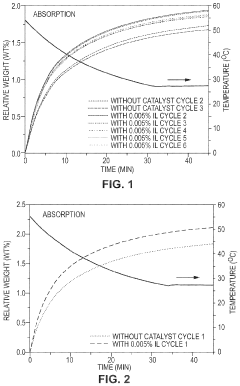
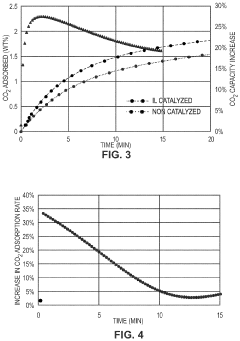
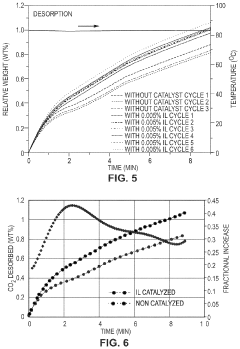
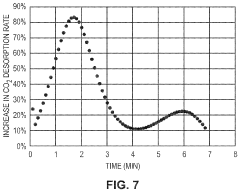
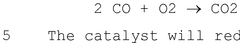Comparison of Carbon Capture Sorbents in Catalytic Performance
OCT 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbon Capture Technology Background and Objectives
Carbon capture technology has evolved significantly over the past several decades, driven by the urgent need to mitigate climate change impacts. Initially developed in the 1970s for enhanced oil recovery applications, carbon capture has transformed into a critical environmental technology aimed at reducing greenhouse gas emissions. The fundamental principle involves capturing carbon dioxide from point sources such as power plants or industrial facilities before it enters the atmosphere, followed by transportation and secure storage or utilization.
The evolution of carbon capture sorbents represents a particularly dynamic area within this field. Early technologies primarily relied on amine-based liquid solvents, which while effective, presented challenges related to energy consumption and equipment corrosion. This led to the development of solid sorbents with enhanced catalytic performance characteristics, including metal-organic frameworks (MOFs), zeolites, activated carbons, and various functionalized materials.
Current technological objectives in carbon capture sorbent development focus on several key parameters: increased CO2 selectivity, improved adsorption capacity, enhanced regeneration efficiency, and superior mechanical and chemical stability under industrial conditions. The ideal sorbent must demonstrate high performance across these metrics while maintaining cost-effectiveness at scale. Additionally, researchers aim to develop materials that can function efficiently across varying temperature and pressure conditions typical in industrial settings.
The comparative analysis of carbon capture sorbents in terms of catalytic performance has become increasingly important as the technology advances toward commercial viability. This comparison encompasses not only the fundamental adsorption properties but also the catalytic behavior that can enhance the capture process through chemical transformations that facilitate either the capture or release phases of the carbon cycle.
Global research efforts are currently directed toward developing next-generation sorbents that combine high selectivity with rapid kinetics and low regeneration energy requirements. Particular attention is being paid to hybrid materials that incorporate both physical adsorption capabilities and chemical reaction pathways, potentially offering synergistic benefits. These advanced materials aim to overcome the traditional trade-offs between adsorption capacity and regeneration energy.
The ultimate technological goal remains the development of carbon capture systems that can achieve significant CO2 removal at costs below $50 per ton, making the technology economically viable without substantial subsidies. This cost target represents a critical threshold for widespread adoption across various industrial sectors and would position carbon capture as a mainstream climate change mitigation strategy alongside renewable energy technologies.
The evolution of carbon capture sorbents represents a particularly dynamic area within this field. Early technologies primarily relied on amine-based liquid solvents, which while effective, presented challenges related to energy consumption and equipment corrosion. This led to the development of solid sorbents with enhanced catalytic performance characteristics, including metal-organic frameworks (MOFs), zeolites, activated carbons, and various functionalized materials.
Current technological objectives in carbon capture sorbent development focus on several key parameters: increased CO2 selectivity, improved adsorption capacity, enhanced regeneration efficiency, and superior mechanical and chemical stability under industrial conditions. The ideal sorbent must demonstrate high performance across these metrics while maintaining cost-effectiveness at scale. Additionally, researchers aim to develop materials that can function efficiently across varying temperature and pressure conditions typical in industrial settings.
The comparative analysis of carbon capture sorbents in terms of catalytic performance has become increasingly important as the technology advances toward commercial viability. This comparison encompasses not only the fundamental adsorption properties but also the catalytic behavior that can enhance the capture process through chemical transformations that facilitate either the capture or release phases of the carbon cycle.
Global research efforts are currently directed toward developing next-generation sorbents that combine high selectivity with rapid kinetics and low regeneration energy requirements. Particular attention is being paid to hybrid materials that incorporate both physical adsorption capabilities and chemical reaction pathways, potentially offering synergistic benefits. These advanced materials aim to overcome the traditional trade-offs between adsorption capacity and regeneration energy.
The ultimate technological goal remains the development of carbon capture systems that can achieve significant CO2 removal at costs below $50 per ton, making the technology economically viable without substantial subsidies. This cost target represents a critical threshold for widespread adoption across various industrial sectors and would position carbon capture as a mainstream climate change mitigation strategy alongside renewable energy technologies.
Market Analysis for Carbon Capture Solutions
The global carbon capture market is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. As of 2023, the market size has reached approximately $7 billion, with projections indicating expansion to $20 billion by 2030, representing a compound annual growth rate of 16.3%. This growth trajectory is supported by substantial government investments, with the United States allocating $12 billion for carbon capture development through the Infrastructure Investment and Jobs Act.
The market for carbon capture sorbents specifically is witnessing heightened interest due to their critical role in improving capture efficiency and reducing operational costs. Amine-based sorbents currently dominate with roughly 65% market share, while metal-organic frameworks (MOFs) and zeolites are gaining traction with annual growth rates exceeding 20%. These advanced materials offer superior catalytic performance, which directly addresses industry demands for more efficient carbon capture solutions.
Regional analysis reveals North America leads the carbon capture market with 40% share, followed by Europe at 30% and Asia-Pacific at 25%. China has emerged as the fastest-growing market, investing heavily in research and deployment of novel sorbent technologies. The Middle East, despite its fossil fuel dependency, is increasingly adopting carbon capture technologies to maintain export competitiveness in a carbon-constrained global economy.
Industry segmentation shows power generation remains the largest application sector (45%), followed by industrial processes (30%) and natural gas processing (15%). Cement and steel manufacturing represent rapidly expanding segments due to their carbon-intensive processes and limited alternative decarbonization options.
Customer demand is increasingly focused on sorbents that demonstrate superior catalytic performance metrics, including high CO2 selectivity, rapid adsorption-desorption kinetics, and minimal energy requirements for regeneration. Market research indicates customers are willing to pay premium prices (20-30% higher) for sorbents that can reduce overall operational costs by improving energy efficiency during the capture process.
Competitive pricing analysis shows significant variation, with conventional amine-based sorbents priced at $2,000-3,000 per ton, while advanced MOFs and specialized zeolites command $5,000-8,000 per ton due to their enhanced performance characteristics. This price differential is expected to narrow as manufacturing scales and technology matures, potentially reaching price parity by 2028.
The market for carbon capture sorbents specifically is witnessing heightened interest due to their critical role in improving capture efficiency and reducing operational costs. Amine-based sorbents currently dominate with roughly 65% market share, while metal-organic frameworks (MOFs) and zeolites are gaining traction with annual growth rates exceeding 20%. These advanced materials offer superior catalytic performance, which directly addresses industry demands for more efficient carbon capture solutions.
Regional analysis reveals North America leads the carbon capture market with 40% share, followed by Europe at 30% and Asia-Pacific at 25%. China has emerged as the fastest-growing market, investing heavily in research and deployment of novel sorbent technologies. The Middle East, despite its fossil fuel dependency, is increasingly adopting carbon capture technologies to maintain export competitiveness in a carbon-constrained global economy.
Industry segmentation shows power generation remains the largest application sector (45%), followed by industrial processes (30%) and natural gas processing (15%). Cement and steel manufacturing represent rapidly expanding segments due to their carbon-intensive processes and limited alternative decarbonization options.
Customer demand is increasingly focused on sorbents that demonstrate superior catalytic performance metrics, including high CO2 selectivity, rapid adsorption-desorption kinetics, and minimal energy requirements for regeneration. Market research indicates customers are willing to pay premium prices (20-30% higher) for sorbents that can reduce overall operational costs by improving energy efficiency during the capture process.
Competitive pricing analysis shows significant variation, with conventional amine-based sorbents priced at $2,000-3,000 per ton, while advanced MOFs and specialized zeolites command $5,000-8,000 per ton due to their enhanced performance characteristics. This price differential is expected to narrow as manufacturing scales and technology matures, potentially reaching price parity by 2028.
Current Sorbent Technologies and Challenges
Carbon capture technologies have evolved significantly over the past decades, with various sorbent materials being developed to address the growing concern of carbon emissions. Currently, the market is dominated by several key sorbent technologies, each with distinct advantages and limitations in their catalytic performance for carbon capture.
Amine-based sorbents remain the most widely deployed technology, particularly monoethanolamine (MEA) which offers high CO2 absorption capacity and relatively fast kinetics. However, these materials face significant challenges including high energy requirements for regeneration (typically 3.5-4.0 GJ/tCO2), corrosion issues, and degradation over multiple cycles. The performance deterioration after repeated use necessitates frequent replacement, increasing operational costs substantially.
Metal-organic frameworks (MOFs) represent a promising alternative with exceptional surface areas exceeding 6,000 m²/g and tunable pore structures. Their catalytic performance for selective CO2 capture has shown remarkable potential in laboratory settings, achieving capacities of 1.5-2.0 mmol/g under ambient conditions. Nevertheless, MOFs struggle with stability issues in humid environments and face scalability challenges that have limited their industrial implementation.
Zeolites and activated carbons offer more mature solutions with moderate capture capacities (0.8-1.2 mmol/g) and excellent thermal stability. Their advantage lies in lower regeneration energy requirements (approximately 2.0-2.5 GJ/tCO2) compared to amine systems. However, these materials exhibit diminished performance at higher temperatures and in the presence of impurities commonly found in flue gas streams.
Emerging calcium-based sorbents demonstrate high theoretical capacities but suffer from rapid performance degradation due to sintering during the calcination-carbonation cycles. Research indicates that after just 20 cycles, capacity can decrease by up to 70%, presenting a significant barrier to commercial viability despite their low material cost.
Ionic liquids have garnered attention for their negligible vapor pressure and good thermal stability, but their high viscosity impedes mass transfer rates, resulting in slower CO2 absorption kinetics compared to conventional sorbents. Additionally, their synthesis costs remain prohibitively high for large-scale deployment.
The technical challenges across all sorbent technologies include finding the optimal balance between selectivity, capacity, and regeneration energy requirements. Most current materials excel in one or two parameters but fail to deliver comprehensive performance. Furthermore, the presence of contaminants such as SOx, NOx, and water vapor significantly impacts long-term stability and catalytic performance, creating a substantial gap between laboratory results and real-world industrial applications.
Amine-based sorbents remain the most widely deployed technology, particularly monoethanolamine (MEA) which offers high CO2 absorption capacity and relatively fast kinetics. However, these materials face significant challenges including high energy requirements for regeneration (typically 3.5-4.0 GJ/tCO2), corrosion issues, and degradation over multiple cycles. The performance deterioration after repeated use necessitates frequent replacement, increasing operational costs substantially.
Metal-organic frameworks (MOFs) represent a promising alternative with exceptional surface areas exceeding 6,000 m²/g and tunable pore structures. Their catalytic performance for selective CO2 capture has shown remarkable potential in laboratory settings, achieving capacities of 1.5-2.0 mmol/g under ambient conditions. Nevertheless, MOFs struggle with stability issues in humid environments and face scalability challenges that have limited their industrial implementation.
Zeolites and activated carbons offer more mature solutions with moderate capture capacities (0.8-1.2 mmol/g) and excellent thermal stability. Their advantage lies in lower regeneration energy requirements (approximately 2.0-2.5 GJ/tCO2) compared to amine systems. However, these materials exhibit diminished performance at higher temperatures and in the presence of impurities commonly found in flue gas streams.
Emerging calcium-based sorbents demonstrate high theoretical capacities but suffer from rapid performance degradation due to sintering during the calcination-carbonation cycles. Research indicates that after just 20 cycles, capacity can decrease by up to 70%, presenting a significant barrier to commercial viability despite their low material cost.
Ionic liquids have garnered attention for their negligible vapor pressure and good thermal stability, but their high viscosity impedes mass transfer rates, resulting in slower CO2 absorption kinetics compared to conventional sorbents. Additionally, their synthesis costs remain prohibitively high for large-scale deployment.
The technical challenges across all sorbent technologies include finding the optimal balance between selectivity, capacity, and regeneration energy requirements. Most current materials excel in one or two parameters but fail to deliver comprehensive performance. Furthermore, the presence of contaminants such as SOx, NOx, and water vapor significantly impacts long-term stability and catalytic performance, creating a substantial gap between laboratory results and real-world industrial applications.
Comparative Analysis of Sorbent Catalytic Performance
01 Metal-organic frameworks (MOFs) for carbon capture
Metal-organic frameworks (MOFs) are highly porous materials that show exceptional performance as carbon capture sorbents. Their tunable pore size, high surface area, and customizable metal centers make them effective for selective CO2 adsorption. These materials can be modified with specific functional groups to enhance their catalytic performance and CO2 binding affinity. The incorporation of open metal sites within MOFs further improves their carbon capture efficiency under various temperature and pressure conditions.- Metal-organic frameworks (MOFs) for carbon capture: Metal-organic frameworks (MOFs) are highly porous materials that can be engineered for selective carbon dioxide adsorption. These materials offer high surface area and tunable pore sizes that enhance CO2 capture efficiency. The catalytic performance of MOFs can be improved by incorporating metal centers that facilitate CO2 binding and conversion. These frameworks demonstrate promising stability under various operating conditions and can be regenerated multiple times without significant loss of performance.
- Amine-functionalized sorbents for enhanced CO2 capture: Amine-functionalized materials show excellent selectivity for CO2 capture through chemical adsorption mechanisms. These sorbents can be designed with various amine groups that form carbamates or bicarbonates upon reaction with CO2. The catalytic performance of these materials can be enhanced by optimizing the amine loading, distribution, and accessibility. Supported amine sorbents demonstrate improved heat management during adsorption-desorption cycles and can operate effectively at lower regeneration temperatures compared to traditional solvents.
- Zeolite-based catalytic sorbents for carbon capture: Zeolites offer unique molecular sieving properties that can be exploited for selective carbon dioxide capture. These aluminosilicate materials can be modified with various cations to enhance their CO2 adsorption capacity and selectivity. The catalytic performance of zeolites can be improved by controlling the Si/Al ratio and introducing specific functional groups. These materials demonstrate good thermal stability and can withstand multiple adsorption-desorption cycles, making them suitable for industrial carbon capture applications.
- Novel composite materials for dual-function capture and conversion: Composite materials combining multiple functional components can simultaneously capture CO2 and catalyze its conversion to valuable products. These materials integrate adsorption sites with catalytic centers to enable one-pot carbon capture and utilization. The catalytic performance of these composites can be enhanced by optimizing the interface between different components and controlling the spatial distribution of active sites. These innovative materials offer potential for energy-efficient carbon capture processes by reducing the need for separate capture and conversion steps.
- Regeneration and stability enhancement of carbon capture sorbents: Advanced regeneration techniques can significantly improve the long-term catalytic performance of carbon capture sorbents. These methods focus on minimizing energy requirements while maintaining sorbent integrity over multiple cycles. Stability enhancement strategies include incorporating support materials, controlling operating conditions, and adding stabilizing agents. The catalytic performance can be preserved by preventing common degradation mechanisms such as amine leaching, pore blocking, and thermal decomposition. These approaches extend sorbent lifetime and improve the economic viability of carbon capture technologies.
02 Amine-functionalized sorbents for enhanced CO2 capture
Amine-functionalized materials represent a significant advancement in carbon capture technology. These sorbents contain various amine groups that chemically bind with CO2 through carbamate formation. The catalytic performance of these materials can be enhanced by optimizing the amine loading, type of amine (primary, secondary, or tertiary), and support material characteristics. These sorbents demonstrate improved selectivity, capacity, and regeneration properties compared to conventional materials, making them suitable for post-combustion carbon capture applications.Expand Specific Solutions03 Novel composite materials with dual functionality
Composite materials combining different components offer dual functionality for carbon capture processes. These innovative sorbents integrate adsorptive properties with catalytic capabilities, enabling both CO2 capture and potential conversion in a single material. By incorporating catalytic nanoparticles into porous supports, these composites can facilitate the transformation of captured CO2 into valuable chemicals or fuels. The synergistic effect between the components enhances overall performance, stability, and reusability under industrial conditions.Expand Specific Solutions04 Temperature-responsive sorbents with controlled release
Temperature-responsive carbon capture sorbents offer significant advantages in energy efficiency during the capture-release cycle. These materials can adsorb CO2 at lower temperatures and release it when heated, with the temperature swing carefully controlled to optimize energy consumption. The catalytic performance of these sorbents is enhanced through the incorporation of specific metal centers or functional groups that modify the binding energy with CO2. This approach reduces the energy penalty associated with traditional carbon capture methods while maintaining high selectivity and capacity.Expand Specific Solutions05 Regeneration and stability enhancement techniques
Improving the regeneration capabilities and long-term stability of carbon capture sorbents is crucial for industrial applications. Various techniques have been developed to enhance the catalytic performance and durability of these materials during multiple capture-release cycles. These include structural reinforcement, surface modification to prevent degradation, and the incorporation of stabilizing agents. Advanced regeneration methods using novel heating approaches, pressure swing techniques, or combination strategies significantly reduce energy requirements while maintaining the sorbent's capture capacity over extended periods.Expand Specific Solutions
Leading Organizations in Carbon Capture Industry
Carbon capture sorbent technology is currently in a growth phase, with the market expected to expand significantly as global decarbonization efforts intensify. The global carbon capture market is projected to reach $7-10 billion by 2030, driven by climate policies and net-zero commitments. Technologically, the field shows varying maturity levels across different approaches. Research institutions like Tsinghua University, Korea Institute of Energy Research, and East China University of Science & Technology are advancing fundamental sorbent research, while commercial players demonstrate different stages of development. Climeworks has deployed commercial direct air capture plants, while energy giants like Sinopec, Shell, and PETRONAS are scaling up industrial carbon capture solutions. Specialized technology providers such as Topsoe and Susteon are bridging research-to-market gaps by developing novel sorbent materials with enhanced catalytic performance and durability for various capture applications.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed proprietary solid adsorbents for carbon capture focusing on post-combustion applications at their refineries and petrochemical facilities. Their technology employs structured porous materials including modified zeolites and metal-organic frameworks (MOFs) with tailored pore structures and surface chemistry. Sinopec's carbon capture approach utilizes a pressure/temperature swing adsorption process with CO2 adsorption occurring at near-ambient temperatures (20-40°C) and desorption through either pressure reduction or moderate temperature increase (80-120°C). Their advanced sorbents demonstrate CO2 capacities of 2-4 mmol/g with high selectivity over nitrogen and water vapor. Sinopec has implemented this technology at their Qilu Petrochemical facility, capturing over 40,000 tons of CO2 annually with reported energy consumption of 2.5-3.0 GJ/ton CO2, significantly lower than conventional amine scrubbing processes. The captured CO2 is utilized for enhanced oil recovery operations, creating a closed carbon loop within their operations. Recent developments include composite sorbents incorporating alkaline components that achieve breakthrough capacities approaching 6 mmol/g while maintaining structural integrity over 1000+ adsorption-desorption cycles.
Strengths: Lower energy requirements compared to solvent-based systems; minimal liquid waste generation; tolerance to oxygen in flue gas streams; integration with existing refinery infrastructure. Weaknesses: Potential for reduced capacity in humid conditions; requires careful pressure management for optimal performance; sorbent manufacturing at industrial scale presents quality control challenges.
Climeworks AG
Technical Solution: Climeworks has developed a direct air capture (DAC) technology using solid sorbent filters that selectively capture CO2 from ambient air. Their approach employs amine-functionalized sorbents arranged in modular air contactor units. The process works in cycles where ambient air passes through the collectors, CO2 binds to the sorbent surface, and then heat (around 100°C) is applied to release concentrated CO2 for storage or utilization. Climeworks' commercial plants (like Orca in Iceland) integrate with geothermal energy sources, using waste heat for the regeneration process while storing captured CO2 underground through mineralization in basaltic rock formations. Their sorbents demonstrate high selectivity for CO2 even at low atmospheric concentrations (approximately 400ppm) and maintain performance over thousands of adsorption-desorption cycles, with minimal degradation rates reported at less than 10% capacity loss over several years of operation.
Strengths: High selectivity for CO2 at atmospheric concentrations; modular, scalable design; integration with renewable energy sources; proven commercial deployment. Weaknesses: Relatively high energy requirements for sorbent regeneration; higher cost per ton of CO2 captured compared to point-source capture technologies; limited by geographical constraints for optimal energy integration.
Key Patents and Research in Sorbent Technology
Co2 capture sorbents with low regeneration temperature and high desorption rates
PatentPendingUS20240009613A1
Innovation
- Development of CO2 capture sorbents comprising a solid support with CO2-sorbing amine and ionic liquid, which enhances CO2 sorption and desorption characteristics, allowing for regeneration at lower temperatures and maintaining high selectivity and capacity through catalytic action.
Reduction of no2 emissions in process gases
PatentWO2025153440A1
Innovation
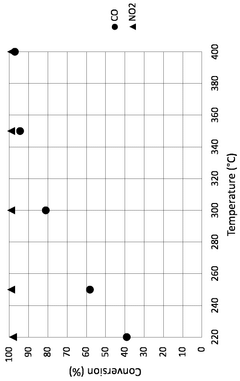
- A process combining SCR with a sulfur-resistant dual-purpose catalyst system that reduces NO2 to NO and oxidizes CO to CO2, using CO and O2 present in the gas, followed by SO2 oxidation to SO3 and condensation, achieving sub-ppm NO2 concentrations suitable for carbon capture.
Environmental Impact Assessment
The environmental impact of carbon capture sorbents extends far beyond their primary function of CO2 sequestration. Different sorbent materials exhibit varying ecological footprints throughout their lifecycle, from production to disposal. Amine-based sorbents, while effective for carbon capture, often require energy-intensive regeneration processes that can partially offset their environmental benefits. The manufacturing of these materials frequently involves toxic precursors that pose risks to ecosystems if improperly managed.
Metal-organic frameworks (MOFs) present a more complex environmental profile. Their synthesis typically requires solvents that may be environmentally harmful, yet their exceptional CO2 selectivity potentially reduces the overall energy requirements for carbon capture operations. Recent advancements in green synthesis methods for MOFs have shown promise in minimizing their environmental impact, though these approaches remain in early development stages.
Zeolites and activated carbons generally demonstrate lower environmental impacts during production compared to synthetic alternatives. Particularly noteworthy is that many activated carbon variants can be derived from waste biomass, creating a circular economy opportunity. However, their regeneration processes still consume significant energy, which must be factored into comprehensive environmental assessments.
Water consumption represents another critical environmental consideration. Aqueous amine systems typically require substantial water resources, raising concerns in water-stressed regions. Solid sorbents generally demand less water during operation but may require water-intensive manufacturing processes. This trade-off necessitates location-specific environmental impact analyses that consider regional water availability.
Land use impacts vary significantly among sorbent types. Large-scale implementation of biologically-derived sorbents could potentially compete with agricultural land, raising food security concerns. Conversely, synthetic sorbents avoid these direct land competition issues but rely on extractive industries for raw materials, which carry their own environmental burdens.
The end-of-life management of spent sorbents presents additional environmental challenges. Many current carbon capture materials lack established recycling pathways, potentially creating new waste streams. Degraded amine sorbents may release harmful compounds if improperly disposed of, while certain MOFs contain heavy metals that could leach into ecosystems. Developing closed-loop systems for sorbent recycling represents a critical research priority for improving the overall environmental sustainability of carbon capture technologies.
Metal-organic frameworks (MOFs) present a more complex environmental profile. Their synthesis typically requires solvents that may be environmentally harmful, yet their exceptional CO2 selectivity potentially reduces the overall energy requirements for carbon capture operations. Recent advancements in green synthesis methods for MOFs have shown promise in minimizing their environmental impact, though these approaches remain in early development stages.
Zeolites and activated carbons generally demonstrate lower environmental impacts during production compared to synthetic alternatives. Particularly noteworthy is that many activated carbon variants can be derived from waste biomass, creating a circular economy opportunity. However, their regeneration processes still consume significant energy, which must be factored into comprehensive environmental assessments.
Water consumption represents another critical environmental consideration. Aqueous amine systems typically require substantial water resources, raising concerns in water-stressed regions. Solid sorbents generally demand less water during operation but may require water-intensive manufacturing processes. This trade-off necessitates location-specific environmental impact analyses that consider regional water availability.
Land use impacts vary significantly among sorbent types. Large-scale implementation of biologically-derived sorbents could potentially compete with agricultural land, raising food security concerns. Conversely, synthetic sorbents avoid these direct land competition issues but rely on extractive industries for raw materials, which carry their own environmental burdens.
The end-of-life management of spent sorbents presents additional environmental challenges. Many current carbon capture materials lack established recycling pathways, potentially creating new waste streams. Degraded amine sorbents may release harmful compounds if improperly disposed of, while certain MOFs contain heavy metals that could leach into ecosystems. Developing closed-loop systems for sorbent recycling represents a critical research priority for improving the overall environmental sustainability of carbon capture technologies.
Cost-Efficiency Analysis
The economic viability of carbon capture technologies hinges significantly on their cost-efficiency profiles. When comparing various carbon capture sorbents, the total cost of ownership must be evaluated comprehensively, including initial investment, operational expenses, and long-term maintenance requirements.
Material costs represent a substantial portion of the economic equation. Traditional amine-based sorbents typically range from $2,000 to $4,000 per ton, while newer metal-organic frameworks (MOFs) can command prices between $5,000 and $20,000 per ton depending on complexity and scale of production. However, the higher initial investment in advanced materials like zeolites and activated carbon derivatives may be offset by their superior durability and regeneration capabilities.
Operational expenditures vary significantly across sorbent types. Energy consumption during the regeneration phase constitutes a critical cost factor, with temperature swing adsorption (TSA) processes requiring 2.5-4.0 GJ/ton CO2 for amine-based systems, compared to 1.8-3.2 GJ/ton CO2 for advanced porous materials. This differential can translate to substantial savings over the operational lifetime of carbon capture installations.
Sorbent degradation rates directly impact replacement frequency and associated costs. Recent studies indicate that novel composite materials exhibit degradation rates of only 0.5-2% per cycle, compared to 3-7% for conventional options. When projected across thousands of adsorption-desorption cycles, this performance advantage yields significant economic benefits despite higher upfront costs.
Scalability considerations further influence cost-efficiency profiles. Industrial-scale implementation requires evaluation of production capacity limitations and economies of scale. Current manufacturing constraints for advanced materials like functionalized MOFs restrict their cost-competitiveness, though technological advancements in synthesis methods are progressively reducing this barrier.
Levelized cost of carbon capture (LCCC) provides a standardized metric for comparison, incorporating capital expenditure, operational costs, and capture efficiency. Analysis of recent pilot projects reveals LCCC ranges of $58-120 per ton CO2 for amine-based systems, $65-140 for zeolites, and $80-180 for early-generation MOFs. However, next-generation hybrid sorbents demonstrate promising potential to reduce these figures to $40-90 per ton CO2.
The economic assessment must also account for secondary benefits, including potential revenue from captured carbon utilization pathways and regulatory incentives. Carbon pricing mechanisms, tax credits, and governmental subsidies significantly alter the cost-benefit equation across different geographical regions and regulatory frameworks.
Material costs represent a substantial portion of the economic equation. Traditional amine-based sorbents typically range from $2,000 to $4,000 per ton, while newer metal-organic frameworks (MOFs) can command prices between $5,000 and $20,000 per ton depending on complexity and scale of production. However, the higher initial investment in advanced materials like zeolites and activated carbon derivatives may be offset by their superior durability and regeneration capabilities.
Operational expenditures vary significantly across sorbent types. Energy consumption during the regeneration phase constitutes a critical cost factor, with temperature swing adsorption (TSA) processes requiring 2.5-4.0 GJ/ton CO2 for amine-based systems, compared to 1.8-3.2 GJ/ton CO2 for advanced porous materials. This differential can translate to substantial savings over the operational lifetime of carbon capture installations.
Sorbent degradation rates directly impact replacement frequency and associated costs. Recent studies indicate that novel composite materials exhibit degradation rates of only 0.5-2% per cycle, compared to 3-7% for conventional options. When projected across thousands of adsorption-desorption cycles, this performance advantage yields significant economic benefits despite higher upfront costs.
Scalability considerations further influence cost-efficiency profiles. Industrial-scale implementation requires evaluation of production capacity limitations and economies of scale. Current manufacturing constraints for advanced materials like functionalized MOFs restrict their cost-competitiveness, though technological advancements in synthesis methods are progressively reducing this barrier.
Levelized cost of carbon capture (LCCC) provides a standardized metric for comparison, incorporating capital expenditure, operational costs, and capture efficiency. Analysis of recent pilot projects reveals LCCC ranges of $58-120 per ton CO2 for amine-based systems, $65-140 for zeolites, and $80-180 for early-generation MOFs. However, next-generation hybrid sorbents demonstrate promising potential to reduce these figures to $40-90 per ton CO2.
The economic assessment must also account for secondary benefits, including potential revenue from captured carbon utilization pathways and regulatory incentives. Carbon pricing mechanisms, tax credits, and governmental subsidies significantly alter the cost-benefit equation across different geographical regions and regulatory frameworks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!