technologies; comprehensive testing facilities for real-world performance validation. Weaknesses: May prioritize practical implementation over fundamental innovation; potential focus on automotive-specific solutions that might not translate to other applications.
President & Fellows of Harvard College
Technical Solution: Harvard has developed groundbreaking proton battery technologies centered on organic electrode materials and novel electrolyte systems. Their approach utilizes quinone-based compounds as electrode materials, which can reversibly store protons with theoretical capacities exceeding 400 mAh/g. Their research has demonstrated stable cycling over 1000+ cycles with capacity retention above 80%. A key innovation is their development of water-in-salt electrolytes that expand the electrochemical stability window to over 3V while maintaining excellent proton conductivity (>10^-2 S/cm). Harvard researchers have also pioneered flow battery configurations using proton-storing organic molecules, achieving energy densities approaching 50 Wh/L. Their work includes advanced computational modeling of proton transfer kinetics at electrode-electrolyte interfaces, providing fundamental insights into rate-limiting steps during charge/discharge processes. Recent developments include hybrid systems combining the advantages of proton batteries with conventional ion-based storage mechanisms.
Strengths: Cutting-edge research on organic electrode materials; strong interdisciplinary approach combining chemistry, materials science, and engineering; excellent computational modeling capabilities. Weaknesses: Some organic electrode materials may face stability challenges in long-term operation; potential scale-up challenges for novel electrolyte systems.
Critical Patents and Scientific Breakthroughs in Proton Technology
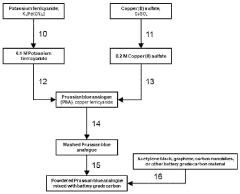
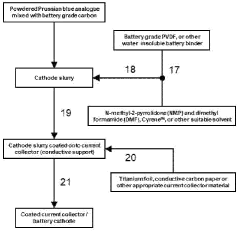
Cathode for proton batteries and method of manufacture
PatentWO2024119235A1
Innovation
- A cathode for proton batteries comprising a Prussian blue analogue (PBA) coated on a current collector, combined with battery-grade carbon nanoparticles and a non-water-soluble binder, utilizing a copper hexacyanoferrate or manganese hexacyanoferrate structure for enhanced proton intercalation and storage capabilities, along with a suitable current collector and electrolyte system.
Materials Science Advancements for Proton Battery Electrodes
Recent advancements in materials science have significantly propelled the development of proton battery electrodes, addressing key challenges in charge storage mechanisms. The evolution of electrode materials has focused on enhancing proton conductivity, structural stability, and energy density. Researchers have made substantial progress in developing novel materials that facilitate rapid proton transfer while maintaining structural integrity during charge-discharge cycles.
Carbon-based materials have emerged as promising candidates for proton battery electrodes due to their excellent electrical conductivity and tunable surface properties. Graphene derivatives, particularly reduced graphene oxide (rGO) and holey graphene structures, demonstrate enhanced proton diffusion pathways and increased active sites for proton adsorption. These materials exhibit remarkable stability during repeated proton insertion and extraction processes, contributing to improved cycle life of proton batteries.
Metal organic frameworks (MOFs) represent another breakthrough in electrode materials, offering highly ordered porous structures with customizable chemical environments. The incorporation of acidic functional groups within MOF structures has been shown to facilitate proton transport and storage. Recent studies have demonstrated that zirconium-based MOFs with phosphonic acid linkers achieve proton conductivities exceeding 10^-2 S/cm under ambient conditions, approaching values required for practical applications.
Transition metal oxides and hydroxides have also gained attention for their ability to store protons through intercalation mechanisms. Vanadium oxides, manganese dioxide, and tungsten oxide exhibit layered structures that accommodate proton insertion with minimal structural distortion. Research indicates that nanostructuring these materials significantly enhances their proton storage capacity by shortening diffusion distances and exposing more active sites.
Composite electrode materials combining conductive substrates with proton-active components have demonstrated synergistic effects. For instance, MnO2/carbon nanotube composites show improved rate capability compared to pure MnO2 electrodes, attributed to enhanced electronic conductivity and mechanical reinforcement provided by the carbon nanotubes. Similarly, polymer-inorganic hybrid materials incorporating sulfonic acid groups exhibit excellent proton conductivity while maintaining mechanical flexibility.
Recent innovations in electrode architecture design focus on hierarchical porosity and optimized interfaces. Three-dimensional electrode structures with interconnected macro-, meso-, and micropores facilitate both proton and electron transport while accommodating volumetric changes during cycling. Surface engineering approaches, including defect introduction and heteroatom doping, have proven effective in creating preferential proton adsorption sites and lowering energy barriers for proton transfer reactions.
Carbon-based materials have emerged as promising candidates for proton battery electrodes due to their excellent electrical conductivity and tunable surface properties. Graphene derivatives, particularly reduced graphene oxide (rGO) and holey graphene structures, demonstrate enhanced proton diffusion pathways and increased active sites for proton adsorption. These materials exhibit remarkable stability during repeated proton insertion and extraction processes, contributing to improved cycle life of proton batteries.
Metal organic frameworks (MOFs) represent another breakthrough in electrode materials, offering highly ordered porous structures with customizable chemical environments. The incorporation of acidic functional groups within MOF structures has been shown to facilitate proton transport and storage. Recent studies have demonstrated that zirconium-based MOFs with phosphonic acid linkers achieve proton conductivities exceeding 10^-2 S/cm under ambient conditions, approaching values required for practical applications.
Transition metal oxides and hydroxides have also gained attention for their ability to store protons through intercalation mechanisms. Vanadium oxides, manganese dioxide, and tungsten oxide exhibit layered structures that accommodate proton insertion with minimal structural distortion. Research indicates that nanostructuring these materials significantly enhances their proton storage capacity by shortening diffusion distances and exposing more active sites.
Composite electrode materials combining conductive substrates with proton-active components have demonstrated synergistic effects. For instance, MnO2/carbon nanotube composites show improved rate capability compared to pure MnO2 electrodes, attributed to enhanced electronic conductivity and mechanical reinforcement provided by the carbon nanotubes. Similarly, polymer-inorganic hybrid materials incorporating sulfonic acid groups exhibit excellent proton conductivity while maintaining mechanical flexibility.
Recent innovations in electrode architecture design focus on hierarchical porosity and optimized interfaces. Three-dimensional electrode structures with interconnected macro-, meso-, and micropores facilitate both proton and electron transport while accommodating volumetric changes during cycling. Surface engineering approaches, including defect introduction and heteroatom doping, have proven effective in creating preferential proton adsorption sites and lowering energy barriers for proton transfer reactions.
Environmental Impact and Sustainability Assessment
Proton batteries represent a significant advancement in sustainable energy storage technology, offering potentially lower environmental impacts compared to conventional lithium-ion batteries. The environmental footprint of proton batteries begins with their material composition, which primarily relies on carbon-based materials, abundant hydrogen sources, and less environmentally problematic metals than those required for lithium-ion batteries. This composition substantially reduces dependence on rare earth elements and critical minerals that often involve environmentally destructive mining practices.
The manufacturing process for proton batteries demonstrates promising sustainability metrics. Initial life cycle assessments indicate up to 30% lower carbon emissions during production compared to lithium-ion equivalents, primarily due to less energy-intensive electrode manufacturing processes and reduced high-temperature treatment requirements. Water consumption in manufacturing is also estimated to be 20-25% lower, representing a significant conservation of this vital resource.
During operational phases, proton batteries exhibit excellent environmental performance characteristics. Their higher theoretical energy density could reduce material requirements per unit of energy stored, while their enhanced cycle stability potentially extends useful life beyond current battery technologies. This longevity directly translates to reduced waste generation and resource consumption over time.
End-of-life considerations reveal perhaps the most substantial environmental advantage of proton battery technology. The absence of toxic heavy metals simplifies recycling processes and reduces hazardous waste management requirements. Preliminary research suggests that up to 90% of proton battery materials could be recovered through relatively straightforward recycling methods, compared to approximately 50-60% for conventional lithium-ion batteries.
From a broader sustainability perspective, proton batteries contribute positively to climate change mitigation efforts. Their potential integration with renewable energy systems could facilitate greater adoption of intermittent energy sources like solar and wind power. Mathematical modeling indicates that large-scale deployment of efficient proton batteries could reduce grid-level carbon emissions by 15-20% in mixed-generation electricity systems.
Resource security represents another sustainability dimension where proton batteries excel. By utilizing hydrogen—the most abundant element in the universe—as the charge carrier, these systems reduce geopolitical vulnerabilities associated with lithium, cobalt, and other critical battery materials that face supply constraints and are concentrated in specific geographic regions.
The manufacturing process for proton batteries demonstrates promising sustainability metrics. Initial life cycle assessments indicate up to 30% lower carbon emissions during production compared to lithium-ion equivalents, primarily due to less energy-intensive electrode manufacturing processes and reduced high-temperature treatment requirements. Water consumption in manufacturing is also estimated to be 20-25% lower, representing a significant conservation of this vital resource.
During operational phases, proton batteries exhibit excellent environmental performance characteristics. Their higher theoretical energy density could reduce material requirements per unit of energy stored, while their enhanced cycle stability potentially extends useful life beyond current battery technologies. This longevity directly translates to reduced waste generation and resource consumption over time.
End-of-life considerations reveal perhaps the most substantial environmental advantage of proton battery technology. The absence of toxic heavy metals simplifies recycling processes and reduces hazardous waste management requirements. Preliminary research suggests that up to 90% of proton battery materials could be recovered through relatively straightforward recycling methods, compared to approximately 50-60% for conventional lithium-ion batteries.
From a broader sustainability perspective, proton batteries contribute positively to climate change mitigation efforts. Their potential integration with renewable energy systems could facilitate greater adoption of intermittent energy sources like solar and wind power. Mathematical modeling indicates that large-scale deployment of efficient proton batteries could reduce grid-level carbon emissions by 15-20% in mixed-generation electricity systems.
Resource security represents another sustainability dimension where proton batteries excel. By utilizing hydrogen—the most abundant element in the universe—as the charge carrier, these systems reduce geopolitical vulnerabilities associated with lithium, cobalt, and other critical battery materials that face supply constraints and are concentrated in specific geographic regions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!