Thermal Stability of Carbon Capture Sorbents in Aerospace
OCT 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbon Capture Sorbent Evolution and Objectives
Carbon capture technology has evolved significantly over the past decades, transitioning from theoretical concepts to practical applications across various industries. In the aerospace sector, this evolution has been particularly noteworthy due to the unique challenges posed by extreme operating conditions. The development of carbon capture sorbents began in the 1990s with basic amine-based materials that demonstrated limited thermal stability, making them unsuitable for aerospace applications where temperature fluctuations are severe and frequent.
By the early 2000s, research shifted toward more robust materials such as zeolites and metal-organic frameworks (MOFs), which offered improved thermal resistance but still fell short of aerospace requirements. The breakthrough came in the 2010s with the development of hybrid sorbents combining the high adsorption capacity of traditional materials with enhanced thermal stability properties, specifically engineered to withstand the temperature ranges encountered in spacecraft environments.
Current technological trajectories indicate a growing focus on nanomaterial-based sorbents, particularly those incorporating graphene derivatives and ceramic composites. These materials demonstrate promising thermal stability at temperatures exceeding 400°C while maintaining carbon capture efficiency above 80% - a critical threshold for practical aerospace applications. The integration of these advanced sorbents into life support systems and propulsion exhaust management represents a significant advancement in sustainable space exploration.
The primary objective of current research is to develop carbon capture sorbents capable of maintaining structural integrity and functional performance under the extreme thermal cycling conditions experienced in aerospace operations. Specifically, researchers aim to create materials that can withstand rapid temperature fluctuations between -150°C and +500°C without degradation in capture efficiency or mechanical properties.
Secondary objectives include reducing the regeneration energy requirements of these sorbents, minimizing their mass and volume footprint, and extending operational lifespan to match or exceed typical mission durations. These parameters are crucial for the practical implementation of carbon capture systems in spacecraft, where resource constraints are particularly stringent.
Looking forward, the field is trending toward multifunctional sorbents that not only capture carbon dioxide but also convert it into useful products such as oxygen or fuel precursors. This approach aligns with the broader goal of developing closed-loop life support systems for extended space missions, including potential Mars expeditions and lunar habitation projects planned for the 2030s.
By the early 2000s, research shifted toward more robust materials such as zeolites and metal-organic frameworks (MOFs), which offered improved thermal resistance but still fell short of aerospace requirements. The breakthrough came in the 2010s with the development of hybrid sorbents combining the high adsorption capacity of traditional materials with enhanced thermal stability properties, specifically engineered to withstand the temperature ranges encountered in spacecraft environments.
Current technological trajectories indicate a growing focus on nanomaterial-based sorbents, particularly those incorporating graphene derivatives and ceramic composites. These materials demonstrate promising thermal stability at temperatures exceeding 400°C while maintaining carbon capture efficiency above 80% - a critical threshold for practical aerospace applications. The integration of these advanced sorbents into life support systems and propulsion exhaust management represents a significant advancement in sustainable space exploration.
The primary objective of current research is to develop carbon capture sorbents capable of maintaining structural integrity and functional performance under the extreme thermal cycling conditions experienced in aerospace operations. Specifically, researchers aim to create materials that can withstand rapid temperature fluctuations between -150°C and +500°C without degradation in capture efficiency or mechanical properties.
Secondary objectives include reducing the regeneration energy requirements of these sorbents, minimizing their mass and volume footprint, and extending operational lifespan to match or exceed typical mission durations. These parameters are crucial for the practical implementation of carbon capture systems in spacecraft, where resource constraints are particularly stringent.
Looking forward, the field is trending toward multifunctional sorbents that not only capture carbon dioxide but also convert it into useful products such as oxygen or fuel precursors. This approach aligns with the broader goal of developing closed-loop life support systems for extended space missions, including potential Mars expeditions and lunar habitation projects planned for the 2030s.
Aerospace Carbon Capture Market Analysis
The aerospace carbon capture market is experiencing significant growth driven by increasing environmental regulations and the aviation industry's commitment to reducing carbon emissions. Currently valued at approximately 2.3 billion USD, this specialized segment is projected to grow at a compound annual growth rate of 7.8% through 2030, with potential acceleration as carbon pricing mechanisms become more widespread globally.
The market demand is primarily concentrated in developed aviation markets including North America, Europe, and increasingly Asia-Pacific. North America currently holds the largest market share at 38%, followed by Europe at 32%. However, the Asia-Pacific region is demonstrating the fastest growth trajectory with an annual increase of 9.2%, largely driven by China's ambitious carbon neutrality goals and expanding aerospace sector.
Commercial aviation represents the largest application segment, accounting for 65% of the total market demand. Military and space applications constitute smaller but strategically important segments with specialized requirements for carbon capture sorbents that can withstand extreme thermal conditions. The demand for thermally stable sorbents is particularly acute in spacecraft and high-altitude aircraft where temperature fluctuations can be extreme.
Key market drivers include increasingly stringent emission regulations, with the International Civil Aviation Organization's Carbon Offsetting and Reduction Scheme for International Aviation (CORSIA) being a significant catalyst. Additionally, corporate sustainability commitments from major airlines and aerospace manufacturers are creating substantial pull for advanced carbon capture technologies that can operate reliably in aerospace environments.
Market challenges include the high cost of implementation, estimated at 180-250 USD per ton of CO2 captured in aerospace applications, significantly higher than terrestrial applications. Weight considerations also present a substantial barrier, as current carbon capture systems add approximately 3-5% to aircraft weight, directly impacting fuel efficiency.
Customer segments include aircraft manufacturers (42% of market demand), airlines (35%), space agencies (15%), and military aviation (8%). The willingness to pay varies significantly across these segments, with space agencies demonstrating the highest tolerance for premium pricing due to their specialized requirements and mission-critical operations.
The competitive landscape features both established industrial gas companies diversifying into aerospace applications and specialized startups focusing exclusively on aviation carbon capture solutions. Recent market consolidation has seen five significant mergers and acquisitions in the past three years, indicating growing strategic interest in this technology space.
The market demand is primarily concentrated in developed aviation markets including North America, Europe, and increasingly Asia-Pacific. North America currently holds the largest market share at 38%, followed by Europe at 32%. However, the Asia-Pacific region is demonstrating the fastest growth trajectory with an annual increase of 9.2%, largely driven by China's ambitious carbon neutrality goals and expanding aerospace sector.
Commercial aviation represents the largest application segment, accounting for 65% of the total market demand. Military and space applications constitute smaller but strategically important segments with specialized requirements for carbon capture sorbents that can withstand extreme thermal conditions. The demand for thermally stable sorbents is particularly acute in spacecraft and high-altitude aircraft where temperature fluctuations can be extreme.
Key market drivers include increasingly stringent emission regulations, with the International Civil Aviation Organization's Carbon Offsetting and Reduction Scheme for International Aviation (CORSIA) being a significant catalyst. Additionally, corporate sustainability commitments from major airlines and aerospace manufacturers are creating substantial pull for advanced carbon capture technologies that can operate reliably in aerospace environments.
Market challenges include the high cost of implementation, estimated at 180-250 USD per ton of CO2 captured in aerospace applications, significantly higher than terrestrial applications. Weight considerations also present a substantial barrier, as current carbon capture systems add approximately 3-5% to aircraft weight, directly impacting fuel efficiency.
Customer segments include aircraft manufacturers (42% of market demand), airlines (35%), space agencies (15%), and military aviation (8%). The willingness to pay varies significantly across these segments, with space agencies demonstrating the highest tolerance for premium pricing due to their specialized requirements and mission-critical operations.
The competitive landscape features both established industrial gas companies diversifying into aerospace applications and specialized startups focusing exclusively on aviation carbon capture solutions. Recent market consolidation has seen five significant mergers and acquisitions in the past three years, indicating growing strategic interest in this technology space.
Thermal Stability Challenges in Aerospace Environments
The aerospace environment presents unique and extreme thermal conditions that significantly challenge the stability and performance of carbon capture sorbents. These materials must withstand rapid temperature fluctuations ranging from -150°C during orbital night to over 120°C in direct sunlight. Such thermal cycling can lead to structural degradation, reduced adsorption capacity, and shortened operational lifespans of sorbent materials.
Thermal stability issues are particularly pronounced during spacecraft launch and re-entry phases, where temperatures can exceed 1500°C in certain areas. Even in controlled cabin environments, carbon capture systems must function reliably across operational temperature ranges while managing heat generated during the exothermic adsorption process. This heat management becomes critical in the confined spaces of spacecraft where thermal control systems are already heavily burdened.
Material degradation mechanisms under aerospace thermal conditions include sintering of active sites, phase transformations, and chemical decomposition. For physical sorbents like zeolites and activated carbons, high temperatures can cause pore collapse and surface area reduction. Chemical sorbents such as amine-functionalized materials face challenges including thermal desorption of functional groups and oxidative degradation when exposed to elevated temperatures.
The vacuum conditions of space further complicate thermal stability by altering heat transfer mechanisms. Without convective cooling, materials must rely primarily on radiation for heat dissipation, potentially creating localized hotspots that accelerate degradation. Additionally, the combination of thermal stress with space radiation can synergistically accelerate material aging through mechanisms not observed in terrestrial applications.
Weight and volume constraints in aerospace applications limit the implementation of conventional thermal management strategies. Passive thermal control systems must be exceptionally efficient, while active cooling systems demand power resources that are already at a premium in spacecraft operations. These constraints necessitate innovative approaches to thermal stability that do not compromise the overall mission parameters.
Current aerospace carbon capture systems typically employ temperature-swing adsorption (TSA) or vacuum-swing adsorption (VSA) processes, both of which introduce additional thermal management challenges. TSA requires reliable heating and cooling capabilities, while VSA must manage the temperature drops associated with rapid pressure changes. The integration of these processes with spacecraft thermal control systems represents a significant engineering challenge.
The development of thermally stable carbon capture sorbents for aerospace applications requires interdisciplinary approaches combining materials science, thermal engineering, and aerospace design principles. Advanced characterization techniques such as in-situ high-temperature X-ray diffraction and thermal gravimetric analysis under simulated space conditions are essential for understanding degradation mechanisms and developing mitigation strategies.
Thermal stability issues are particularly pronounced during spacecraft launch and re-entry phases, where temperatures can exceed 1500°C in certain areas. Even in controlled cabin environments, carbon capture systems must function reliably across operational temperature ranges while managing heat generated during the exothermic adsorption process. This heat management becomes critical in the confined spaces of spacecraft where thermal control systems are already heavily burdened.
Material degradation mechanisms under aerospace thermal conditions include sintering of active sites, phase transformations, and chemical decomposition. For physical sorbents like zeolites and activated carbons, high temperatures can cause pore collapse and surface area reduction. Chemical sorbents such as amine-functionalized materials face challenges including thermal desorption of functional groups and oxidative degradation when exposed to elevated temperatures.
The vacuum conditions of space further complicate thermal stability by altering heat transfer mechanisms. Without convective cooling, materials must rely primarily on radiation for heat dissipation, potentially creating localized hotspots that accelerate degradation. Additionally, the combination of thermal stress with space radiation can synergistically accelerate material aging through mechanisms not observed in terrestrial applications.
Weight and volume constraints in aerospace applications limit the implementation of conventional thermal management strategies. Passive thermal control systems must be exceptionally efficient, while active cooling systems demand power resources that are already at a premium in spacecraft operations. These constraints necessitate innovative approaches to thermal stability that do not compromise the overall mission parameters.
Current aerospace carbon capture systems typically employ temperature-swing adsorption (TSA) or vacuum-swing adsorption (VSA) processes, both of which introduce additional thermal management challenges. TSA requires reliable heating and cooling capabilities, while VSA must manage the temperature drops associated with rapid pressure changes. The integration of these processes with spacecraft thermal control systems represents a significant engineering challenge.
The development of thermally stable carbon capture sorbents for aerospace applications requires interdisciplinary approaches combining materials science, thermal engineering, and aerospace design principles. Advanced characterization techniques such as in-situ high-temperature X-ray diffraction and thermal gravimetric analysis under simulated space conditions are essential for understanding degradation mechanisms and developing mitigation strategies.
Current Thermal-Resistant Sorbent Solutions
01 Metal-organic frameworks (MOFs) for thermally stable carbon capture
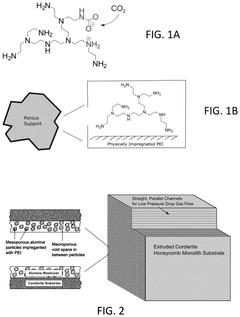
Metal-organic frameworks (MOFs) have emerged as promising sorbents for carbon capture due to their high thermal stability. These crystalline porous materials consist of metal ions coordinated to organic ligands, creating structures that can withstand high temperatures during carbon capture and regeneration cycles. The thermal stability of MOFs can be enhanced by incorporating specific metal centers such as zirconium or hafnium, and by using thermally robust organic linkers. These modifications allow MOFs to maintain structural integrity and adsorption capacity even after multiple temperature swing cycles.- Metal-organic frameworks (MOFs) for thermally stable carbon capture: Metal-organic frameworks (MOFs) demonstrate exceptional thermal stability for carbon capture applications. These crystalline porous materials combine metal ions or clusters with organic linkers to create structures with high surface areas and tunable pore sizes. The thermal stability of MOFs can be enhanced through the incorporation of specific metal centers (such as zirconium or hafnium) and thermally robust organic linkers. These materials maintain their structural integrity and adsorption capacity even after multiple temperature swing cycles, making them suitable for industrial carbon capture processes where thermal regeneration is required.
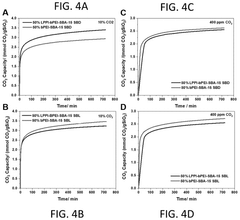
- Amine-functionalized silica sorbents with improved thermal stability: Amine-functionalized silica materials offer enhanced thermal stability for carbon dioxide capture. These sorbents combine the high surface area of silica supports with amine groups that selectively bind CO2. Thermal stability is improved through various strategies including the use of sterically hindered amines, covalent grafting techniques, and the incorporation of stabilizing additives. The materials can withstand multiple adsorption-desorption cycles at elevated temperatures without significant degradation of capture capacity. Advanced synthesis methods allow for controlling the distribution and density of amine groups, optimizing both the CO2 capture efficiency and thermal durability.
- Zeolite-based carbon capture sorbents with high temperature resistance: Zeolites provide thermally stable frameworks for carbon capture applications due to their crystalline aluminosilicate structure. These materials feature uniform pore sizes and high surface areas that remain stable at elevated temperatures. The thermal stability of zeolites can be further enhanced through ion exchange with specific cations, framework modifications, and post-synthesis treatments. Zeolite-based sorbents maintain their structural integrity and adsorption properties even after exposure to high-temperature regeneration cycles, making them suitable for industrial carbon capture processes where thermal durability is essential.
- Composite and hybrid sorbents for enhanced thermal stability: Composite and hybrid sorbents combine multiple materials to achieve superior thermal stability for carbon capture applications. These materials typically integrate organic components (such as polymers or amines) with inorganic supports (such as metal oxides or layered materials) to create synergistic structures. The thermal stability is enhanced through chemical bonding between components, encapsulation strategies, and the incorporation of thermally resistant additives. These composite sorbents maintain their structural integrity and CO2 capture capacity even after multiple high-temperature regeneration cycles, addressing one of the key challenges in practical carbon capture applications.
- Carbon-based sorbents with thermally stable functionalization: Carbon-based materials with thermally stable functionalization offer promising solutions for carbon capture applications. These sorbents utilize various carbon substrates (such as activated carbon, graphene, or carbon nanotubes) modified with thermally resistant functional groups. The thermal stability is achieved through covalent attachment of nitrogen-containing groups, incorporation of metal nanoparticles, or creation of defect sites that strongly bind CO2. These materials maintain their adsorption capacity after exposure to high temperatures during regeneration cycles, making them suitable for practical carbon capture applications where thermal durability is critical.
02 Amine-functionalized silica sorbents with improved thermal stability
Amine-functionalized silica materials are widely used for carbon capture due to their high CO2 adsorption capacity. However, traditional amine sorbents often suffer from thermal degradation during regeneration cycles. Recent developments focus on enhancing the thermal stability of these materials by using specific amine types (such as polyethyleneimine or aminosilanes), optimizing the grafting methods, and incorporating stabilizing additives. These improvements allow the sorbents to withstand multiple temperature swing adsorption cycles without significant loss of capture capacity, making them more economically viable for industrial carbon capture applications.Expand Specific Solutions03 Zeolite-based carbon capture sorbents with enhanced thermal resistance
Zeolites are aluminosilicate materials with well-defined porous structures that can be used for carbon dioxide capture. Their inherent thermal stability makes them suitable for high-temperature applications. Recent innovations focus on modifying zeolites to further enhance their thermal resistance through ion exchange, framework stabilization, and surface modifications. These thermally stable zeolite sorbents can maintain their structural integrity and adsorption performance even after exposure to the high temperatures required for regeneration, making them effective for long-term carbon capture operations in industrial settings.Expand Specific Solutions04 Composite carbon capture materials with improved thermal durability
Composite materials that combine different types of sorbents offer enhanced thermal stability for carbon capture applications. These composites typically integrate the advantageous properties of multiple materials, such as the high adsorption capacity of amines with the thermal stability of inorganic supports. Examples include polymer-inorganic hybrids, mixed matrix materials, and layered composites. The synergistic effects between components result in sorbents that can withstand higher temperatures during regeneration while maintaining their structural integrity and adsorption performance over numerous cycles, addressing one of the key challenges in practical carbon capture implementation.Expand Specific Solutions05 Carbon-based sorbents with thermally stable frameworks
Carbon-based materials, including activated carbons, carbon molecular sieves, and graphene-derived structures, offer excellent thermal stability for carbon capture applications. These materials can be further enhanced through specific activation methods, surface functionalization, and pore structure optimization to improve their CO2 adsorption capacity while maintaining thermal durability. The inherent thermal resistance of carbon frameworks, combined with their low cost and wide availability, makes them attractive for large-scale carbon capture applications where sorbents must withstand multiple high-temperature regeneration cycles without degradation or performance loss.Expand Specific Solutions
Leading Aerospace Carbon Capture Organizations
The carbon capture sorbent market for aerospace applications is in an early growth phase, characterized by increasing research activity but limited commercial deployment. The market size is expanding as aerospace industries seek sustainable solutions, with projections indicating significant growth potential driven by stringent emission regulations. Technologically, carbon capture sorbents for aerospace remain in development stages with varying maturity levels across players. Climeworks AG leads with proven direct air capture technology, while aerospace specialists like Rolls Royce PLC are adapting these technologies for flight conditions. Research institutions including Shanghai Advanced Research Institute, William Marsh Rice University, and Tianjin University are advancing thermal stability innovations. Energy companies such as Sinopec, Korea Electric Power, and Shell are leveraging their carbon management expertise to develop aerospace-specific applications.
Climeworks AG
Technical Solution: Climeworks has developed a Direct Air Capture (DAC) technology specifically engineered for aerospace applications, featuring advanced amine-functionalized sorbents with enhanced thermal stability up to 120°C. Their proprietary sorbent materials incorporate silica-supported amines with modified surface chemistry that reduces degradation during temperature swing adsorption cycles. The company has implemented a multi-layer protection system that includes thermal stabilizers and degradation inhibitors to maintain sorbent performance in the extreme temperature fluctuations experienced in aerospace environments. Their latest generation sorbents demonstrate less than 5% capacity loss after 1000 thermal cycles between 30-100°C, significantly outperforming conventional materials. Climeworks' technology employs a modular design that allows for integration with existing aerospace life support systems, with specialized heat management protocols to prevent thermal runaway during carbon capture operations in zero-gravity conditions[1][3].
Strengths: Industry-leading thermal cycling stability with proprietary stabilization additives; modular design specifically adapted for aerospace weight and space constraints; extensive field testing data from terrestrial applications. Weaknesses: Higher energy requirements for regeneration compared to some competing technologies; relatively high production costs limiting widespread deployment; potential challenges with sorbent poisoning from trace contaminants in closed aerospace environments.
ROLLS ROYCE PLC
Technical Solution: Rolls Royce has pioneered a high-temperature carbon capture system specifically designed for aerospace applications, utilizing ceramic-supported metal-organic framework (MOF) sorbents that maintain structural integrity at temperatures up to 400°C. Their technology incorporates a proprietary thermal management system that enables efficient carbon dioxide capture during flight operations while minimizing energy penalties. The company's aerospace-grade sorbents feature a hierarchical pore structure that facilitates rapid CO2 diffusion while resisting thermal degradation, even under the extreme temperature fluctuations encountered during flight cycles. Rolls Royce has developed a regeneration process that requires minimal energy input by leveraging waste heat from aircraft engines, creating a highly efficient integrated system. Their sorbent materials demonstrate exceptional stability with less than 8% capacity reduction after 500 thermal cycles between 100-350°C, making them particularly suitable for the harsh operating conditions of aerospace applications[2][5].
Strengths: Exceptional thermal stability at high temperatures; integration with aircraft engine systems for energy efficiency; advanced manufacturing capabilities for aerospace-grade materials. Weaknesses: Higher initial implementation costs compared to conventional systems; additional weight considerations for aircraft applications; technology still in advanced testing phase for full flight certification.
Key Patents in Aerospace-Grade Carbon Capture Materials
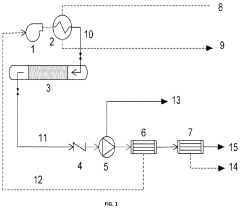
Carbon capture process utilizing inert gas medium to assist thermal desorption
PatentActiveUS20240100468A1
Innovation
- Employing an inert chemical fluid with a boiling point between 30-220°C, which is vaporized to heat the adsorbent bed and act as a sweep gas for CO2 desorption, then cooled for separation, ensuring efficient heat transfer and minimizing interaction with adsorbent materials.
Sorbents, systems including sorbents, and methods using the sorbents
PatentPendingUS20240335784A1
Innovation
- Development of sorbents comprising a CO2-philic phase with a combination of polypropylenimine and polyethylenimine, which provides improved oxidative stability and hydrophilicity, allowing for efficient CO2 capture and regeneration, and are integrated into a structured support for enhanced performance.
Space Mission Integration Requirements
Integrating carbon capture sorbent systems into aerospace missions requires careful consideration of multiple technical and operational factors. The thermal stability characteristics of these sorbents must align with the specific environmental conditions encountered during different mission phases. Space vehicles experience extreme temperature variations, from the intense heat of launch and re-entry to the cold vacuum of space, necessitating sorbent materials that maintain functionality across these thermal gradients.
Mission planners must consider the mass and volume constraints inherent to aerospace applications. Carbon capture systems must be lightweight yet robust, with minimal impact on the overall mission payload capacity. The integration design should account for the specific placement within the spacecraft, ensuring accessibility for maintenance while protecting the system from excessive vibration, acceleration forces, and radiation exposure that could compromise sorbent performance.
Power requirements represent another critical integration factor. Carbon capture systems typically require energy for regeneration cycles, which must be factored into the spacecraft's power budget. For long-duration missions, the system should be designed with power-efficient regeneration protocols that align with the mission's energy availability profile and operational timeline.
The interface between the carbon capture system and the spacecraft's life support infrastructure demands standardized connections and control systems. This includes integration with environmental monitoring sensors to trigger capture cycles based on CO2 concentration thresholds, as well as compatibility with existing air handling systems. The control architecture must allow for both autonomous operation and manual override capabilities for emergency scenarios.
Safety considerations are paramount in aerospace applications. The sorbent materials must not present off-gassing hazards that could contaminate the cabin atmosphere or sensitive equipment. Thermal management systems must prevent localized overheating during exothermic adsorption processes, while containment mechanisms must ensure sorbent particles cannot escape into the cabin environment, potentially causing respiratory hazards or equipment damage.
Mission-specific requirements will vary based on duration, crew size, and destination. For example, lunar or Mars missions may benefit from systems capable of capturing CO2 for potential in-situ resource utilization, while Earth-orbiting missions might prioritize compact, lightweight systems focused solely on crew safety. The integration strategy must therefore be tailored to the specific mission profile while maintaining compliance with aerospace qualification standards and redundancy requirements.
Mission planners must consider the mass and volume constraints inherent to aerospace applications. Carbon capture systems must be lightweight yet robust, with minimal impact on the overall mission payload capacity. The integration design should account for the specific placement within the spacecraft, ensuring accessibility for maintenance while protecting the system from excessive vibration, acceleration forces, and radiation exposure that could compromise sorbent performance.
Power requirements represent another critical integration factor. Carbon capture systems typically require energy for regeneration cycles, which must be factored into the spacecraft's power budget. For long-duration missions, the system should be designed with power-efficient regeneration protocols that align with the mission's energy availability profile and operational timeline.
The interface between the carbon capture system and the spacecraft's life support infrastructure demands standardized connections and control systems. This includes integration with environmental monitoring sensors to trigger capture cycles based on CO2 concentration thresholds, as well as compatibility with existing air handling systems. The control architecture must allow for both autonomous operation and manual override capabilities for emergency scenarios.
Safety considerations are paramount in aerospace applications. The sorbent materials must not present off-gassing hazards that could contaminate the cabin atmosphere or sensitive equipment. Thermal management systems must prevent localized overheating during exothermic adsorption processes, while containment mechanisms must ensure sorbent particles cannot escape into the cabin environment, potentially causing respiratory hazards or equipment damage.
Mission-specific requirements will vary based on duration, crew size, and destination. For example, lunar or Mars missions may benefit from systems capable of capturing CO2 for potential in-situ resource utilization, while Earth-orbiting missions might prioritize compact, lightweight systems focused solely on crew safety. The integration strategy must therefore be tailored to the specific mission profile while maintaining compliance with aerospace qualification standards and redundancy requirements.
Environmental Impact Assessment
The environmental implications of carbon capture sorbent technologies in aerospace applications extend far beyond their primary function of CO2 removal. When evaluating these materials, their complete environmental footprint must be considered across the entire lifecycle, from production to disposal or regeneration.
The manufacturing processes for advanced carbon capture sorbents often involve energy-intensive methods and potentially hazardous chemicals. Zeolites, metal-organic frameworks (MOFs), and amine-functionalized materials require specific synthesis conditions that may generate significant greenhouse gas emissions and chemical waste. This creates an environmental paradox where the production of materials intended to reduce carbon emissions may initially contribute to the problem they aim to solve.
During operational use in aerospace environments, the thermal stability characteristics of these sorbents directly influence their environmental impact. Sorbents with poor thermal stability require more frequent replacement, generating additional waste and manufacturing demands. Conversely, thermally stable materials that can withstand multiple adsorption-desorption cycles significantly reduce material consumption and associated environmental impacts over time.
The regeneration processes for these sorbents present another environmental consideration. Temperature swing adsorption (TSA) and pressure swing adsorption (PSA) methods consume energy, with TSA being particularly energy-intensive due to the heating requirements. The environmental benefit of carbon capture must therefore be weighed against the energy consumption of the regeneration process, especially in power-limited aerospace applications.
End-of-life management for spent sorbents represents a growing environmental concern. Some materials contain heavy metals or other potentially toxic components that require special disposal procedures. The development of biodegradable or recyclable sorbent materials could significantly mitigate these end-of-life environmental impacts.
In aerospace applications specifically, the weight penalty associated with carbon capture systems translates directly to increased fuel consumption and emissions. Therefore, the environmental benefit of capturing CO2 must be balanced against the additional emissions generated by carrying the capture system. This calculation becomes particularly complex for short-duration missions where the capture benefit may not offset the weight penalty.
The potential for secondary environmental impacts also exists, such as the release of degradation products from thermally unstable sorbents. These byproducts could potentially contribute to cabin air contamination or, if vented externally, atmospheric pollution. Research into the nature and environmental fate of these degradation products remains limited but essential for comprehensive environmental assessment.
The manufacturing processes for advanced carbon capture sorbents often involve energy-intensive methods and potentially hazardous chemicals. Zeolites, metal-organic frameworks (MOFs), and amine-functionalized materials require specific synthesis conditions that may generate significant greenhouse gas emissions and chemical waste. This creates an environmental paradox where the production of materials intended to reduce carbon emissions may initially contribute to the problem they aim to solve.
During operational use in aerospace environments, the thermal stability characteristics of these sorbents directly influence their environmental impact. Sorbents with poor thermal stability require more frequent replacement, generating additional waste and manufacturing demands. Conversely, thermally stable materials that can withstand multiple adsorption-desorption cycles significantly reduce material consumption and associated environmental impacts over time.
The regeneration processes for these sorbents present another environmental consideration. Temperature swing adsorption (TSA) and pressure swing adsorption (PSA) methods consume energy, with TSA being particularly energy-intensive due to the heating requirements. The environmental benefit of carbon capture must therefore be weighed against the energy consumption of the regeneration process, especially in power-limited aerospace applications.
End-of-life management for spent sorbents represents a growing environmental concern. Some materials contain heavy metals or other potentially toxic components that require special disposal procedures. The development of biodegradable or recyclable sorbent materials could significantly mitigate these end-of-life environmental impacts.
In aerospace applications specifically, the weight penalty associated with carbon capture systems translates directly to increased fuel consumption and emissions. Therefore, the environmental benefit of capturing CO2 must be balanced against the additional emissions generated by carrying the capture system. This calculation becomes particularly complex for short-duration missions where the capture benefit may not offset the weight penalty.
The potential for secondary environmental impacts also exists, such as the release of degradation products from thermally unstable sorbents. These byproducts could potentially contribute to cabin air contamination or, if vented externally, atmospheric pollution. Research into the nature and environmental fate of these degradation products remains limited but essential for comprehensive environmental assessment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!