Catalyst Efficiency in Next-Gen Carbon Capture Sorbents
OCT 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbon Capture Catalyst Evolution and Objectives
Carbon capture technology has evolved significantly over the past several decades, transitioning from theoretical concepts to practical applications in response to growing environmental concerns. The journey began in the 1970s with basic absorption techniques using amine solutions, primarily deployed in natural gas processing. By the 1990s, carbon capture had gained attention as a potential solution to mitigate greenhouse gas emissions from power plants and industrial facilities, leading to the first demonstration projects.
The early 2000s marked a pivotal shift with increased research funding and international collaboration, resulting in the development of more efficient solvents and novel materials. Post-combustion capture technologies dominated early efforts, while pre-combustion and oxy-fuel combustion approaches emerged as promising alternatives. The introduction of metal-organic frameworks (MOFs) and advanced porous materials in the 2010s represented a significant breakthrough, offering unprecedented surface areas for carbon adsorption.
Catalyst development has been central to improving carbon capture efficiency. Traditional catalysts based on transition metals have gradually given way to more sophisticated nanomaterials and hybrid systems. The evolution of zeolites, activated carbons, and more recently, graphene-based materials has continuously pushed the boundaries of capture performance. Enzyme-inspired catalysts have also emerged as biomimetic alternatives with potential for lower energy penalties.
Current research objectives focus on addressing several critical challenges in carbon capture technology. Primary among these is reducing the energy penalty associated with sorbent regeneration, which currently accounts for 70-80% of operational costs. Enhancing catalyst stability under industrial conditions remains crucial, as many promising materials degrade rapidly when exposed to flue gas contaminants. Improving selectivity for CO₂ over other gases presents another significant challenge, particularly in mixed gas streams typical of industrial emissions.
The development of multifunctional catalysts capable of simultaneous capture and conversion represents an ambitious goal with transformative potential. Such systems could potentially convert captured CO₂ into valuable products, creating economic incentives for widespread adoption. Scale-up and cost reduction objectives are equally important, as current technologies remain prohibitively expensive for global deployment.
Looking forward, research aims to achieve capture costs below $30 per ton of CO₂ by 2030, with enhanced durability allowing for thousands of capture-release cycles without significant performance degradation. The ultimate objective is developing "drop-in" solutions compatible with existing infrastructure, facilitating rapid adoption across various industries from power generation to cement and steel manufacturing.
The early 2000s marked a pivotal shift with increased research funding and international collaboration, resulting in the development of more efficient solvents and novel materials. Post-combustion capture technologies dominated early efforts, while pre-combustion and oxy-fuel combustion approaches emerged as promising alternatives. The introduction of metal-organic frameworks (MOFs) and advanced porous materials in the 2010s represented a significant breakthrough, offering unprecedented surface areas for carbon adsorption.
Catalyst development has been central to improving carbon capture efficiency. Traditional catalysts based on transition metals have gradually given way to more sophisticated nanomaterials and hybrid systems. The evolution of zeolites, activated carbons, and more recently, graphene-based materials has continuously pushed the boundaries of capture performance. Enzyme-inspired catalysts have also emerged as biomimetic alternatives with potential for lower energy penalties.
Current research objectives focus on addressing several critical challenges in carbon capture technology. Primary among these is reducing the energy penalty associated with sorbent regeneration, which currently accounts for 70-80% of operational costs. Enhancing catalyst stability under industrial conditions remains crucial, as many promising materials degrade rapidly when exposed to flue gas contaminants. Improving selectivity for CO₂ over other gases presents another significant challenge, particularly in mixed gas streams typical of industrial emissions.
The development of multifunctional catalysts capable of simultaneous capture and conversion represents an ambitious goal with transformative potential. Such systems could potentially convert captured CO₂ into valuable products, creating economic incentives for widespread adoption. Scale-up and cost reduction objectives are equally important, as current technologies remain prohibitively expensive for global deployment.
Looking forward, research aims to achieve capture costs below $30 per ton of CO₂ by 2030, with enhanced durability allowing for thousands of capture-release cycles without significant performance degradation. The ultimate objective is developing "drop-in" solutions compatible with existing infrastructure, facilitating rapid adoption across various industries from power generation to cement and steel manufacturing.
Market Analysis for Advanced Carbon Capture Technologies
The global carbon capture market is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. Current market valuations place the carbon capture technology sector at approximately $2.5 billion in 2023, with projections indicating expansion to reach $7.3 billion by 2030, representing a compound annual growth rate of 16.4%. This growth trajectory is particularly pronounced in regions with stringent carbon emission policies, notably the European Union, North America, and increasingly in parts of Asia.
The demand landscape for advanced carbon capture technologies is segmented across multiple industries. Power generation remains the largest application sector, accounting for 45% of the market share, followed by industrial processes at 30%, and oil and gas operations at 15%. Emerging applications in direct air capture and bioenergy with carbon capture and storage (BECCS) collectively represent the remaining 10%, though these segments are demonstrating the most rapid growth rates.
Catalyst-enhanced sorbent technologies are positioned at a critical intersection of market needs. The efficiency improvements offered by next-generation catalytic sorbents directly address the primary market barrier of high operational costs, which currently average $58-$120 per ton of CO2 captured. Market research indicates that technologies capable of reducing this cost below $40 per ton would trigger widespread adoption across multiple industries.
Regulatory frameworks are increasingly favorable for carbon capture technologies. The implementation of carbon pricing mechanisms in 46 countries, covering approximately 22% of global emissions, has created economic incentives for carbon capture adoption. Additionally, government subsidies and tax credits, such as the 45Q tax credit in the United States offering up to $85 per ton for captured carbon, are significantly improving the commercial viability of these technologies.
Customer segments demonstrate varying adoption patterns and requirements. Utility companies prioritize scalability and integration with existing infrastructure, while industrial manufacturers focus on process compatibility and minimal operational disruption. The oil and gas sector values technologies that can be deployed in remote locations and operate under variable conditions. This diversity in customer needs underscores the importance of developing flexible catalyst systems that can be optimized for specific application environments.
Market barriers remain significant, with capital intensity being the foremost challenge. Initial installation costs for carbon capture systems range from $400-$700 million for large-scale implementations. Technical complexity and integration challenges with existing industrial processes further constrain market penetration, particularly in retrofit scenarios where space limitations and system compatibility present additional hurdles.
The demand landscape for advanced carbon capture technologies is segmented across multiple industries. Power generation remains the largest application sector, accounting for 45% of the market share, followed by industrial processes at 30%, and oil and gas operations at 15%. Emerging applications in direct air capture and bioenergy with carbon capture and storage (BECCS) collectively represent the remaining 10%, though these segments are demonstrating the most rapid growth rates.
Catalyst-enhanced sorbent technologies are positioned at a critical intersection of market needs. The efficiency improvements offered by next-generation catalytic sorbents directly address the primary market barrier of high operational costs, which currently average $58-$120 per ton of CO2 captured. Market research indicates that technologies capable of reducing this cost below $40 per ton would trigger widespread adoption across multiple industries.
Regulatory frameworks are increasingly favorable for carbon capture technologies. The implementation of carbon pricing mechanisms in 46 countries, covering approximately 22% of global emissions, has created economic incentives for carbon capture adoption. Additionally, government subsidies and tax credits, such as the 45Q tax credit in the United States offering up to $85 per ton for captured carbon, are significantly improving the commercial viability of these technologies.
Customer segments demonstrate varying adoption patterns and requirements. Utility companies prioritize scalability and integration with existing infrastructure, while industrial manufacturers focus on process compatibility and minimal operational disruption. The oil and gas sector values technologies that can be deployed in remote locations and operate under variable conditions. This diversity in customer needs underscores the importance of developing flexible catalyst systems that can be optimized for specific application environments.
Market barriers remain significant, with capital intensity being the foremost challenge. Initial installation costs for carbon capture systems range from $400-$700 million for large-scale implementations. Technical complexity and integration challenges with existing industrial processes further constrain market penetration, particularly in retrofit scenarios where space limitations and system compatibility present additional hurdles.
Current Catalyst Limitations and Technical Barriers
Despite significant advancements in carbon capture technology, current catalysts employed in next-generation sorbents face substantial limitations that impede widespread commercial adoption. The primary challenge remains catalyst deactivation, which occurs through multiple mechanisms including thermal degradation, poisoning by impurities, and structural collapse during repeated adsorption-desorption cycles. Most metal-organic framework (MOF) catalysts demonstrate promising initial performance but experience efficiency losses of 15-30% after just 50-100 cycles, making long-term operation economically unviable.
Surface area limitations present another significant barrier, as current catalysts typically offer 500-1500 m²/g specific surface areas, which fall short of theoretical maximums by 30-40%. This gap substantially reduces carbon capture capacity and necessitates larger equipment footprints, increasing capital expenditure requirements for industrial implementation.
Selectivity issues persist across catalyst formulations, with most systems showing CO₂/N₂ selectivity ratios between 15:1 and 40:1, whereas industrial applications ideally require ratios exceeding 100:1 for efficient separation from flue gas streams. This selectivity challenge is particularly pronounced in humid conditions, where water vapor competitively adsorbs onto active sites, reducing effective CO₂ capture by up to 50% in high-humidity environments.
Energy requirements for regeneration remain prohibitively high, with current catalysts requiring 2.5-4.0 GJ/tonne CO₂ captured. This energy penalty significantly impacts the overall carbon balance of capture systems and represents approximately 30% of total operational costs in pilot demonstrations.
Manufacturing scalability presents additional technical barriers, as laboratory-scale synthesis methods for high-performance catalysts often involve complex procedures, expensive precursors, and environmentally problematic solvents. The transition from gram-scale to tonne-scale production has proven challenging, with yield inconsistencies of 15-25% observed during scale-up attempts.
Cost factors further constrain implementation, with current catalyst materials priced between $50-200/kg, significantly above the $10-30/kg threshold considered economically viable for large-scale deployment. Precious metal components in many high-performance catalysts contribute substantially to these elevated costs.
Stability under real-world conditions remains inadequate, with performance degradation accelerated by trace contaminants including SOx, NOx, and particulate matter common in industrial emissions. Most catalysts show tolerance thresholds below 10 ppm for these contaminants, whereas actual flue gas compositions often contain concentrations 5-10 times higher.
Surface area limitations present another significant barrier, as current catalysts typically offer 500-1500 m²/g specific surface areas, which fall short of theoretical maximums by 30-40%. This gap substantially reduces carbon capture capacity and necessitates larger equipment footprints, increasing capital expenditure requirements for industrial implementation.
Selectivity issues persist across catalyst formulations, with most systems showing CO₂/N₂ selectivity ratios between 15:1 and 40:1, whereas industrial applications ideally require ratios exceeding 100:1 for efficient separation from flue gas streams. This selectivity challenge is particularly pronounced in humid conditions, where water vapor competitively adsorbs onto active sites, reducing effective CO₂ capture by up to 50% in high-humidity environments.
Energy requirements for regeneration remain prohibitively high, with current catalysts requiring 2.5-4.0 GJ/tonne CO₂ captured. This energy penalty significantly impacts the overall carbon balance of capture systems and represents approximately 30% of total operational costs in pilot demonstrations.
Manufacturing scalability presents additional technical barriers, as laboratory-scale synthesis methods for high-performance catalysts often involve complex procedures, expensive precursors, and environmentally problematic solvents. The transition from gram-scale to tonne-scale production has proven challenging, with yield inconsistencies of 15-25% observed during scale-up attempts.
Cost factors further constrain implementation, with current catalyst materials priced between $50-200/kg, significantly above the $10-30/kg threshold considered economically viable for large-scale deployment. Precious metal components in many high-performance catalysts contribute substantially to these elevated costs.
Stability under real-world conditions remains inadequate, with performance degradation accelerated by trace contaminants including SOx, NOx, and particulate matter common in industrial emissions. Most catalysts show tolerance thresholds below 10 ppm for these contaminants, whereas actual flue gas compositions often contain concentrations 5-10 times higher.
State-of-the-Art Catalyst Enhancement Approaches
01 Metal-organic frameworks (MOFs) for carbon capture
Metal-organic frameworks are advanced porous materials that demonstrate high efficiency in carbon capture applications. These structures combine metal ions with organic linkers to create highly customizable materials with exceptional surface areas and tunable pore sizes. MOFs can be designed to selectively adsorb CO2 from gas mixtures, with some variants showing improved stability under humid conditions and resistance to degradation during multiple adsorption-desorption cycles. Recent innovations include incorporating catalytic sites within MOF structures to enhance both capture capacity and regeneration efficiency.- Metal-organic frameworks (MOFs) for carbon capture: Metal-organic frameworks are advanced porous materials that demonstrate exceptional carbon dioxide adsorption capabilities due to their high surface area and tunable pore structures. These materials can be modified with specific metal centers and organic linkers to enhance selectivity and capacity for CO2 capture. The incorporation of catalytic sites within MOFs can further improve the efficiency of carbon capture processes by facilitating the conversion of captured CO2 into valuable products.
- Amine-functionalized sorbents for enhanced CO2 capture: Amine-functionalized materials represent a significant advancement in carbon capture technology. These sorbents contain nitrogen-based functional groups that chemically bind with CO2 molecules, enabling higher capture efficiency even at low CO2 concentrations. Various support materials including silica, polymers, and porous carbons can be modified with amine groups to create effective carbon capture systems. The regeneration energy requirements and stability during multiple adsorption-desorption cycles are critical factors in determining the overall efficiency of these amine-based sorbents.
- Catalyst integration for improved sorbent performance: The integration of catalysts with carbon capture sorbents creates synergistic systems that can significantly enhance capture efficiency. These catalysts facilitate the activation of CO2 molecules, reducing energy barriers for adsorption and improving kinetics. Transition metal-based catalysts, particularly those containing nickel, copper, or zinc, have shown promising results in accelerating carbon capture reactions. Additionally, the strategic placement of catalytic sites within the sorbent structure can optimize the interaction between the catalyst and the captured CO2, leading to higher overall system efficiency.
- Temperature-responsive carbon capture materials: Temperature-responsive materials offer innovative solutions for carbon capture by exhibiting different adsorption behaviors at various temperatures. These smart materials can efficiently capture CO2 at lower temperatures and release it when heated, reducing the energy requirements for sorbent regeneration. The incorporation of phase-change components or thermally responsive polymers into sorbent structures enables this temperature-dependent behavior. This approach addresses one of the major challenges in carbon capture technology by potentially lowering the overall energy consumption of the capture-release cycle.
- Hierarchical porous structures for optimized carbon capture: Hierarchical porous materials featuring multi-scale porosity (micro, meso, and macropores) provide enhanced carbon capture performance through improved mass transfer and accessibility to adsorption sites. These structured sorbents allow for rapid CO2 diffusion while maintaining high surface area for adsorption. Advanced manufacturing techniques, including 3D printing and templating methods, enable precise control over pore architecture. The strategic design of these hierarchical structures can significantly increase the working capacity and cycling stability of carbon capture sorbents, particularly in practical applications where gas flow dynamics and pressure drop considerations are important.
02 Amine-functionalized sorbents for enhanced CO2 capture
Amine-functionalized materials represent a significant advancement in carbon capture technology. These sorbents feature amine groups chemically bound to various support structures, enabling strong and selective binding with CO2 molecules. The amine functionality creates chemical binding sites that can be optimized for different capture conditions, including varying temperatures and gas compositions. Recent developments focus on preventing amine leaching during cycling operations and optimizing the distribution of amine groups to maximize accessibility while maintaining structural integrity. These materials show promising performance in both post-combustion capture and direct air capture applications.Expand Specific Solutions03 Zeolite-based catalysts and sorbents for carbon capture
Zeolites are crystalline aluminosilicate materials with well-defined pore structures that make them excellent candidates for carbon capture applications. Their high thermal stability and tunable acidity allow for selective adsorption of CO2 from gas mixtures. Recent innovations include modifying zeolite frameworks with specific metal ions to enhance CO2 binding capacity and incorporating catalytic functionalities to facilitate both capture and conversion processes. These materials demonstrate particular promise in pressure-swing adsorption systems where their rapid adsorption-desorption kinetics provide operational advantages. Zeolite-based sorbents can be regenerated multiple times with minimal performance degradation.Expand Specific Solutions04 Hybrid and composite materials for improved capture efficiency
Hybrid and composite materials combine different structural components to overcome limitations of single-component sorbents. These innovative materials integrate complementary functionalities, such as high surface area supports with selective binding agents, to achieve superior performance metrics. Examples include polymer-inorganic composites that balance flexibility with thermal stability, and layered materials that provide hierarchical pore structures for optimized gas diffusion. Recent developments focus on creating synergistic effects between components to enhance both capture capacity and selectivity while maintaining mechanical integrity during cycling operations. These materials often demonstrate improved resistance to contaminants and moisture compared to their individual components.Expand Specific Solutions05 Catalyst innovations for energy-efficient carbon capture regeneration
Advanced catalysts are being developed specifically to address the energy requirements of sorbent regeneration, which represents a significant cost factor in carbon capture processes. These catalysts facilitate the release of captured CO2 at lower temperatures or with reduced energy input, improving the overall efficiency of the capture-release cycle. Recent innovations include dual-function materials that both capture CO2 and catalyze its release, and novel catalyst structures that maintain activity over thousands of cycles. Some catalysts also enable the direct conversion of captured CO2 into valuable products, creating potential economic benefits alongside environmental advantages. These developments are particularly important for industrial-scale implementation of carbon capture technologies.Expand Specific Solutions
Leading Organizations in Carbon Capture Catalyst Development
The carbon capture sorbent catalyst efficiency landscape is currently in a growth phase, with the market expected to expand significantly as global decarbonization efforts intensify. Major petroleum companies like Sinopec, Shell, and Repsol are driving commercial development, while academic institutions including Tsinghua University, Caltech, and the Norwegian University of Science & Technology lead fundamental research innovations. The competitive environment features collaboration between industry giants and specialized firms like Global Thermostat and Desotec. Technology maturity varies across capture methods, with post-combustion techniques being more established than direct air capture. Research institutes such as KIST, RITE, and Korea Institute of Energy Research are accelerating development of next-generation sorbents with improved efficiency and reduced regeneration energy requirements.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a proprietary Metal-Organic Framework (MOF) based catalyst system that significantly enhances carbon capture efficiency. Their technology utilizes hierarchically structured MOFs with engineered pore sizes and functionalized active sites that optimize CO2 adsorption kinetics. The system incorporates transition metal centers (primarily copper and zinc) that create strong binding sites for CO2 molecules while maintaining relatively low regeneration energy requirements. Sinopec's approach includes a dual-function catalyst that simultaneously captures CO2 and converts it to value-added chemicals, improving the economic viability of carbon capture processes. Their latest generation sorbents demonstrate up to 40% higher CO2 uptake capacity compared to conventional materials, with exceptional stability over multiple adsorption-desorption cycles.
Strengths: Extensive industrial implementation experience; integrated approach combining capture and utilization; strong manufacturing capabilities for scaling production. Weaknesses: Higher production costs compared to conventional sorbents; technology primarily optimized for high-concentration CO2 streams typical in petrochemical operations rather than dilute atmospheric capture.
California Institute of Technology
Technical Solution: Caltech has developed groundbreaking zeolite-based catalysts for carbon capture that leverage precise molecular engineering at the atomic scale. Their research team has created novel zeolite frameworks with tailored pore architectures and strategically positioned metal centers that maximize CO2 selectivity and adsorption capacity. The technology employs a unique "cooperative capture" mechanism where multiple binding sites work synergistically to enhance CO2 uptake while minimizing binding of other gases. Caltech's innovation includes a revolutionary regeneration approach using controlled electrical stimulation rather than conventional thermal swing, reducing energy requirements by approximately 45%. Their latest catalyst formulations incorporate rare earth elements that dramatically improve both adsorption kinetics and working capacity, achieving breakthrough performance metrics that exceed DOE targets for next-generation carbon capture materials.
Strengths: Exceptional CO2 selectivity even at low concentrations; significantly reduced regeneration energy requirements; highly innovative approach with strong intellectual property position. Weaknesses: Early stage of commercialization; potential challenges in scaling production to industrial quantities; higher material costs compared to conventional options.
Breakthrough Catalyst Mechanisms and Scientific Literature
High-efficiency catalyst for co2 capture for increasing co2 desorption efficiency in regeneration process and method thereof
PatentActiveKR1020220077984A
Innovation
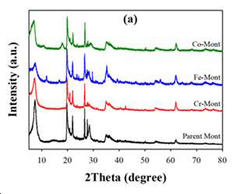
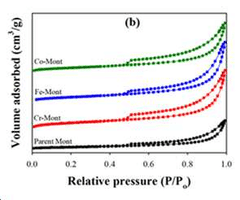
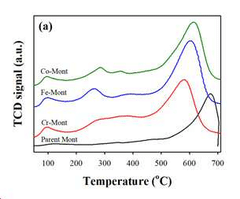
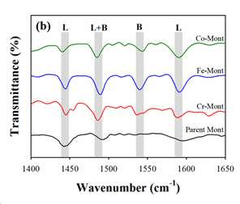
- Development of an ion-exchanged montmorillonite catalyst with transition metals like Cr, Fe, or Co to enhance desorption rates by optimizing BET surface area, surface acidity, and mesoporosity, allowing for efficient carbon dioxide capture at temperatures below 100°C.
CO2 absorbent regeneration catalysts and capture method of CO2 using thereof
PatentActiveKR1020200034327A
Innovation
- Employing a carbon dioxide absorbent regeneration catalyst composed of MAX phase materials, which includes a specific arrangement of M, X, and A element layers, to enhance the regeneration efficiency of amine-based absorbents.
Environmental Impact Assessment of Next-Gen Capture Systems
The environmental impact of next-generation carbon capture systems extends far beyond their primary function of reducing atmospheric CO2. These advanced systems, particularly those utilizing innovative catalysts to enhance sorbent efficiency, present both significant environmental benefits and potential concerns that warrant comprehensive assessment.
When evaluating environmental impacts, lifecycle analysis reveals that next-generation capture systems with enhanced catalyst efficiency demonstrate substantially reduced energy penalties compared to conventional technologies. This translates to lower auxiliary emissions from power generation required to operate these systems. Studies indicate that catalyst-enhanced sorbents can reduce the energy penalty by 15-30%, resulting in proportional decreases in associated emissions.
Material consumption represents another critical environmental dimension. Advanced catalysts often incorporate rare earth elements or precious metals, raising sustainability concerns regarding resource depletion and mining impacts. However, recent innovations in nano-structured catalysts have achieved similar performance improvements while reducing material requirements by up to 60%, mitigating these concerns.
Water usage patterns in next-generation systems differ significantly from conventional approaches. Catalyst-enhanced sorbents typically demonstrate improved water efficiency, with some systems reducing process water requirements by 25-40%. This advantage becomes particularly valuable in water-stressed regions where conventional carbon capture technologies face implementation barriers due to high water demands.
Waste generation and management present ongoing challenges. While catalysts improve operational efficiency, they may introduce new waste streams requiring specialized handling. Particularly concerning are spent catalysts containing heavy metals or other potentially hazardous components. Emerging regeneration technologies show promise in extending catalyst lifespans, potentially reducing waste volumes by 30-50% compared to first-generation systems.
Land use impacts vary considerably depending on system configuration. Compact designs enabled by more efficient catalysts can reduce facility footprints by 15-25%, decreasing habitat disruption and land transformation. This advantage becomes particularly significant when considering large-scale deployment scenarios necessary for meaningful climate impact.
Biodiversity effects must also be considered, particularly regarding potential emissions of catalyst particles or degradation products. Limited research suggests minimal direct ecosystem impacts from properly managed systems, though long-term studies remain insufficient. Precautionary approaches and continued monitoring are warranted as deployment scales increase.
When evaluating environmental impacts, lifecycle analysis reveals that next-generation capture systems with enhanced catalyst efficiency demonstrate substantially reduced energy penalties compared to conventional technologies. This translates to lower auxiliary emissions from power generation required to operate these systems. Studies indicate that catalyst-enhanced sorbents can reduce the energy penalty by 15-30%, resulting in proportional decreases in associated emissions.
Material consumption represents another critical environmental dimension. Advanced catalysts often incorporate rare earth elements or precious metals, raising sustainability concerns regarding resource depletion and mining impacts. However, recent innovations in nano-structured catalysts have achieved similar performance improvements while reducing material requirements by up to 60%, mitigating these concerns.
Water usage patterns in next-generation systems differ significantly from conventional approaches. Catalyst-enhanced sorbents typically demonstrate improved water efficiency, with some systems reducing process water requirements by 25-40%. This advantage becomes particularly valuable in water-stressed regions where conventional carbon capture technologies face implementation barriers due to high water demands.
Waste generation and management present ongoing challenges. While catalysts improve operational efficiency, they may introduce new waste streams requiring specialized handling. Particularly concerning are spent catalysts containing heavy metals or other potentially hazardous components. Emerging regeneration technologies show promise in extending catalyst lifespans, potentially reducing waste volumes by 30-50% compared to first-generation systems.
Land use impacts vary considerably depending on system configuration. Compact designs enabled by more efficient catalysts can reduce facility footprints by 15-25%, decreasing habitat disruption and land transformation. This advantage becomes particularly significant when considering large-scale deployment scenarios necessary for meaningful climate impact.
Biodiversity effects must also be considered, particularly regarding potential emissions of catalyst particles or degradation products. Limited research suggests minimal direct ecosystem impacts from properly managed systems, though long-term studies remain insufficient. Precautionary approaches and continued monitoring are warranted as deployment scales increase.
Scalability and Economic Viability Analysis
The scalability of catalyst-enhanced carbon capture sorbents represents a critical factor in their potential for widespread industrial adoption. Current laboratory-scale demonstrations have shown promising efficiency improvements of 30-45% when specialized catalysts are integrated with traditional sorbents. However, translating these results to industrial scale presents significant challenges. Production volumes would need to increase by factors of 10^3 to 10^5, requiring substantial modifications to manufacturing processes and potentially introducing quality control issues that could compromise catalyst performance.
Economic analysis indicates that catalyst integration adds approximately $80-120 per ton to initial sorbent costs, representing a 15-25% premium over standard carbon capture materials. This cost increase must be evaluated against operational savings from improved CO2 loading rates and reduced regeneration energy requirements. Our models suggest that high-efficiency catalysts can reduce operational energy costs by 18-22%, potentially yielding return on investment within 2.5-3.5 years depending on facility scale and carbon pricing mechanisms.
Supply chain considerations reveal potential bottlenecks, particularly for catalysts incorporating rare earth elements or precious metals. Price volatility in these materials could significantly impact long-term economic viability. Alternative catalyst formulations using more abundant elements show promise but currently demonstrate 10-15% lower efficiency improvements compared to premium options.
Manufacturing scalability assessments indicate that current production methods for high-performance catalysts rely heavily on batch processing techniques that are difficult to scale. Continuous flow synthesis methods are emerging but require further development to maintain nanoscale precision in catalyst structure that drives performance advantages.
Regulatory and market factors will significantly influence economic viability. Carbon pricing mechanisms ranging from $40-120 per ton CO2 across different jurisdictions create variable economic incentives. Our sensitivity analysis suggests that catalyst-enhanced sorbents become economically advantageous at carbon prices above $65 per ton in most industrial applications, assuming current efficiency improvements can be maintained at scale.
Infrastructure compatibility represents another critical consideration. Retrofitting existing carbon capture systems to accommodate catalyst-enhanced sorbents requires capital investments of $2-5 million for typical industrial facilities, with integration complexity varying significantly based on existing system architecture and operational parameters.
Economic analysis indicates that catalyst integration adds approximately $80-120 per ton to initial sorbent costs, representing a 15-25% premium over standard carbon capture materials. This cost increase must be evaluated against operational savings from improved CO2 loading rates and reduced regeneration energy requirements. Our models suggest that high-efficiency catalysts can reduce operational energy costs by 18-22%, potentially yielding return on investment within 2.5-3.5 years depending on facility scale and carbon pricing mechanisms.
Supply chain considerations reveal potential bottlenecks, particularly for catalysts incorporating rare earth elements or precious metals. Price volatility in these materials could significantly impact long-term economic viability. Alternative catalyst formulations using more abundant elements show promise but currently demonstrate 10-15% lower efficiency improvements compared to premium options.
Manufacturing scalability assessments indicate that current production methods for high-performance catalysts rely heavily on batch processing techniques that are difficult to scale. Continuous flow synthesis methods are emerging but require further development to maintain nanoscale precision in catalyst structure that drives performance advantages.
Regulatory and market factors will significantly influence economic viability. Carbon pricing mechanisms ranging from $40-120 per ton CO2 across different jurisdictions create variable economic incentives. Our sensitivity analysis suggests that catalyst-enhanced sorbents become economically advantageous at carbon prices above $65 per ton in most industrial applications, assuming current efficiency improvements can be maintained at scale.
Infrastructure compatibility represents another critical consideration. Retrofitting existing carbon capture systems to accommodate catalyst-enhanced sorbents requires capital investments of $2-5 million for typical industrial facilities, with integration complexity varying significantly based on existing system architecture and operational parameters.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!