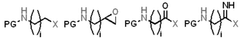Analysis of Carbon Capture Sorbent Materials in Renewable Energy
OCT 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbon Capture Sorbent Evolution and Objectives
Carbon capture technology has evolved significantly over the past several decades, transitioning from theoretical concepts to practical applications in response to growing climate change concerns. The initial development of carbon capture technologies in the 1970s focused primarily on enhanced oil recovery applications rather than environmental mitigation. By the 1990s, as climate science advanced, carbon capture research expanded to address greenhouse gas emissions directly, with early sorbent materials being predominantly amine-based solutions.
The 2000s marked a pivotal shift toward solid sorbents, including activated carbons, zeolites, and metal-organic frameworks (MOFs), which offered improved efficiency and reduced energy penalties compared to liquid systems. The past decade has witnessed accelerated innovation in nanoporous materials and hybrid sorbents that combine the advantages of multiple capture mechanisms, significantly enhancing both selectivity and capacity for CO2.
Current research trajectories are focused on developing sorbent materials that can operate effectively within renewable energy systems, addressing the intermittent nature of renewable power sources while maintaining capture efficiency. This integration represents a critical evolution from standalone carbon capture systems to holistic approaches that complement clean energy generation.
The primary objective of modern carbon capture sorbent development is to achieve materials with high CO2 selectivity, rapid adsorption-desorption kinetics, and long-term stability under variable operating conditions typical in renewable energy applications. Researchers aim to reduce the energy penalty associated with sorbent regeneration below 1.0 GJ/tonne CO2, a threshold that would make carbon capture economically viable at scale.
Additional technical goals include developing sorbents that maintain performance under moisture conditions, resist degradation from impurities common in industrial emissions, and can be manufactured from abundant, low-cost precursors. The ideal sorbent material should also demonstrate minimal environmental impact throughout its lifecycle, aligning with broader sustainability objectives.
The evolution of these materials is increasingly guided by computational modeling and high-throughput screening methodologies, allowing researchers to predict performance characteristics before synthesis. This approach has accelerated development cycles and enabled more targeted research efforts, particularly in identifying promising metal-organic frameworks and functionalized porous polymers with exceptional CO2 capture properties.
Looking forward, the field is moving toward biomimetic approaches that draw inspiration from natural carbon fixation processes, potentially revolutionizing how we conceptualize and implement carbon capture technologies within renewable energy systems.
The 2000s marked a pivotal shift toward solid sorbents, including activated carbons, zeolites, and metal-organic frameworks (MOFs), which offered improved efficiency and reduced energy penalties compared to liquid systems. The past decade has witnessed accelerated innovation in nanoporous materials and hybrid sorbents that combine the advantages of multiple capture mechanisms, significantly enhancing both selectivity and capacity for CO2.
Current research trajectories are focused on developing sorbent materials that can operate effectively within renewable energy systems, addressing the intermittent nature of renewable power sources while maintaining capture efficiency. This integration represents a critical evolution from standalone carbon capture systems to holistic approaches that complement clean energy generation.
The primary objective of modern carbon capture sorbent development is to achieve materials with high CO2 selectivity, rapid adsorption-desorption kinetics, and long-term stability under variable operating conditions typical in renewable energy applications. Researchers aim to reduce the energy penalty associated with sorbent regeneration below 1.0 GJ/tonne CO2, a threshold that would make carbon capture economically viable at scale.
Additional technical goals include developing sorbents that maintain performance under moisture conditions, resist degradation from impurities common in industrial emissions, and can be manufactured from abundant, low-cost precursors. The ideal sorbent material should also demonstrate minimal environmental impact throughout its lifecycle, aligning with broader sustainability objectives.
The evolution of these materials is increasingly guided by computational modeling and high-throughput screening methodologies, allowing researchers to predict performance characteristics before synthesis. This approach has accelerated development cycles and enabled more targeted research efforts, particularly in identifying promising metal-organic frameworks and functionalized porous polymers with exceptional CO2 capture properties.
Looking forward, the field is moving toward biomimetic approaches that draw inspiration from natural carbon fixation processes, potentially revolutionizing how we conceptualize and implement carbon capture technologies within renewable energy systems.
Market Analysis for Carbon Capture Technologies
The global carbon capture market is experiencing significant growth, driven by increasing environmental concerns and regulatory pressures to reduce greenhouse gas emissions. As of 2023, the carbon capture, utilization, and storage (CCUS) market is valued at approximately $7.5 billion, with projections indicating growth to reach $35.9 billion by 2030, representing a compound annual growth rate (CAGR) of 25.3%. This rapid expansion reflects the urgent need for effective carbon management solutions across various industries.
The market for carbon capture sorbent materials specifically is becoming increasingly competitive, with demand primarily coming from power generation, cement production, steel manufacturing, and chemical processing sectors. These industries collectively account for over 70% of global industrial carbon emissions, creating substantial market opportunities for advanced sorbent technologies.
Regional analysis reveals that North America currently leads the carbon capture market, holding approximately 40% of the global share, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region is expected to witness the fastest growth rate in the coming years due to rapid industrialization and increasing environmental regulations in countries like China and India.
Investment patterns in carbon capture technologies have shifted significantly, with venture capital funding increasing from $1.9 billion in 2021 to $3.2 billion in 2023. This investment surge is particularly focused on developing more efficient and cost-effective sorbent materials that can overcome the current economic barriers to widespread adoption.
Customer segmentation within the carbon capture market reveals three primary buyer categories: large industrial emitters seeking compliance with emissions regulations, energy companies pursuing carbon-neutral operations, and government entities implementing climate action plans. Each segment has distinct requirements regarding capture efficiency, operational costs, and integration capabilities with existing infrastructure.
Pricing trends indicate that the cost of carbon capture using current sorbent technologies ranges from $40-100 per ton of CO2 captured, depending on the application and scale. However, emerging sorbent materials show promise for reducing this cost to $25-30 per ton by 2028, which would significantly expand market adoption.
Market barriers include high initial capital requirements, uncertain regulatory frameworks in some regions, and competition from alternative decarbonization strategies. Nevertheless, the increasing implementation of carbon pricing mechanisms globally and corporate net-zero commitments are creating strong market pull for advanced carbon capture solutions, particularly those utilizing innovative sorbent materials optimized for renewable energy applications.
The market for carbon capture sorbent materials specifically is becoming increasingly competitive, with demand primarily coming from power generation, cement production, steel manufacturing, and chemical processing sectors. These industries collectively account for over 70% of global industrial carbon emissions, creating substantial market opportunities for advanced sorbent technologies.
Regional analysis reveals that North America currently leads the carbon capture market, holding approximately 40% of the global share, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region is expected to witness the fastest growth rate in the coming years due to rapid industrialization and increasing environmental regulations in countries like China and India.
Investment patterns in carbon capture technologies have shifted significantly, with venture capital funding increasing from $1.9 billion in 2021 to $3.2 billion in 2023. This investment surge is particularly focused on developing more efficient and cost-effective sorbent materials that can overcome the current economic barriers to widespread adoption.
Customer segmentation within the carbon capture market reveals three primary buyer categories: large industrial emitters seeking compliance with emissions regulations, energy companies pursuing carbon-neutral operations, and government entities implementing climate action plans. Each segment has distinct requirements regarding capture efficiency, operational costs, and integration capabilities with existing infrastructure.
Pricing trends indicate that the cost of carbon capture using current sorbent technologies ranges from $40-100 per ton of CO2 captured, depending on the application and scale. However, emerging sorbent materials show promise for reducing this cost to $25-30 per ton by 2028, which would significantly expand market adoption.
Market barriers include high initial capital requirements, uncertain regulatory frameworks in some regions, and competition from alternative decarbonization strategies. Nevertheless, the increasing implementation of carbon pricing mechanisms globally and corporate net-zero commitments are creating strong market pull for advanced carbon capture solutions, particularly those utilizing innovative sorbent materials optimized for renewable energy applications.
Current Sorbent Materials and Technical Barriers
Carbon capture sorbent materials have evolved significantly over the past decade, with several categories currently dominating the market. Physical sorbents such as activated carbon, zeolites, and metal-organic frameworks (MOFs) represent the first generation of widely deployed materials. These function primarily through physical adsorption mechanisms, offering moderate CO2 selectivity and capacity under specific conditions. Activated carbon remains popular due to its low cost and wide availability, though its CO2 selectivity is relatively low compared to newer alternatives.
Chemical sorbents, including amine-functionalized materials and alkali metal-based sorbents, constitute the second major category. These materials form chemical bonds with CO2, enabling higher selectivity and capacity even at low CO2 concentrations. Amine-functionalized silica has gained particular attention for its promising performance in post-combustion capture scenarios, demonstrating CO2 capacities of 2-4 mmol/g under relevant conditions.
Hybrid sorbents combining physical and chemical capture mechanisms have emerged as a promising third category. These materials aim to leverage the high capacity of physical sorbents with the selectivity of chemical sorbents, though optimization remains challenging.
Despite these advances, significant technical barriers persist. Thermal stability represents a critical challenge, as many high-performing sorbents degrade after multiple temperature-swing cycles, particularly amine-based materials which can lose 20-30% capacity after just 100 cycles. Moisture sensitivity also poses problems, with water vapor often competing with CO2 for adsorption sites, reducing overall efficiency in real-world applications.
Scalability and manufacturing constraints further limit widespread adoption. Many advanced materials with excellent laboratory performance, particularly MOFs and specialized hybrid sorbents, face prohibitive production costs and complex synthesis procedures that hinder industrial-scale implementation. Current production methods typically yield kilogram quantities, whereas commercial deployment requires ton-scale production.
Energy requirements for regeneration remain suboptimal, with most current sorbents requiring significant thermal energy input (3-4 GJ/ton CO2) for the desorption phase. This high energy penalty reduces the net environmental benefit of the capture process, particularly when fossil fuels provide the regeneration energy.
Mechanical stability issues also plague many high-performance materials, with pelletized or structured sorbents often showing degradation under pressure and flow conditions typical in industrial settings. Attrition rates of 1-2% per cycle have been observed in fluidized bed applications, necessitating frequent material replacement and increasing operational costs.
Chemical sorbents, including amine-functionalized materials and alkali metal-based sorbents, constitute the second major category. These materials form chemical bonds with CO2, enabling higher selectivity and capacity even at low CO2 concentrations. Amine-functionalized silica has gained particular attention for its promising performance in post-combustion capture scenarios, demonstrating CO2 capacities of 2-4 mmol/g under relevant conditions.
Hybrid sorbents combining physical and chemical capture mechanisms have emerged as a promising third category. These materials aim to leverage the high capacity of physical sorbents with the selectivity of chemical sorbents, though optimization remains challenging.
Despite these advances, significant technical barriers persist. Thermal stability represents a critical challenge, as many high-performing sorbents degrade after multiple temperature-swing cycles, particularly amine-based materials which can lose 20-30% capacity after just 100 cycles. Moisture sensitivity also poses problems, with water vapor often competing with CO2 for adsorption sites, reducing overall efficiency in real-world applications.
Scalability and manufacturing constraints further limit widespread adoption. Many advanced materials with excellent laboratory performance, particularly MOFs and specialized hybrid sorbents, face prohibitive production costs and complex synthesis procedures that hinder industrial-scale implementation. Current production methods typically yield kilogram quantities, whereas commercial deployment requires ton-scale production.
Energy requirements for regeneration remain suboptimal, with most current sorbents requiring significant thermal energy input (3-4 GJ/ton CO2) for the desorption phase. This high energy penalty reduces the net environmental benefit of the capture process, particularly when fossil fuels provide the regeneration energy.
Mechanical stability issues also plague many high-performance materials, with pelletized or structured sorbents often showing degradation under pressure and flow conditions typical in industrial settings. Attrition rates of 1-2% per cycle have been observed in fluidized bed applications, necessitating frequent material replacement and increasing operational costs.
Mainstream Sorbent Material Solutions
01 Metal-organic frameworks (MOFs) for carbon capture
Metal-organic frameworks are advanced porous materials with high surface area and tunable pore structures that can effectively capture CO2. These crystalline materials consist of metal ions or clusters coordinated with organic ligands, creating a framework with exceptional adsorption properties. MOFs can be designed with specific functional groups to enhance CO2 selectivity and capacity, making them promising candidates for carbon capture applications in industrial settings.- Metal-organic frameworks (MOFs) for carbon capture: Metal-organic frameworks are advanced porous materials with high surface area that can effectively capture carbon dioxide. These crystalline structures consist of metal ions coordinated to organic ligands, creating a framework with tunable pore sizes and functionalities. MOFs can be designed with specific binding sites for CO2, allowing for selective adsorption even in the presence of other gases. Their high adsorption capacity and regeneration capabilities make them promising candidates for industrial carbon capture applications.
- Amine-functionalized sorbents for CO2 capture: Amine-functionalized materials represent a significant class of carbon capture sorbents that utilize the chemical reaction between amines and CO2 to form carbamates or bicarbonates. These materials can be created by grafting or impregnating various amine compounds onto porous supports such as silica, activated carbon, or polymers. The amine groups provide strong binding sites for CO2, enabling efficient capture even at low CO2 concentrations. These sorbents typically offer high selectivity for CO2 over other gases and can be regenerated through temperature or pressure swing processes.
- Zeolite-based carbon capture materials: Zeolites are crystalline aluminosilicate materials with well-defined pore structures that can be utilized for carbon dioxide adsorption. These materials capture CO2 through physical adsorption mechanisms based on their microporous structure and surface properties. Zeolites can be modified with various cations to enhance their CO2 selectivity and capacity. Their thermal stability and resistance to harsh conditions make them suitable for industrial carbon capture applications, particularly in pressure swing adsorption systems where rapid adsorption-desorption cycles are required.
- Novel composite and hybrid sorbent materials: Composite and hybrid sorbent materials combine different components to create carbon capture systems with enhanced properties. These materials often integrate the advantages of multiple sorbent types, such as the high capacity of porous materials with the selectivity of functionalized surfaces. Examples include polymer-inorganic composites, layered double hydroxides, and hybrid membranes. By combining different materials, these sorbents can achieve improved thermal stability, mechanical strength, and resistance to degradation while maintaining high CO2 capture performance. The synergistic effects between components often result in superior adsorption properties compared to single-component materials.
- Regeneration and cyclic stability of carbon capture sorbents: The development of carbon capture sorbents with improved regeneration capabilities and cyclic stability is crucial for practical applications. These materials are designed to maintain their CO2 capture performance over multiple adsorption-desorption cycles while minimizing energy requirements for regeneration. Various approaches include incorporating heat-conducting elements, designing hierarchical pore structures for improved mass transfer, and developing materials with lower heat of adsorption. Advanced regeneration methods such as vacuum swing adsorption, temperature swing adsorption, and combinations thereof are being optimized to reduce the energy penalty associated with sorbent regeneration.
02 Amine-functionalized sorbent materials
Amine-functionalized materials represent a significant class of carbon capture sorbents that operate through chemical adsorption mechanisms. These materials contain amine groups that react with CO2 to form carbamates or bicarbonates under appropriate conditions. The amine functionality can be incorporated into various support structures including silica, polymers, and porous carbons. These materials typically offer high CO2 selectivity and can operate effectively at lower temperatures compared to traditional capture methods.Expand Specific Solutions03 Zeolite and molecular sieve-based carbon capture
Zeolites and molecular sieves are aluminosilicate materials with well-defined microporous structures that can selectively adsorb CO2 based on molecular size and polarity. These materials offer high thermal stability and can be regenerated multiple times without significant performance degradation. Modified zeolites with enhanced hydrophobicity or incorporated metal ions can improve CO2 selectivity in the presence of moisture, addressing a common challenge in carbon capture applications.Expand Specific Solutions04 Novel composite and hybrid sorbent materials
Composite and hybrid sorbent materials combine different components to achieve enhanced carbon capture performance. These materials often integrate the advantages of multiple sorbent types, such as the high capacity of physical adsorbents with the selectivity of chemical sorbents. Examples include polymer-inorganic composites, layered double hydroxides combined with amines, and carbon-based composites with metal oxides. These hybrid approaches can overcome limitations of individual materials while providing synergistic effects for improved capture efficiency.Expand Specific Solutions05 Regeneration and cyclic stability of carbon capture sorbents
The development of sorbent materials with improved regeneration capabilities and cyclic stability is crucial for practical carbon capture applications. This includes materials designed to release captured CO2 with minimal energy input and maintain performance over multiple adsorption-desorption cycles. Innovations in this area focus on reducing the energy penalty associated with sorbent regeneration, preventing degradation mechanisms such as amine leaching or pore blocking, and developing materials that can withstand industrial operating conditions over extended periods.Expand Specific Solutions
Leading Companies and Research Institutions
The carbon capture sorbent materials market in renewable energy is in a growth phase, with increasing global focus on decarbonization driving market expansion. The competitive landscape features diverse players across the value chain, from specialized startups like Climeworks AG and Susteon focusing on direct air capture technologies, to established corporations such as Microsoft, IBM, and Google investing in carbon removal solutions. Academic institutions including Arizona State University, Cornell, and Columbia University are advancing fundamental research on novel sorbent materials. The technology maturity varies significantly, with commercial deployment by Climeworks (Orca and Mammoth plants) representing early market validation, while many solutions remain in research and development stages. Korean power companies (KEPCO and subsidiaries) are increasingly investing in this space, indicating growing international market interest.
Climeworks AG
Technical Solution: Climeworks has developed a Direct Air Capture (DAC) technology using proprietary amine-functionalized filter materials as sorbents to selectively capture CO2 from ambient air. Their modular collectors draw air through the sorbent using fans, where CO2 binds to the surface while other air components pass through. Once saturated, the collectors are heated to approximately 100°C using low-grade waste heat or renewable energy sources, releasing concentrated CO2 for storage or utilization. Climeworks has deployed commercial plants in Switzerland, Iceland (Orca plant), and is developing their larger Mammoth plant with 36,000 tons/year capacity. Their technology achieves capture costs around $600-800 per ton CO2, with pathways identified to reduce this to $200-300 per ton at scale.
Strengths: Modular, scalable design allows flexible deployment; proven commercial implementation; high-purity CO2 output (>99%); low temperature regeneration requirements; long sorbent lifespan (several thousand cycles). Weaknesses: Currently high capture costs compared to point-source capture; significant energy requirements for regeneration; requires substantial land area for large-scale deployment; dependent on renewable energy availability for climate-positive operation.
Susteon, Inc.
Technical Solution: Susteon has pioneered advanced structured sorbent materials for carbon capture applications in renewable energy systems. Their proprietary technology focuses on metal-organic frameworks (MOFs) and functionalized porous materials with tailored pore structures that maximize CO2 adsorption capacity while minimizing regeneration energy. Susteon's sorbents feature high surface area (>1000 m²/g) and CO2 selectivity ratios exceeding 50:1 over nitrogen. Their innovative manufacturing process incorporates these advanced materials into structured contactors like monoliths and 3D-printed structures, overcoming traditional pressure drop and heat/mass transfer limitations. Susteon has demonstrated their technology at pilot scale with capture rates of 90%+ and regeneration temperatures below 80°C, making them compatible with low-grade waste heat sources. Their materials show stability over 1000+ adsorption-desorption cycles with minimal performance degradation, addressing a key challenge for commercial deployment.
Strengths: Exceptional CO2 selectivity and capacity compared to conventional sorbents; structured formats reduce pressure drop and improve system efficiency; low regeneration energy requirements compatible with renewable heat sources; demonstrated durability over many cycles. Weaknesses: Limited large-scale commercial deployment experience; manufacturing costs may be higher than conventional sorbents; requires specialized production facilities; performance in real-world conditions with contaminants needs further validation.
Key Patents and Innovations in Sorbent Technology
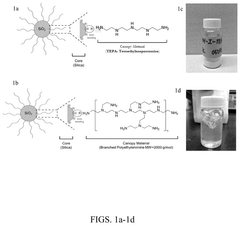
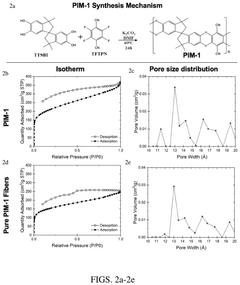
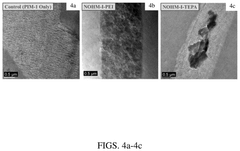
Fiber-encapsulated hybrid materials for capture of carbon dioxide
PatentPendingUS20240326017A1
Innovation
- Development of encapsulated fiber compositions with a microporous sheath surrounding an amine-containing core, produced through electrospinning, which enhances thermal stability, reduces water absorption, and minimizes pressure drop, enabling efficient CO2 capture and scalable deployment.
Sorbent material for co2 capture, uses thereof and methods for making same
PatentWO2025124872A1
Innovation
- A sorbent material composed of a mixture of 75-98 wt.% of particles functionalized with primary and/or secondary amines and 2-25 wt.% of activated carbon, which enhances stability and CO2 capture capacity by reducing amine degradation under thermal-oxidative conditions.
Environmental Impact Assessment
The environmental impact of carbon capture sorbent materials extends far beyond their primary function of CO2 sequestration. When evaluating these materials for renewable energy applications, a comprehensive assessment reveals both positive and negative ecological implications across their lifecycle.
Carbon capture technologies fundamentally contribute to greenhouse gas reduction, with advanced sorbent materials potentially removing 85-95% of CO2 from emission sources. This direct climate benefit must be weighed against the environmental footprint of manufacturing these materials. Production of zeolites, metal-organic frameworks (MOFs), and amine-functionalized sorbents often involves energy-intensive processes and potentially toxic precursors that can generate significant upstream emissions.
Water consumption presents another critical consideration. Certain sorbent materials, particularly amine-based solutions, require substantial water for regeneration cycles. In water-stressed regions, this demand could exacerbate existing resource pressures. Conversely, solid sorbents like activated carbons typically demonstrate lower water requirements, offering environmental advantages in appropriate deployment contexts.
Land use impacts vary significantly between sorbent types. Direct air capture systems utilizing solid sorbents may require substantial land area for installation, potentially competing with agricultural or conservation priorities. However, when integrated with existing renewable energy infrastructure such as solar farms, these systems can achieve land use synergies that minimize additional environmental disruption.
Waste management challenges emerge at end-of-life stages for these materials. Chemical degradation of amine-based sorbents can produce hazardous byproducts requiring specialized disposal protocols. More promising are recent developments in MOF recycling techniques, which have demonstrated up to 90% material recovery rates in laboratory settings, substantially reducing waste streams.
Biodiversity considerations must also factor into environmental assessments. Mining operations for raw materials like zeolites can disrupt local ecosystems, while chemical leaching from improperly managed sorbent disposal sites poses contamination risks to soil and water systems. These impacts necessitate rigorous containment strategies and site remediation planning.
The net environmental benefit ultimately depends on lifecycle efficiency. Recent studies indicate that carbon capture systems employing high-performance sorbents can achieve carbon neutrality within 2-5 years of operation, after which they deliver progressive climate benefits. This timeline varies significantly based on sorbent composition, energy source for regeneration, and operational parameters.
Carbon capture technologies fundamentally contribute to greenhouse gas reduction, with advanced sorbent materials potentially removing 85-95% of CO2 from emission sources. This direct climate benefit must be weighed against the environmental footprint of manufacturing these materials. Production of zeolites, metal-organic frameworks (MOFs), and amine-functionalized sorbents often involves energy-intensive processes and potentially toxic precursors that can generate significant upstream emissions.
Water consumption presents another critical consideration. Certain sorbent materials, particularly amine-based solutions, require substantial water for regeneration cycles. In water-stressed regions, this demand could exacerbate existing resource pressures. Conversely, solid sorbents like activated carbons typically demonstrate lower water requirements, offering environmental advantages in appropriate deployment contexts.
Land use impacts vary significantly between sorbent types. Direct air capture systems utilizing solid sorbents may require substantial land area for installation, potentially competing with agricultural or conservation priorities. However, when integrated with existing renewable energy infrastructure such as solar farms, these systems can achieve land use synergies that minimize additional environmental disruption.
Waste management challenges emerge at end-of-life stages for these materials. Chemical degradation of amine-based sorbents can produce hazardous byproducts requiring specialized disposal protocols. More promising are recent developments in MOF recycling techniques, which have demonstrated up to 90% material recovery rates in laboratory settings, substantially reducing waste streams.
Biodiversity considerations must also factor into environmental assessments. Mining operations for raw materials like zeolites can disrupt local ecosystems, while chemical leaching from improperly managed sorbent disposal sites poses contamination risks to soil and water systems. These impacts necessitate rigorous containment strategies and site remediation planning.
The net environmental benefit ultimately depends on lifecycle efficiency. Recent studies indicate that carbon capture systems employing high-performance sorbents can achieve carbon neutrality within 2-5 years of operation, after which they deliver progressive climate benefits. This timeline varies significantly based on sorbent composition, energy source for regeneration, and operational parameters.
Cost-Benefit Analysis of Sorbent Implementation
The implementation of carbon capture sorbent materials in renewable energy systems requires careful economic evaluation to determine viability across different scales and applications. Initial capital expenditure for sorbent-based carbon capture systems varies significantly based on technology maturity, with physical sorbents like activated carbon typically requiring $400-600 per ton of CO2 capture capacity, while more advanced chemical sorbents such as metal-organic frameworks (MOFs) can cost $800-1,500 per ton initially.
Operational expenses present a more nuanced picture. Traditional amine-based sorbents incur replacement costs of $30-50 per ton of CO2 captured due to degradation under thermal cycling, while newer zeolite and MOF materials demonstrate superior durability with replacement costs potentially reduced to $15-25 per ton. Energy penalties—a critical consideration—range from 15-30% for first-generation sorbents to 8-15% for advanced materials, directly impacting the overall efficiency of renewable energy systems.
Long-term economic benefits materialize through multiple pathways. Carbon credits and regulatory compliance represent immediate financial returns, with current carbon pricing in developed markets ranging from $25-85 per ton. The captured CO2 itself becomes a valuable commodity in applications such as enhanced oil recovery ($20-40 per ton), food and beverage industries ($100-150 per ton), or as feedstock for synthetic fuels and chemicals.
Scalability economics reveal important threshold effects. Small-scale implementations (<10,000 tons CO2/year) typically show unfavorable economics with costs exceeding $120 per ton captured. Medium-scale operations (10,000-100,000 tons/year) achieve better efficiency with costs potentially reduced to $70-90 per ton. Large industrial applications (>100,000 tons/year) demonstrate the most favorable economics, potentially reaching $40-60 per ton through economies of scale.
Lifecycle assessment indicates that most advanced sorbent systems achieve carbon payback within 1.5-3 years, depending on application context and energy source. The environmental return on investment improves significantly when sorbents are integrated with renewable energy systems rather than fossil fuel operations, with net positive carbon impact increasing by 30-45%.
Integration costs with existing renewable infrastructure vary by technology. Wind energy systems show the lowest integration costs at $5-15 per kW of installed capacity, while solar thermal systems demonstrate higher complexity with integration costs of $20-40 per kW. Biomass energy systems present the most favorable economics for sorbent integration due to concentrated CO2 streams, reducing capture costs by 25-40% compared to more dilute sources.
Operational expenses present a more nuanced picture. Traditional amine-based sorbents incur replacement costs of $30-50 per ton of CO2 captured due to degradation under thermal cycling, while newer zeolite and MOF materials demonstrate superior durability with replacement costs potentially reduced to $15-25 per ton. Energy penalties—a critical consideration—range from 15-30% for first-generation sorbents to 8-15% for advanced materials, directly impacting the overall efficiency of renewable energy systems.
Long-term economic benefits materialize through multiple pathways. Carbon credits and regulatory compliance represent immediate financial returns, with current carbon pricing in developed markets ranging from $25-85 per ton. The captured CO2 itself becomes a valuable commodity in applications such as enhanced oil recovery ($20-40 per ton), food and beverage industries ($100-150 per ton), or as feedstock for synthetic fuels and chemicals.
Scalability economics reveal important threshold effects. Small-scale implementations (<10,000 tons CO2/year) typically show unfavorable economics with costs exceeding $120 per ton captured. Medium-scale operations (10,000-100,000 tons/year) achieve better efficiency with costs potentially reduced to $70-90 per ton. Large industrial applications (>100,000 tons/year) demonstrate the most favorable economics, potentially reaching $40-60 per ton through economies of scale.
Lifecycle assessment indicates that most advanced sorbent systems achieve carbon payback within 1.5-3 years, depending on application context and energy source. The environmental return on investment improves significantly when sorbents are integrated with renewable energy systems rather than fossil fuel operations, with net positive carbon impact increasing by 30-45%.
Integration costs with existing renewable infrastructure vary by technology. Wind energy systems show the lowest integration costs at $5-15 per kW of installed capacity, while solar thermal systems demonstrate higher complexity with integration costs of $20-40 per kW. Biomass energy systems present the most favorable economics for sorbent integration due to concentrated CO2 streams, reducing capture costs by 25-40% compared to more dilute sources.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!