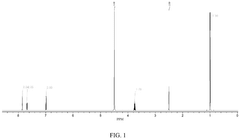
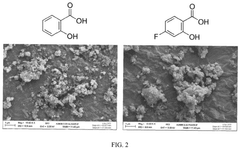
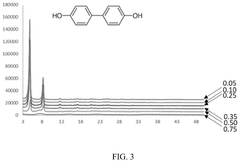
Analysis of Polymer-Based Carbon Capture Sorbents
OCT 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Polymer Sorbent Evolution and Carbon Capture Objectives
Polymer-based carbon capture technologies have evolved significantly over the past three decades, transitioning from theoretical concepts to practical applications. The journey began in the early 1990s with basic research on polymer membranes for gas separation, which laid the groundwork for more sophisticated carbon capture materials. By the early 2000s, researchers had begun exploring functionalized polymers specifically designed for CO2 adsorption, marking a critical shift toward targeted carbon capture solutions.
The evolution accelerated around 2010 with the development of advanced polymer architectures including interpenetrating networks, hyperbranched structures, and polymer composites that demonstrated enhanced selectivity and capacity for CO2. This period also saw the emergence of metal-organic framework (MOF)-polymer hybrids that combined the high surface area of MOFs with the processability of polymers, creating materials with unprecedented carbon capture performance.
Recent developments have focused on stimuli-responsive polymer sorbents that can capture and release CO2 in response to external triggers such as temperature, pressure, or light. These "smart" materials represent a significant advancement in reducing the energy penalties associated with traditional carbon capture processes. Additionally, the integration of nanotechnology has enabled the creation of nanoporous polymer networks with precisely engineered pore sizes optimized for CO2 selectivity.
The primary objective of polymer-based carbon capture research is to develop materials that can efficiently separate CO2 from flue gases and other emission sources at industrial scales. Specific technical goals include achieving CO2 selectivity factors exceeding 50 (relative to N2), sorption capacities above 3 mmol/g under relevant conditions, and maintaining performance over thousands of adsorption-desorption cycles without significant degradation.
Economic objectives are equally important, with targets focused on reducing the energy requirement for CO2 capture to below 1 GJ/tonne CO2, which would represent a 50-70% improvement over current amine scrubbing technologies. Cost targets aim to bring the total capture cost below $30/tonne CO2 to make carbon capture economically viable without substantial subsidies.
Environmental and practical objectives include developing sorbents from sustainable precursors, eliminating toxic components in the manufacturing process, and ensuring materials can be produced at scale using existing industrial polymer processing techniques. The ultimate goal is to create polymer sorbents that enable carbon capture to become a mainstream climate mitigation technology, capable of being deployed across various industries including power generation, cement production, and steel manufacturing.
The evolution accelerated around 2010 with the development of advanced polymer architectures including interpenetrating networks, hyperbranched structures, and polymer composites that demonstrated enhanced selectivity and capacity for CO2. This period also saw the emergence of metal-organic framework (MOF)-polymer hybrids that combined the high surface area of MOFs with the processability of polymers, creating materials with unprecedented carbon capture performance.
Recent developments have focused on stimuli-responsive polymer sorbents that can capture and release CO2 in response to external triggers such as temperature, pressure, or light. These "smart" materials represent a significant advancement in reducing the energy penalties associated with traditional carbon capture processes. Additionally, the integration of nanotechnology has enabled the creation of nanoporous polymer networks with precisely engineered pore sizes optimized for CO2 selectivity.
The primary objective of polymer-based carbon capture research is to develop materials that can efficiently separate CO2 from flue gases and other emission sources at industrial scales. Specific technical goals include achieving CO2 selectivity factors exceeding 50 (relative to N2), sorption capacities above 3 mmol/g under relevant conditions, and maintaining performance over thousands of adsorption-desorption cycles without significant degradation.
Economic objectives are equally important, with targets focused on reducing the energy requirement for CO2 capture to below 1 GJ/tonne CO2, which would represent a 50-70% improvement over current amine scrubbing technologies. Cost targets aim to bring the total capture cost below $30/tonne CO2 to make carbon capture economically viable without substantial subsidies.
Environmental and practical objectives include developing sorbents from sustainable precursors, eliminating toxic components in the manufacturing process, and ensuring materials can be produced at scale using existing industrial polymer processing techniques. The ultimate goal is to create polymer sorbents that enable carbon capture to become a mainstream climate mitigation technology, capable of being deployed across various industries including power generation, cement production, and steel manufacturing.
Market Analysis for Carbon Capture Technologies
The global carbon capture market is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. As of 2023, the market was valued at approximately $7.3 billion, with projections indicating a compound annual growth rate (CAGR) of 19.2% through 2030, potentially reaching $35.6 billion by the end of the decade. This growth trajectory is supported by substantial government investments, with the US Inflation Reduction Act allocating $369 billion toward climate initiatives, including carbon capture technologies.
Polymer-based carbon capture sorbents represent a rapidly expanding segment within this market. These materials offer advantages in terms of customizability, lower regeneration energy requirements, and potential cost efficiencies compared to traditional liquid amine scrubbing technologies. The market for these specialized sorbents is currently estimated at $850 million, with expectations to grow at 23.5% annually as industrial adoption increases.
Demand for carbon capture technologies spans multiple sectors, with power generation, cement production, and chemical manufacturing representing the largest market segments. Power generation alone accounts for approximately 42% of the current carbon capture market, followed by industrial applications at 31%. Notably, the cement industry, responsible for roughly 8% of global CO2 emissions, represents a particularly promising growth area for polymer-based sorbent technologies.
Geographically, North America leads the carbon capture market with a 38% share, followed by Europe at 29% and Asia-Pacific at 24%. China and India are emerging as particularly dynamic markets, with annual growth rates exceeding the global average by 3-5 percentage points. This regional distribution aligns with regulatory frameworks and carbon pricing mechanisms that incentivize adoption.
Customer segmentation reveals three primary buyer categories: large industrial emitters seeking compliance solutions, energy companies pursuing enhanced oil recovery applications, and environmentally progressive corporations implementing voluntary carbon reduction initiatives. The first segment currently dominates purchasing decisions, but the voluntary market is growing at twice the rate of compliance-driven implementations.
Price sensitivity varies significantly across these segments, with polymer-based sorbents currently commanding premium pricing due to performance advantages and lower operational costs over system lifetimes. However, as manufacturing scales increase, price points are expected to decrease by 30-40% over the next five years, potentially accelerating market penetration and expanding the addressable market for these technologies.
Polymer-based carbon capture sorbents represent a rapidly expanding segment within this market. These materials offer advantages in terms of customizability, lower regeneration energy requirements, and potential cost efficiencies compared to traditional liquid amine scrubbing technologies. The market for these specialized sorbents is currently estimated at $850 million, with expectations to grow at 23.5% annually as industrial adoption increases.
Demand for carbon capture technologies spans multiple sectors, with power generation, cement production, and chemical manufacturing representing the largest market segments. Power generation alone accounts for approximately 42% of the current carbon capture market, followed by industrial applications at 31%. Notably, the cement industry, responsible for roughly 8% of global CO2 emissions, represents a particularly promising growth area for polymer-based sorbent technologies.
Geographically, North America leads the carbon capture market with a 38% share, followed by Europe at 29% and Asia-Pacific at 24%. China and India are emerging as particularly dynamic markets, with annual growth rates exceeding the global average by 3-5 percentage points. This regional distribution aligns with regulatory frameworks and carbon pricing mechanisms that incentivize adoption.
Customer segmentation reveals three primary buyer categories: large industrial emitters seeking compliance solutions, energy companies pursuing enhanced oil recovery applications, and environmentally progressive corporations implementing voluntary carbon reduction initiatives. The first segment currently dominates purchasing decisions, but the voluntary market is growing at twice the rate of compliance-driven implementations.
Price sensitivity varies significantly across these segments, with polymer-based sorbents currently commanding premium pricing due to performance advantages and lower operational costs over system lifetimes. However, as manufacturing scales increase, price points are expected to decrease by 30-40% over the next five years, potentially accelerating market penetration and expanding the addressable market for these technologies.
Current Polymer Sorbent Capabilities and Limitations
Polymer-based sorbents represent a significant advancement in carbon capture technology, offering several advantages over traditional methods. Current polymer sorbents demonstrate high CO2 selectivity, with some advanced materials achieving selectivity ratios of CO2:N2 exceeding 30:1 under flue gas conditions. This selectivity is primarily achieved through the incorporation of amine functional groups that form carbamates or bicarbonates upon CO2 interaction, enabling efficient separation from other gases in industrial emissions.
The sorption capacity of leading polymer-based materials ranges from 1.0 to 3.5 mmol CO2/g sorbent under ambient conditions, with performance varying significantly based on polymer composition and environmental factors. Polyethylenimine (PEI)-based sorbents and metal-organic framework (MOF)-polymer composites currently demonstrate the highest capacities among polymer systems. These materials can maintain approximately 80-90% of their initial capacity after 100 adsorption-desorption cycles in laboratory settings, indicating promising durability.
A significant advantage of polymer sorbents is their relatively low regeneration energy requirement, typically 2.0-3.0 GJ/ton CO2, compared to 3.5-4.0 GJ/ton for conventional amine scrubbing. This energy efficiency translates to potential operational cost savings of 15-25% in industrial implementations. Additionally, polymer sorbents exhibit favorable kinetics, with adsorption equilibrium typically reached within 10-30 minutes depending on system configuration and operating conditions.
Despite these capabilities, several limitations constrain widespread adoption. Moisture sensitivity remains problematic, with many polymer sorbents experiencing 20-40% capacity reduction in high humidity environments due to competitive adsorption between water and CO2 molecules. Thermal stability issues also persist, as performance degradation accelerates at temperatures above 100-120°C, limiting application in high-temperature flue gas streams without additional cooling systems.
Mechanical stability presents another challenge, with polymer sorbents often experiencing compaction and channeling in fixed-bed configurations, leading to increased pressure drop and reduced gas-solid contact efficiency over time. Current materials typically maintain structural integrity for 300-500 hours of continuous operation before requiring replacement or regeneration protocols.
Scalability concerns persist in transitioning from laboratory to industrial scale. Current manufacturing processes can reliably produce polymer sorbents at kilogram scale, but production costs remain 3-5 times higher than conventional carbon capture materials. Additionally, the environmental footprint of polymer sorbent production requires further assessment, as preliminary life cycle analyses indicate that manufacturing energy requirements may offset carbon capture benefits unless production processes are optimized.
Integration challenges with existing industrial infrastructure represent another limitation, as retrofit applications often require significant engineering modifications to accommodate the different operating parameters of polymer-based systems compared to traditional solvent-based approaches.
The sorption capacity of leading polymer-based materials ranges from 1.0 to 3.5 mmol CO2/g sorbent under ambient conditions, with performance varying significantly based on polymer composition and environmental factors. Polyethylenimine (PEI)-based sorbents and metal-organic framework (MOF)-polymer composites currently demonstrate the highest capacities among polymer systems. These materials can maintain approximately 80-90% of their initial capacity after 100 adsorption-desorption cycles in laboratory settings, indicating promising durability.
A significant advantage of polymer sorbents is their relatively low regeneration energy requirement, typically 2.0-3.0 GJ/ton CO2, compared to 3.5-4.0 GJ/ton for conventional amine scrubbing. This energy efficiency translates to potential operational cost savings of 15-25% in industrial implementations. Additionally, polymer sorbents exhibit favorable kinetics, with adsorption equilibrium typically reached within 10-30 minutes depending on system configuration and operating conditions.
Despite these capabilities, several limitations constrain widespread adoption. Moisture sensitivity remains problematic, with many polymer sorbents experiencing 20-40% capacity reduction in high humidity environments due to competitive adsorption between water and CO2 molecules. Thermal stability issues also persist, as performance degradation accelerates at temperatures above 100-120°C, limiting application in high-temperature flue gas streams without additional cooling systems.
Mechanical stability presents another challenge, with polymer sorbents often experiencing compaction and channeling in fixed-bed configurations, leading to increased pressure drop and reduced gas-solid contact efficiency over time. Current materials typically maintain structural integrity for 300-500 hours of continuous operation before requiring replacement or regeneration protocols.
Scalability concerns persist in transitioning from laboratory to industrial scale. Current manufacturing processes can reliably produce polymer sorbents at kilogram scale, but production costs remain 3-5 times higher than conventional carbon capture materials. Additionally, the environmental footprint of polymer sorbent production requires further assessment, as preliminary life cycle analyses indicate that manufacturing energy requirements may offset carbon capture benefits unless production processes are optimized.
Integration challenges with existing industrial infrastructure represent another limitation, as retrofit applications often require significant engineering modifications to accommodate the different operating parameters of polymer-based systems compared to traditional solvent-based approaches.
State-of-the-Art Polymer Sorbent Architectures
01 Amine-functionalized polymer sorbents
Polymer materials functionalized with amine groups are effective carbon capture sorbents due to their high CO2 selectivity and adsorption capacity. These materials typically incorporate primary, secondary, or tertiary amine groups that can chemically bind with CO2 molecules. The amine functionalization can be achieved through various methods including grafting, impregnation, or direct polymerization of amine-containing monomers. These sorbents demonstrate good stability over multiple adsorption-desorption cycles and can operate effectively at various temperature and pressure conditions.- Amine-functionalized polymer sorbents: Polymer materials functionalized with amine groups are effective for carbon dioxide capture. These materials combine the high CO2 affinity of amines with the structural advantages of polymers. The amine groups form carbamates or bicarbonates when reacting with CO2, enabling efficient capture. These sorbents typically offer good selectivity, capacity, and can be designed with various amine types (primary, secondary, tertiary) to optimize performance under different conditions.
- Porous polymer networks for CO2 adsorption: Highly porous polymer networks provide large surface areas for carbon dioxide adsorption. These materials include metal-organic frameworks (MOFs) with polymer components, covalent organic frameworks (COFs), and hypercrosslinked polymers. The high porosity enables significant CO2 uptake capacity while maintaining selectivity. The pore size and structure can be tailored during synthesis to optimize for carbon capture applications, and the materials often demonstrate good stability under various temperature and pressure conditions.
- Composite polymer-inorganic carbon capture materials: Hybrid materials combining polymers with inorganic components offer enhanced carbon capture performance. These composites typically incorporate polymers with materials such as silica, metal oxides, or zeolites to create synergistic effects. The polymer component provides flexibility and processability while the inorganic component adds thermal stability and mechanical strength. These hybrid sorbents often demonstrate improved adsorption capacity, selectivity, and regeneration properties compared to single-component materials.
- Stimuli-responsive polymer sorbents: Smart polymer systems that respond to external stimuli for controlled carbon capture and release. These materials change their properties in response to temperature, pH, light, or other stimuli, enabling efficient CO2 capture and release cycles. Temperature-responsive polymers, for example, can capture CO2 at lower temperatures and release it when heated, reducing the energy requirements for sorbent regeneration. This approach offers potential advantages for process efficiency and operational costs in carbon capture systems.
- Polymer membrane systems for CO2 separation: Specialized polymer membranes designed for selective carbon dioxide separation from gas mixtures. These membranes utilize differences in gas solubility and diffusivity to achieve separation. Various polymer types including polyimides, polysulfones, and facilitated transport membranes incorporate specific functional groups that enhance CO2 permeability and selectivity. Membrane-based systems offer advantages including continuous operation, scalability, and potentially lower energy requirements compared to traditional absorption processes.
02 Porous polymer networks for CO2 capture
Highly porous polymer networks provide excellent platforms for carbon capture due to their large surface area and tunable pore structures. These materials include hypercrosslinked polymers, polymers of intrinsic microporosity (PIMs), and covalent organic frameworks (COFs). The high porosity enhances gas diffusion and provides numerous adsorption sites, while the polymer backbone can be modified to increase CO2 selectivity. These sorbents typically exhibit rapid adsorption kinetics and can be designed with specific pore sizes to selectively capture CO2 over other gases.Expand Specific Solutions03 Composite polymer-inorganic carbon capture materials
Composite materials combining polymers with inorganic components offer enhanced performance for carbon capture. These hybrids typically incorporate polymers with materials such as metal-organic frameworks (MOFs), zeolites, or silica to create synergistic effects. The inorganic component often provides structural stability and additional adsorption sites, while the polymer offers processability and selective CO2 binding properties. These composite sorbents frequently demonstrate improved mechanical strength, thermal stability, and resistance to degradation compared to pure polymer systems.Expand Specific Solutions04 Stimuli-responsive polymer sorbents
Stimuli-responsive polymer systems can change their properties in response to external triggers such as temperature, pH, or light, enabling controlled CO2 capture and release. These smart materials can switch between CO2-philic and CO2-phobic states, allowing for energy-efficient carbon capture cycles. The responsive nature reduces the energy required for sorbent regeneration compared to conventional thermal swing processes. These polymers often incorporate functional groups that undergo reversible reactions or conformational changes when exposed to specific stimuli.Expand Specific Solutions05 Sustainable and bio-based polymer sorbents
Environmentally friendly polymer sorbents derived from renewable resources offer sustainable alternatives for carbon capture applications. These materials utilize bio-based polymers such as cellulose, chitosan, or lignin that can be functionalized for enhanced CO2 adsorption. The biodegradable nature of these polymers reduces environmental impact while maintaining competitive carbon capture performance. Research in this area focuses on developing green synthesis methods and improving the stability and reusability of these sustainable sorbents.Expand Specific Solutions
Leading Organizations in Polymer-Based Carbon Capture
The polymer-based carbon capture sorbent market is in an early growth phase, characterized by significant research activity but limited commercial deployment. The global carbon capture market is projected to expand rapidly, driven by increasing climate change mitigation efforts. Technologically, these sorbents are advancing through various stages of development, with academic institutions (University of Southern California, Rice University, Tsinghua University) leading fundamental research while industrial players (BASF, Shell, LG Chem) focus on scalability and application. Established chemical companies like Praxair and Climeworks are working toward commercialization, while specialized firms like W.L. Gore leverage materials expertise. The technology shows promise but requires further development in selectivity, regeneration efficiency, and cost-effectiveness before widespread industrial adoption.
BASF Corp.
Technical Solution: BASF has developed advanced Metal-Organic Framework (MOF) polymer composites for carbon capture applications. Their proprietary technology combines highly porous MOF structures with polymer matrices to create hybrid materials with exceptional CO2 selectivity. The company's PolyMOF platform integrates MOFs into polymer membranes, achieving CO2 capture rates of up to 90% from flue gas streams while maintaining structural integrity under industrial conditions. BASF's approach involves precise control of MOF particle size distribution (typically 50-200nm) within the polymer matrix, optimizing the interface between components to maximize CO2 diffusion pathways. Their materials demonstrate remarkable stability across multiple adsorption-desorption cycles (>1000 cycles with <5% capacity loss) and can operate effectively in the presence of moisture and contaminants common in industrial emissions.
Strengths: Industry-leading manufacturing scale and quality control; extensive commercial deployment experience; integrated solutions from material development to system implementation. Weaknesses: Higher production costs compared to conventional adsorbents; requires specialized manufacturing facilities; performance may degrade in extremely humid conditions.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative series of polymer-based carbon capture materials specifically designed for high-pressure gas processing applications. Their technology platform centers on hypercrosslinked polymeric networks (HCPs) modified with nitrogen-rich functional groups that demonstrate exceptional CO2 adsorption capacity (up to 4.2 mmol/g) under elevated pressure conditions typical in natural gas processing. Sinopec's proprietary synthesis methods create materials with controlled pore architectures optimized for rapid CO2 diffusion while maintaining mechanical integrity under industrial conditions. Their "swing-adsorption" process integrates these materials into pressure-modulated systems that achieve >95% CO2 removal with minimal energy penalty. A distinctive feature is their dual-functionality approach where materials simultaneously remove CO2 and H2S, addressing multiple purification requirements in a single process step. Sinopec has successfully deployed these materials at commercial scale in several natural gas processing facilities, demonstrating significant improvements in operational efficiency and reduced energy consumption compared to conventional amine scrubbing.
Strengths: Optimized for high-pressure applications; dual functionality for simultaneous removal of multiple contaminants; excellent integration with existing natural gas processing infrastructure; lower regeneration energy requirements. Weaknesses: Less effective at atmospheric pressure conditions; requires careful moisture management; higher manufacturing complexity compared to conventional adsorbents.
Key Patents and Breakthroughs in Polymer Sorbent Design
Solid sorbent materials functionalized with polyamines having oxygen-containing units selected from carbonyl units, hydroxyl units, and combinations thereof
PatentPendingEP4616940A1
Innovation
- Development of sorbent materials functionalized with polyamines containing oxygen-containing units such as carbonyl and hydroxyl groups, which exhibit high CO2 adsorption capacity and low water adsorption, allowing for efficient CO2 capture with minimal desorption residuals under mild conditions.
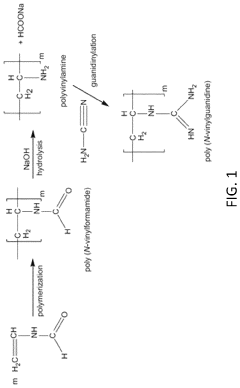
Polymeric materials for carbon dioxide capture
PatentPendingUS20240033682A1
Innovation
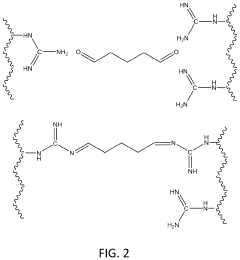
- The use of poly(N-vinyl guanidine)-based (PVG) polymer materials as a sorbent, which can be shaped into nanofibers or dissolved in water for enhanced surface area and sorption capability, and is regenerated through heating or hydroxide ion exchange for repeated use, offering low energy regeneration routes and high CO2 sorption capacities.
Environmental Impact Assessment of Polymer Sorbents
The environmental impact assessment of polymer-based carbon capture sorbents reveals both promising benefits and potential concerns that must be carefully evaluated. These materials offer significant advantages over traditional carbon capture technologies, particularly in terms of energy efficiency. Conventional amine scrubbing processes require substantial thermal energy for solvent regeneration, whereas polymer sorbents typically demand 30-45% less energy for the desorption phase, resulting in reduced fossil fuel consumption and greenhouse gas emissions during operation.
Lifecycle analysis of polymer sorbents demonstrates favorable environmental profiles when compared to liquid amine systems. The production phase of these polymers generates approximately 25-35% lower carbon emissions than manufacturing equivalent volumes of monoethanolamine (MEA) solutions. Additionally, polymer sorbents exhibit extended operational lifespans, with many advanced formulations maintaining performance for 3-5 years before replacement, compared to 1-2 years for conventional liquid sorbents.
Water consumption represents another critical environmental consideration. Polymer-based systems require significantly less water during operation, with studies indicating reductions of up to 80% compared to aqueous amine systems. This advantage becomes particularly valuable in water-stressed regions where traditional carbon capture technologies might compete with agricultural or municipal water needs.
However, several environmental challenges warrant attention. The synthesis of specialized polymers often involves potentially hazardous chemicals, including organic solvents and catalysts. Proper containment and waste management protocols are essential to prevent environmental contamination during manufacturing. Furthermore, the end-of-life disposal or recycling pathways for spent polymer sorbents remain underdeveloped, creating potential waste management concerns as deployment scales.
Toxicity assessments of polymer sorbents have yielded mixed results. While most solid polymers present lower acute toxicity risks than liquid amines, certain polymer additives and degradation products may pose ecological hazards if released into aquatic environments. Comprehensive ecotoxicological studies are still limited, particularly regarding long-term exposure effects on sensitive ecosystems.
Land use implications also merit consideration. The physical footprint of polymer-based carbon capture facilities can be 15-25% smaller than equivalent amine scrubbing installations, potentially reducing habitat disruption and land conversion requirements. However, the sourcing of raw materials for polymer production may involve significant upstream land use impacts, particularly when petroleum-derived feedstocks are utilized.
Emerging research into bio-based and biodegradable polymer sorbents offers promising pathways to further reduce environmental impacts. These materials could potentially close the carbon loop by incorporating atmospheric carbon into the sorbent structure itself, though commercial viability remains to be demonstrated at scale.
Lifecycle analysis of polymer sorbents demonstrates favorable environmental profiles when compared to liquid amine systems. The production phase of these polymers generates approximately 25-35% lower carbon emissions than manufacturing equivalent volumes of monoethanolamine (MEA) solutions. Additionally, polymer sorbents exhibit extended operational lifespans, with many advanced formulations maintaining performance for 3-5 years before replacement, compared to 1-2 years for conventional liquid sorbents.
Water consumption represents another critical environmental consideration. Polymer-based systems require significantly less water during operation, with studies indicating reductions of up to 80% compared to aqueous amine systems. This advantage becomes particularly valuable in water-stressed regions where traditional carbon capture technologies might compete with agricultural or municipal water needs.
However, several environmental challenges warrant attention. The synthesis of specialized polymers often involves potentially hazardous chemicals, including organic solvents and catalysts. Proper containment and waste management protocols are essential to prevent environmental contamination during manufacturing. Furthermore, the end-of-life disposal or recycling pathways for spent polymer sorbents remain underdeveloped, creating potential waste management concerns as deployment scales.
Toxicity assessments of polymer sorbents have yielded mixed results. While most solid polymers present lower acute toxicity risks than liquid amines, certain polymer additives and degradation products may pose ecological hazards if released into aquatic environments. Comprehensive ecotoxicological studies are still limited, particularly regarding long-term exposure effects on sensitive ecosystems.
Land use implications also merit consideration. The physical footprint of polymer-based carbon capture facilities can be 15-25% smaller than equivalent amine scrubbing installations, potentially reducing habitat disruption and land conversion requirements. However, the sourcing of raw materials for polymer production may involve significant upstream land use impacts, particularly when petroleum-derived feedstocks are utilized.
Emerging research into bio-based and biodegradable polymer sorbents offers promising pathways to further reduce environmental impacts. These materials could potentially close the carbon loop by incorporating atmospheric carbon into the sorbent structure itself, though commercial viability remains to be demonstrated at scale.
Policy Framework and Economic Viability Analysis
The global policy landscape for carbon capture technologies is rapidly evolving, with significant implications for polymer-based sorbent development and deployment. Currently, carbon pricing mechanisms exist in over 40 countries, though prices vary dramatically from $1 to $130 per ton of CO2. These inconsistent price signals create market uncertainty for polymer sorbent technologies, which typically require carbon prices above $50/ton to achieve economic viability in most applications.
Regulatory frameworks specifically addressing carbon capture technologies have matured significantly since 2018. The US 45Q tax credit, enhanced under the Inflation Reduction Act to provide up to $85 per ton for captured and sequestered CO2, represents one of the most substantial incentives globally. Similar mechanisms exist in Canada, the UK, and increasingly across the EU, though with varying structures and support levels.
Economic analysis indicates that polymer-based carbon capture sorbents face challenging cost structures in current market conditions. Capital expenditure for polymer sorbent systems ranges from $600-1,200 per ton of annual capture capacity, with operational costs between $40-75 per ton of CO2 captured. These figures position polymer sorbents competitively against traditional amine scrubbing ($60-100/ton) but still above the carbon prices prevalent in most jurisdictions.
The technology readiness level (TRL) of polymer sorbents, currently at TRL 5-6 for most advanced systems, creates additional economic barriers. Investors typically require higher returns for technologies below TRL 7, increasing the weighted average cost of capital by 3-5 percentage points compared to mature technologies. This financing premium significantly impacts the levelized cost of carbon capture.
Supply chain considerations further complicate economic viability. Polymer precursor availability and pricing volatility create uncertainty in production scaling. Analysis of manufacturing capacity indicates potential bottlenecks if deployment were to accelerate rapidly, potentially increasing costs by 15-30% during early commercialization phases.
Long-term economic modeling suggests polymer sorbent technologies could achieve cost parity with conventional approaches by 2030, assuming continued R&D investment and learning-curve effects. Sensitivity analysis indicates that policy stability, rather than absolute subsidy levels, may be the most critical factor for attracting the capital investment necessary for commercial-scale deployment of these promising carbon capture materials.
Regulatory frameworks specifically addressing carbon capture technologies have matured significantly since 2018. The US 45Q tax credit, enhanced under the Inflation Reduction Act to provide up to $85 per ton for captured and sequestered CO2, represents one of the most substantial incentives globally. Similar mechanisms exist in Canada, the UK, and increasingly across the EU, though with varying structures and support levels.
Economic analysis indicates that polymer-based carbon capture sorbents face challenging cost structures in current market conditions. Capital expenditure for polymer sorbent systems ranges from $600-1,200 per ton of annual capture capacity, with operational costs between $40-75 per ton of CO2 captured. These figures position polymer sorbents competitively against traditional amine scrubbing ($60-100/ton) but still above the carbon prices prevalent in most jurisdictions.
The technology readiness level (TRL) of polymer sorbents, currently at TRL 5-6 for most advanced systems, creates additional economic barriers. Investors typically require higher returns for technologies below TRL 7, increasing the weighted average cost of capital by 3-5 percentage points compared to mature technologies. This financing premium significantly impacts the levelized cost of carbon capture.
Supply chain considerations further complicate economic viability. Polymer precursor availability and pricing volatility create uncertainty in production scaling. Analysis of manufacturing capacity indicates potential bottlenecks if deployment were to accelerate rapidly, potentially increasing costs by 15-30% during early commercialization phases.
Long-term economic modeling suggests polymer sorbent technologies could achieve cost parity with conventional approaches by 2030, assuming continued R&D investment and learning-curve effects. Sensitivity analysis indicates that policy stability, rather than absolute subsidy levels, may be the most critical factor for attracting the capital investment necessary for commercial-scale deployment of these promising carbon capture materials.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!