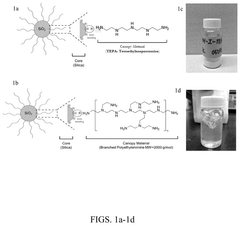
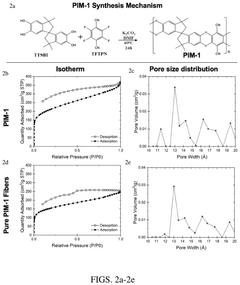
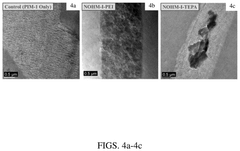
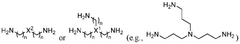Carbon Capture Sorbents and Their Semiconductor Interfaces
OCT 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbon Capture Technology Evolution and Objectives
Carbon capture technology has evolved significantly over the past several decades, transitioning from theoretical concepts to practical applications in response to growing environmental concerns. The earliest carbon capture methods emerged in the 1970s, primarily focusing on separating CO2 from natural gas streams using amine-based solvents. These initial approaches were not specifically designed for climate change mitigation but rather for industrial gas purification processes.
By the 1990s, as climate change awareness increased, carbon capture research expanded to include post-combustion capture from power plants. This period saw the development of first-generation sorbent materials, predominantly liquid amines and solid adsorbents with limited capacity and selectivity. The early 2000s marked a turning point with increased research funding and industrial interest in carbon capture technologies.
The evolution of carbon capture sorbents has followed several distinct technological waves. First-generation materials focused primarily on chemical absorption using aqueous amine solutions. Second-generation approaches introduced advanced solid sorbents including metal-organic frameworks (MOFs), zeolites, and activated carbons with improved CO2 selectivity. The current third-generation materials represent a paradigm shift toward hybrid systems that combine the advantages of multiple capture mechanisms.
Recent innovations have increasingly focused on the interface between carbon capture sorbents and semiconductor materials, creating new possibilities for electrically-switchable capture systems and photocatalytic conversion processes. This integration represents a significant departure from traditional passive sorbent technologies toward active, controllable capture systems with potential for lower energy penalties.
The primary objectives of current carbon capture sorbent research include developing materials with substantially higher CO2 selectivity, faster adsorption-desorption kinetics, and greater stability over thousands of cycles. Researchers aim to reduce regeneration energy requirements below 1.5 GJ/ton CO2, compared to approximately 3.5 GJ/ton for conventional amine systems. Additionally, there is growing emphasis on creating sorbents capable of functioning effectively in diverse industrial environments beyond power generation.
For semiconductor interfaces specifically, key objectives include developing materials that can facilitate CO2 capture and conversion using renewable electricity or direct solar energy. These interfaces aim to enable precise control over capture-release cycles through applied voltage or illumination, potentially eliminating the energy-intensive thermal swing processes that dominate conventional approaches. The ultimate goal is to transform carbon capture from an energy-consuming process to an energy-neutral or even energy-producing technology through integration with renewable energy systems.
By the 1990s, as climate change awareness increased, carbon capture research expanded to include post-combustion capture from power plants. This period saw the development of first-generation sorbent materials, predominantly liquid amines and solid adsorbents with limited capacity and selectivity. The early 2000s marked a turning point with increased research funding and industrial interest in carbon capture technologies.
The evolution of carbon capture sorbents has followed several distinct technological waves. First-generation materials focused primarily on chemical absorption using aqueous amine solutions. Second-generation approaches introduced advanced solid sorbents including metal-organic frameworks (MOFs), zeolites, and activated carbons with improved CO2 selectivity. The current third-generation materials represent a paradigm shift toward hybrid systems that combine the advantages of multiple capture mechanisms.
Recent innovations have increasingly focused on the interface between carbon capture sorbents and semiconductor materials, creating new possibilities for electrically-switchable capture systems and photocatalytic conversion processes. This integration represents a significant departure from traditional passive sorbent technologies toward active, controllable capture systems with potential for lower energy penalties.
The primary objectives of current carbon capture sorbent research include developing materials with substantially higher CO2 selectivity, faster adsorption-desorption kinetics, and greater stability over thousands of cycles. Researchers aim to reduce regeneration energy requirements below 1.5 GJ/ton CO2, compared to approximately 3.5 GJ/ton for conventional amine systems. Additionally, there is growing emphasis on creating sorbents capable of functioning effectively in diverse industrial environments beyond power generation.
For semiconductor interfaces specifically, key objectives include developing materials that can facilitate CO2 capture and conversion using renewable electricity or direct solar energy. These interfaces aim to enable precise control over capture-release cycles through applied voltage or illumination, potentially eliminating the energy-intensive thermal swing processes that dominate conventional approaches. The ultimate goal is to transform carbon capture from an energy-consuming process to an energy-neutral or even energy-producing technology through integration with renewable energy systems.
Market Analysis for Carbon Capture Solutions
The global carbon capture market is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. As of 2023, the market was valued at approximately $7.5 billion, with projections indicating a compound annual growth rate (CAGR) of 19.2% through 2030, potentially reaching $35.6 billion by the end of the decade. This growth trajectory is supported by substantial government investments, with the US Inflation Reduction Act allocating $369 billion toward climate initiatives, including carbon capture technologies.
The market for carbon capture sorbents specifically represents about 40% of the overall carbon capture technology market, with particular growth in materials that can interface with semiconductor technologies for enhanced efficiency and monitoring capabilities. These integrated solutions are gaining traction due to their potential for real-time performance optimization and reduced operational costs.
Demand is primarily concentrated in power generation (34%), industrial applications (28%), and oil and gas operations (22%), with emerging interest from the transportation and construction sectors. Geographically, North America leads with 38% market share, followed by Europe (29%) and Asia-Pacific (24%), with the latter showing the fastest growth rate at 22.3% annually.
Customer segments can be categorized into three tiers: large industrial emitters seeking compliance solutions (55% of market), mid-sized companies pursuing sustainability credentials (30%), and innovative technology adopters exploring competitive advantages through advanced carbon management (15%). The willingness to pay varies significantly across these segments, with price sensitivity decreasing as regulatory pressures increase.
The economic viability of carbon capture solutions continues to improve, with the cost per ton of CO₂ captured decreasing from $80-120 in 2018 to $58-75 in 2023. Solutions incorporating semiconductor interfaces for monitoring and optimization have demonstrated an additional 12-18% efficiency improvement, strengthening their value proposition despite a 15-20% higher initial investment cost.
Market barriers include high capital expenditure requirements, uncertain regulatory frameworks in developing markets, and technical challenges in scaling solutions across diverse industrial applications. However, the emergence of carbon pricing mechanisms in 46 countries and growing corporate net-zero commitments are creating strong market pull factors that are expected to accelerate adoption rates by 27% over the next five years.
The market for carbon capture sorbents specifically represents about 40% of the overall carbon capture technology market, with particular growth in materials that can interface with semiconductor technologies for enhanced efficiency and monitoring capabilities. These integrated solutions are gaining traction due to their potential for real-time performance optimization and reduced operational costs.
Demand is primarily concentrated in power generation (34%), industrial applications (28%), and oil and gas operations (22%), with emerging interest from the transportation and construction sectors. Geographically, North America leads with 38% market share, followed by Europe (29%) and Asia-Pacific (24%), with the latter showing the fastest growth rate at 22.3% annually.
Customer segments can be categorized into three tiers: large industrial emitters seeking compliance solutions (55% of market), mid-sized companies pursuing sustainability credentials (30%), and innovative technology adopters exploring competitive advantages through advanced carbon management (15%). The willingness to pay varies significantly across these segments, with price sensitivity decreasing as regulatory pressures increase.
The economic viability of carbon capture solutions continues to improve, with the cost per ton of CO₂ captured decreasing from $80-120 in 2018 to $58-75 in 2023. Solutions incorporating semiconductor interfaces for monitoring and optimization have demonstrated an additional 12-18% efficiency improvement, strengthening their value proposition despite a 15-20% higher initial investment cost.
Market barriers include high capital expenditure requirements, uncertain regulatory frameworks in developing markets, and technical challenges in scaling solutions across diverse industrial applications. However, the emergence of carbon pricing mechanisms in 46 countries and growing corporate net-zero commitments are creating strong market pull factors that are expected to accelerate adoption rates by 27% over the next five years.
Current Sorbent Technologies and Challenges
Carbon capture sorbent technologies have evolved significantly over the past decades, with several distinct categories emerging as frontrunners in addressing CO2 emissions. Physical sorbents, including activated carbons, zeolites, and metal-organic frameworks (MOFs), operate primarily through van der Waals forces and offer advantages in regeneration energy requirements. However, they typically suffer from limited selectivity and capacity under practical operating conditions, particularly at low CO2 partial pressures relevant to direct air capture applications.
Chemical sorbents, such as amine-functionalized materials and alkali metal-based sorbents, demonstrate superior CO2 selectivity through the formation of chemical bonds. Amine-based sorbents have shown particular promise due to their high affinity for CO2 even at low concentrations, though they face challenges related to thermal stability during regeneration cycles and susceptibility to degradation in the presence of SOx and NOx contaminants.
Recent advances in hybrid sorbent systems attempt to combine the benefits of both physical and chemical capture mechanisms. These materials often incorporate semiconductor interfaces that can enhance capture performance through electronic interactions or photocatalytic effects. However, the precise mechanisms governing these interfaces remain incompletely understood, presenting both challenges and opportunities for further development.
A significant challenge facing current sorbent technologies is the trade-off between capture capacity and regeneration energy requirements. Materials with high binding energies typically require substantial energy inputs for regeneration, reducing overall process efficiency. Conversely, sorbents with lower binding energies may require less regeneration energy but often demonstrate insufficient capture performance under practical conditions.
Scalability represents another critical challenge, as many promising materials developed in laboratory settings face significant barriers to cost-effective mass production. Manufacturing complexities, raw material availability, and process integration issues have limited commercial deployment of advanced sorbent technologies despite promising research results.
Stability under real-world operating conditions remains problematic for many sorbent materials. Exposure to moisture, thermal cycling, and trace contaminants can lead to significant performance degradation over time. This is particularly challenging for semiconductor-integrated sorbents, where interface stability is crucial for maintaining enhanced capture properties.
The integration of semiconductor interfaces with carbon capture sorbents introduces additional complexities related to charge transfer dynamics, surface chemistry, and long-term stability. While these interfaces offer potential for enhanced capture through electronic effects or light-driven regeneration, controlling and maintaining these interfaces under practical operating conditions presents substantial technical hurdles that must be overcome for successful implementation.
Chemical sorbents, such as amine-functionalized materials and alkali metal-based sorbents, demonstrate superior CO2 selectivity through the formation of chemical bonds. Amine-based sorbents have shown particular promise due to their high affinity for CO2 even at low concentrations, though they face challenges related to thermal stability during regeneration cycles and susceptibility to degradation in the presence of SOx and NOx contaminants.
Recent advances in hybrid sorbent systems attempt to combine the benefits of both physical and chemical capture mechanisms. These materials often incorporate semiconductor interfaces that can enhance capture performance through electronic interactions or photocatalytic effects. However, the precise mechanisms governing these interfaces remain incompletely understood, presenting both challenges and opportunities for further development.
A significant challenge facing current sorbent technologies is the trade-off between capture capacity and regeneration energy requirements. Materials with high binding energies typically require substantial energy inputs for regeneration, reducing overall process efficiency. Conversely, sorbents with lower binding energies may require less regeneration energy but often demonstrate insufficient capture performance under practical conditions.
Scalability represents another critical challenge, as many promising materials developed in laboratory settings face significant barriers to cost-effective mass production. Manufacturing complexities, raw material availability, and process integration issues have limited commercial deployment of advanced sorbent technologies despite promising research results.
Stability under real-world operating conditions remains problematic for many sorbent materials. Exposure to moisture, thermal cycling, and trace contaminants can lead to significant performance degradation over time. This is particularly challenging for semiconductor-integrated sorbents, where interface stability is crucial for maintaining enhanced capture properties.
The integration of semiconductor interfaces with carbon capture sorbents introduces additional complexities related to charge transfer dynamics, surface chemistry, and long-term stability. While these interfaces offer potential for enhanced capture through electronic effects or light-driven regeneration, controlling and maintaining these interfaces under practical operating conditions presents substantial technical hurdles that must be overcome for successful implementation.
Semiconductor-Sorbent Interface Solutions
01 Metal-organic frameworks (MOFs) for carbon capture
Metal-organic frameworks are advanced porous materials with high surface area and tunable pore structures that make them effective for carbon dioxide adsorption. These crystalline materials consist of metal ions coordinated to organic ligands, creating a framework with exceptional CO2 selectivity and capacity. MOFs can be designed with specific functional groups to enhance CO2 binding and can operate under various temperature and pressure conditions, making them versatile carbon capture sorbents.- Metal-organic frameworks (MOFs) for carbon capture: Metal-organic frameworks are advanced porous materials with high surface area and tunable pore structures that make them effective for carbon dioxide adsorption. These crystalline materials consist of metal ions or clusters coordinated with organic ligands, creating a framework with exceptional CO2 selectivity and capacity. Their modular nature allows for customization of binding sites and pore dimensions to optimize carbon capture performance under various conditions.
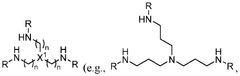
- Amine-functionalized sorbents: Amine-functionalized materials represent a significant class of carbon capture sorbents that operate through chemical adsorption mechanisms. These sorbents incorporate various amine groups onto support materials such as silica, polymers, or porous carbons to create strong binding sites for CO2. The amine groups react with carbon dioxide to form carbamates or bicarbonates, enabling high selectivity even at low CO2 concentrations. These materials can be designed with different amine types and loadings to optimize capture efficiency and regeneration energy requirements.
- Zeolite-based carbon capture materials: Zeolites are crystalline aluminosilicate materials with well-defined microporous structures that make them effective for carbon dioxide separation. Their molecular sieving properties allow for selective adsorption based on molecular size and shape. Zeolites can be modified through ion exchange, dealumination, or incorporation of functional groups to enhance CO2 selectivity and capacity. These materials offer advantages including high thermal stability, resistance to contaminants, and relatively low regeneration energy requirements for sustainable carbon capture applications.
- Novel composite and hybrid sorbent materials: Composite and hybrid sorbent materials combine multiple components to create synergistic effects for enhanced carbon capture performance. These materials integrate different functional elements such as polymers with inorganic supports, mixed metal oxides, or combinations of physical and chemical sorbents. The resulting composites offer improved properties including higher CO2 capacity, better selectivity, enhanced mechanical stability, and more efficient regeneration characteristics. These innovative material designs address multiple challenges in carbon capture technology through their multifunctional nature.
- Carbon-based sorbents and activation methods: Carbon-based materials, including activated carbons, carbon molecular sieves, and graphene-derived structures, serve as effective sorbents for CO2 capture. These materials can be produced from various precursors including biomass, polymers, or industrial byproducts. Different activation methods such as physical activation with steam or CO2, chemical activation with KOH or ZnCl2, and template-based approaches can be employed to develop optimized pore structures. The resulting materials offer advantages including high surface area, tunable pore size distribution, hydrophobicity, and potential for surface functionalization to enhance carbon capture performance.
02 Amine-functionalized sorbents
Amine-functionalized materials represent a significant class of carbon capture sorbents that utilize the chemical affinity between amine groups and CO2 molecules. These sorbents typically consist of a porous support material impregnated or grafted with various amine compounds. The amine groups form carbamates or bicarbonates upon reaction with CO2, enabling efficient capture even at low CO2 concentrations. These materials can be regenerated through temperature or pressure swing processes, making them suitable for cyclic carbon capture applications.Expand Specific Solutions03 Zeolite-based carbon capture materials
Zeolites are crystalline aluminosilicate materials with well-defined microporous structures that can effectively adsorb carbon dioxide. Their molecular sieve properties allow for selective capture of CO2 from gas mixtures based on molecular size and polarity differences. Zeolite-based sorbents can be modified through ion exchange, framework substitution, or surface functionalization to enhance their CO2 adsorption capacity and selectivity. These materials offer advantages including high thermal stability, resistance to contaminants, and relatively low regeneration energy requirements.Expand Specific Solutions04 Novel composite and hybrid sorbent materials
Composite and hybrid sorbent materials combine multiple components to achieve enhanced carbon capture performance beyond what individual materials can provide. These innovative materials often integrate organic and inorganic components, such as polymer-inorganic hybrids, mixed matrix materials, or hierarchical porous structures. The synergistic effects between components can improve CO2 adsorption capacity, selectivity, kinetics, and material stability. These composites can be tailored for specific operating conditions and often demonstrate improved resistance to degradation during multiple adsorption-desorption cycles.Expand Specific Solutions05 Regeneration methods for carbon capture sorbents
Effective regeneration methods are crucial for the practical application of carbon capture sorbents in industrial settings. Various approaches have been developed, including temperature swing adsorption (TSA), pressure swing adsorption (PSA), vacuum swing adsorption (VSA), and combinations thereof. Novel regeneration techniques incorporate microwave heating, electrical swing adsorption, or steam stripping to reduce energy requirements. Advanced process configurations, such as moving bed systems or fluidized bed reactors, can improve the efficiency of sorbent regeneration while maintaining material integrity over multiple capture-release cycles.Expand Specific Solutions
Leading Companies in Carbon Capture Industry
Carbon capture technology is currently in a growth phase, with increasing market demand driven by global decarbonization efforts. The market is projected to expand significantly as regulatory pressures intensify, though commercial-scale deployment remains limited. Among key players, academic institutions like MIT, University of Michigan, and Rice University are advancing fundamental research, while industrial leaders demonstrate varying levels of technological maturity. Samsung Electronics, Infineon, and Applied Materials are leveraging semiconductor expertise to develop advanced interfaces for carbon capture materials. Chemical industry players such as DuPont and Shell are focusing on sorbent development and integration with existing industrial processes. The competitive landscape shows a mix of established corporations and specialized startups like Heliatek, with collaboration between academic and industrial sectors becoming increasingly important for commercialization.
Shell Internationale Research Maatschappij BV
Technical Solution: Shell has developed advanced Metal-Organic Framework (MOF) based carbon capture sorbents with semiconductor interfaces for enhanced CO2 selectivity and capacity. Their proprietary technology combines porous MOF structures with semiconductor materials to create hybrid interfaces that improve charge transfer and CO2 binding. Shell's approach involves modifying the electronic properties of the sorbent-semiconductor junction to optimize CO2 adsorption kinetics while maintaining regeneration efficiency. The company has demonstrated in pilot projects that their MOF-semiconductor hybrid materials can achieve CO2 capture rates of up to 90% with significantly reduced energy penalties compared to conventional amine scrubbing technologies[1]. Shell's research focuses on scalable manufacturing processes for these materials, including solution-based deposition techniques that enable precise control over the semiconductor-sorbent interface properties. Their latest generation materials incorporate nanoscale engineering to maximize surface area (>2000 m²/g) while maintaining mechanical stability during multiple adsorption-desorption cycles[2].
Strengths: Shell's extensive industrial infrastructure provides immediate pathways for large-scale implementation and testing. Their materials show excellent stability over multiple capture-release cycles and can be integrated into existing industrial processes. Weaknesses: The production costs remain relatively high compared to conventional sorbents, and the technology still faces challenges in humid conditions where water competition can reduce CO2 selectivity.
Massachusetts Institute of Technology
Technical Solution: MIT has developed groundbreaking carbon capture sorbent technologies that leverage advanced semiconductor interfaces to dramatically improve capture efficiency and selectivity. Their research focuses on atomically precise engineering of sorbent-semiconductor junctions to optimize electronic interactions with CO2 molecules. MIT's approach utilizes two-dimensional semiconductor materials (including modified transition metal dichalcogenides) as platforms for anchoring specialized CO2-philic functional groups. These hybrid materials create unique electronic environments that strengthen CO2 binding through both physical and chemical mechanisms. Laboratory studies have demonstrated that MIT's engineered interfaces can achieve CO2 capture capacities up to 5.2 mmol/g with exceptional selectivity ratios exceeding 100:1 for CO2 over N2[5]. The semiconductor components enable novel capture mechanisms including light-assisted adsorption, where specific wavelengths can trigger enhanced capture or controlled release, reducing the energy penalty associated with traditional temperature or pressure swing processes. MIT researchers have also pioneered in-situ characterization techniques that provide atomic-level insights into the capture mechanisms, enabling rational design of next-generation materials. Recent developments include self-healing sorbent-semiconductor interfaces that maintain performance even after exposure to common contaminants in industrial gas streams[6].
Strengths: MIT's materials demonstrate unprecedented CO2 selectivity and capacity under realistic conditions and offer multiple pathways for regeneration (thermal, electrical, optical). Their fundamental understanding of interface phenomena enables continuous improvement through rational design. Weaknesses: Current fabrication methods are laboratory-scale and would require significant engineering for industrial production. Some of the more advanced materials rely on rare elements that could limit large-scale deployment.
Key Patents in Sorbent-Semiconductor Integration
Fiber-encapsulated hybrid materials for capture of carbon dioxide
PatentPendingUS20240326017A1
Innovation
- Development of encapsulated fiber compositions with a microporous sheath surrounding an amine-containing core, produced through electrospinning, which enhances thermal stability, reduces water absorption, and minimizes pressure drop, enabling efficient CO2 capture and scalable deployment.
Entrapped small amines in nanoporous materials for gas capture
PatentWO2025101819A1
Innovation
- The development of sorbents comprising a porous substrate entrapping a plurality of amine compounds, with a polyamide film disposed on the substrate surface, enhances gas capture efficiency by entrapping amine compounds and preventing amine loss over time.
Environmental Impact Assessment
The environmental implications of carbon capture sorbents and their semiconductor interfaces extend far beyond their primary function of reducing atmospheric CO2. These technologies, while promising for climate change mitigation, present a complex matrix of environmental considerations that must be thoroughly evaluated.
The production of advanced sorbent materials often involves energy-intensive processes and potentially hazardous chemicals. Metal-organic frameworks (MOFs), zeolites, and amine-functionalized materials require precise synthesis conditions that can generate significant carbon footprints if powered by fossil fuels. This creates a paradoxical situation where carbon capture technologies may initially contribute to the very problem they aim to solve, necessitating life cycle assessments to ensure net environmental benefits.
Water usage represents another critical environmental factor. Many sorbent regeneration processes require substantial water resources, potentially creating competition in water-stressed regions. Additionally, the integration of semiconductors with sorbents introduces electronic waste concerns, as these components contain rare earth elements and potentially toxic materials that require specialized disposal protocols.
Land use changes associated with large-scale deployment of carbon capture facilities must also be considered. Industrial-scale implementation could require significant land area, potentially competing with agricultural or conservation priorities. The ecological footprint extends to habitat disruption and biodiversity impacts in areas where capture facilities are constructed.
The fate of captured carbon presents both opportunities and risks. While permanent sequestration in geological formations is one pathway, the conversion of captured CO2 into value-added products through semiconductor-assisted processes could reduce dependence on fossil carbon sources. However, leakage risks from storage sites must be continuously monitored to prevent re-release of captured carbon.
Energy requirements for sorbent regeneration and semiconductor operation represent a significant environmental consideration. The energy penalty associated with carbon capture can range from 15-30% of a power plant's output, highlighting the importance of renewable energy integration to maximize net climate benefits.
Potential chemical emissions from degraded sorbents or semiconductor materials may introduce new environmental contaminants. Long-term monitoring programs must be established to detect any unforeseen environmental impacts as these technologies scale from laboratory demonstrations to industrial implementation.
The production of advanced sorbent materials often involves energy-intensive processes and potentially hazardous chemicals. Metal-organic frameworks (MOFs), zeolites, and amine-functionalized materials require precise synthesis conditions that can generate significant carbon footprints if powered by fossil fuels. This creates a paradoxical situation where carbon capture technologies may initially contribute to the very problem they aim to solve, necessitating life cycle assessments to ensure net environmental benefits.
Water usage represents another critical environmental factor. Many sorbent regeneration processes require substantial water resources, potentially creating competition in water-stressed regions. Additionally, the integration of semiconductors with sorbents introduces electronic waste concerns, as these components contain rare earth elements and potentially toxic materials that require specialized disposal protocols.
Land use changes associated with large-scale deployment of carbon capture facilities must also be considered. Industrial-scale implementation could require significant land area, potentially competing with agricultural or conservation priorities. The ecological footprint extends to habitat disruption and biodiversity impacts in areas where capture facilities are constructed.
The fate of captured carbon presents both opportunities and risks. While permanent sequestration in geological formations is one pathway, the conversion of captured CO2 into value-added products through semiconductor-assisted processes could reduce dependence on fossil carbon sources. However, leakage risks from storage sites must be continuously monitored to prevent re-release of captured carbon.
Energy requirements for sorbent regeneration and semiconductor operation represent a significant environmental consideration. The energy penalty associated with carbon capture can range from 15-30% of a power plant's output, highlighting the importance of renewable energy integration to maximize net climate benefits.
Potential chemical emissions from degraded sorbents or semiconductor materials may introduce new environmental contaminants. Long-term monitoring programs must be established to detect any unforeseen environmental impacts as these technologies scale from laboratory demonstrations to industrial implementation.
Scalability and Cost Analysis
The scalability and cost considerations for carbon capture sorbents and their semiconductor interfaces present significant challenges for widespread commercial implementation. Current carbon capture technologies utilizing advanced sorbents face substantial economic barriers, with estimated costs ranging from $50-100 per ton of CO2 captured, significantly higher than the market price of carbon in many regions. This cost disparity creates a fundamental economic obstacle to adoption without substantial policy incentives or carbon pricing mechanisms.
Manufacturing scalability represents another critical challenge. Laboratory-scale synthesis of specialized sorbents with semiconductor interfaces often employs precise, controlled conditions that are difficult to replicate in industrial settings. The transition from gram-scale production to tons requires substantial process engineering innovations. Particularly challenging is maintaining consistent quality and performance characteristics across large production volumes, as nanoscale features and surface properties critical to capture efficiency can vary significantly with scaled production methods.
Material availability presents additional constraints, especially for sorbents incorporating rare earth elements or precious metals at semiconductor interfaces. Supply chain vulnerabilities could significantly impact large-scale deployment, with certain critical materials facing potential shortages or geopolitical supply risks. Alternative material pathways using earth-abundant elements show promise but often with performance trade-offs that must be carefully evaluated.
Energy requirements for regeneration cycles represent a substantial portion of operational costs. Current estimates indicate that regeneration energy accounts for 30-40% of total operational expenses in temperature swing adsorption systems. Semiconductor interfaces that can harness light or electrical stimuli for regeneration show potential for reducing these costs, but require further development to achieve energy efficiency at scale.
Durability and lifetime considerations significantly impact long-term economics. Most advanced sorbents demonstrate performance degradation after multiple capture-release cycles, with typical lifespans of 1,000-5,000 cycles before requiring replacement. Extending operational lifetimes through improved material stability could dramatically improve cost structures, as replacement costs currently represent approximately 15-25% of total system costs over a 20-year operational period.
Integration costs with existing infrastructure must also be considered. Retrofitting existing power plants or industrial facilities with carbon capture systems requires significant capital investment beyond the sorbent materials themselves. Modular designs that can be incrementally scaled show promise for reducing initial capital requirements, potentially enabling more widespread adoption through phased implementation approaches.
Manufacturing scalability represents another critical challenge. Laboratory-scale synthesis of specialized sorbents with semiconductor interfaces often employs precise, controlled conditions that are difficult to replicate in industrial settings. The transition from gram-scale production to tons requires substantial process engineering innovations. Particularly challenging is maintaining consistent quality and performance characteristics across large production volumes, as nanoscale features and surface properties critical to capture efficiency can vary significantly with scaled production methods.
Material availability presents additional constraints, especially for sorbents incorporating rare earth elements or precious metals at semiconductor interfaces. Supply chain vulnerabilities could significantly impact large-scale deployment, with certain critical materials facing potential shortages or geopolitical supply risks. Alternative material pathways using earth-abundant elements show promise but often with performance trade-offs that must be carefully evaluated.
Energy requirements for regeneration cycles represent a substantial portion of operational costs. Current estimates indicate that regeneration energy accounts for 30-40% of total operational expenses in temperature swing adsorption systems. Semiconductor interfaces that can harness light or electrical stimuli for regeneration show potential for reducing these costs, but require further development to achieve energy efficiency at scale.
Durability and lifetime considerations significantly impact long-term economics. Most advanced sorbents demonstrate performance degradation after multiple capture-release cycles, with typical lifespans of 1,000-5,000 cycles before requiring replacement. Extending operational lifetimes through improved material stability could dramatically improve cost structures, as replacement costs currently represent approximately 15-25% of total system costs over a 20-year operational period.
Integration costs with existing infrastructure must also be considered. Retrofitting existing power plants or industrial facilities with carbon capture systems requires significant capital investment beyond the sorbent materials themselves. Modular designs that can be incrementally scaled show promise for reducing initial capital requirements, potentially enabling more widespread adoption through phased implementation approaches.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!