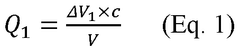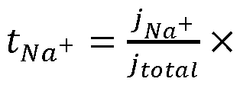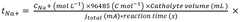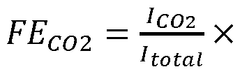Electrochemical Mechanisms within Carbon Capture Sorbents
OCT 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbon Capture Electrochemical Fundamentals and Objectives
Carbon capture technology has evolved significantly over the past decades, transitioning from theoretical concepts to practical applications in response to growing climate change concerns. The electrochemical mechanisms within carbon capture sorbents represent a cutting-edge approach that combines principles from electrochemistry, materials science, and chemical engineering to enhance CO2 capture efficiency and reduce energy requirements for the regeneration process.
The historical trajectory of electrochemical carbon capture began in the late 1990s with rudimentary experiments on electrode-assisted absorption. By the early 2000s, researchers had begun exploring the potential of applying electrical potential to modify sorbent properties during capture and release cycles. The field gained significant momentum after 2010 when concerns about climate change accelerated research into more efficient carbon capture technologies.
Current electrochemical carbon capture technologies primarily operate through three fundamental mechanisms: electrochemically-mediated amine regeneration (EMAR), electrochemically-driven phase change, and electrochemical pH-swing processes. Each mechanism leverages electrical energy to manipulate the binding affinity between sorbent materials and CO2 molecules, offering potentially lower energy penalties compared to conventional thermal regeneration methods.
The technical objectives for advancing electrochemical carbon capture sorbents include developing materials with higher selectivity for CO2 over other gases, improving the kinetics of both capture and release processes, and enhancing the cyclability of sorbent materials under repeated electrochemical cycling. Additionally, reducing the electrical resistance within the system and minimizing parasitic reactions remain critical challenges to overcome.
Recent breakthroughs in conductive polymers, metal-organic frameworks (MOFs), and carbon-based nanomaterials have opened new avenues for designing electrochemically responsive sorbents. These materials can undergo reversible changes in their electronic structure, surface chemistry, or physical conformation upon application of electrical potential, directly influencing their CO2 binding properties.
The integration of electrochemical principles into carbon capture systems aims to achieve several key performance targets: reducing regeneration energy to below 1 GJ/tonne CO2, achieving capture rates exceeding 90% from flue gas streams, maintaining sorbent performance over thousands of cycles, and developing systems that can operate effectively across various industrial settings including power plants, cement factories, and direct air capture applications.
Looking forward, the technological trajectory points toward hybrid systems that combine electrochemical approaches with other capture methods, smart materials that can respond to multiple stimuli, and integrated systems that can convert captured CO2 directly into valuable products through further electrochemical processing, creating a closed-loop carbon utilization pathway.
The historical trajectory of electrochemical carbon capture began in the late 1990s with rudimentary experiments on electrode-assisted absorption. By the early 2000s, researchers had begun exploring the potential of applying electrical potential to modify sorbent properties during capture and release cycles. The field gained significant momentum after 2010 when concerns about climate change accelerated research into more efficient carbon capture technologies.
Current electrochemical carbon capture technologies primarily operate through three fundamental mechanisms: electrochemically-mediated amine regeneration (EMAR), electrochemically-driven phase change, and electrochemical pH-swing processes. Each mechanism leverages electrical energy to manipulate the binding affinity between sorbent materials and CO2 molecules, offering potentially lower energy penalties compared to conventional thermal regeneration methods.
The technical objectives for advancing electrochemical carbon capture sorbents include developing materials with higher selectivity for CO2 over other gases, improving the kinetics of both capture and release processes, and enhancing the cyclability of sorbent materials under repeated electrochemical cycling. Additionally, reducing the electrical resistance within the system and minimizing parasitic reactions remain critical challenges to overcome.
Recent breakthroughs in conductive polymers, metal-organic frameworks (MOFs), and carbon-based nanomaterials have opened new avenues for designing electrochemically responsive sorbents. These materials can undergo reversible changes in their electronic structure, surface chemistry, or physical conformation upon application of electrical potential, directly influencing their CO2 binding properties.
The integration of electrochemical principles into carbon capture systems aims to achieve several key performance targets: reducing regeneration energy to below 1 GJ/tonne CO2, achieving capture rates exceeding 90% from flue gas streams, maintaining sorbent performance over thousands of cycles, and developing systems that can operate effectively across various industrial settings including power plants, cement factories, and direct air capture applications.
Looking forward, the technological trajectory points toward hybrid systems that combine electrochemical approaches with other capture methods, smart materials that can respond to multiple stimuli, and integrated systems that can convert captured CO2 directly into valuable products through further electrochemical processing, creating a closed-loop carbon utilization pathway.
Market Analysis for Carbon Capture Technologies
The global carbon capture market is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. As of 2023, the market was valued at approximately $7.5 billion, with projections indicating expansion to reach $20 billion by 2030, representing a compound annual growth rate of 15.2%. This growth trajectory is primarily fueled by governmental carbon reduction targets established under the Paris Agreement and subsequent climate accords.
The market segmentation for carbon capture technologies reveals distinct categories based on application sectors. Power generation currently dominates with 45% market share, followed by industrial processes (30%), natural gas processing (15%), and emerging applications (10%). Within these segments, post-combustion capture technologies hold the largest market share at 60%, while pre-combustion and oxy-fuel combustion technologies account for 25% and 15% respectively.
Electrochemical mechanisms within carbon capture sorbents represent a rapidly growing subsegment, with current market penetration of approximately 8% but showing the highest growth rate among all carbon capture approaches at 22% annually. This accelerated adoption is attributed to their enhanced energy efficiency and reduced operational costs compared to traditional amine-based systems.
Regional analysis indicates North America leads the market with 35% share, followed by Europe (30%), Asia-Pacific (25%), and rest of the world (10%). However, the Asia-Pacific region demonstrates the fastest growth rate at 18% annually, driven primarily by China's aggressive decarbonization policies and substantial investments in clean energy infrastructure.
Customer segmentation reveals three primary buyer categories: large industrial emitters (65% of market demand), utility companies (25%), and government/research institutions (10%). The purchasing decision factors vary significantly across these segments, with cost efficiency being paramount for industrial users, while regulatory compliance drives utility company adoption.
The pricing structure for electrochemical carbon capture solutions ranges from $40-80 per ton of CO2 captured, representing a 30% premium over conventional technologies but offering 40% lower operational costs over a typical 15-year installation lifecycle. This favorable total cost of ownership is accelerating market penetration despite higher initial capital requirements.
Market barriers include high upfront investment costs, technological complexity requiring specialized expertise, and infrastructure limitations for carbon transport and storage. Nevertheless, the market demonstrates strong growth potential as technological advancements in electrochemical mechanisms continue to improve efficiency and reduce implementation costs.
The market segmentation for carbon capture technologies reveals distinct categories based on application sectors. Power generation currently dominates with 45% market share, followed by industrial processes (30%), natural gas processing (15%), and emerging applications (10%). Within these segments, post-combustion capture technologies hold the largest market share at 60%, while pre-combustion and oxy-fuel combustion technologies account for 25% and 15% respectively.
Electrochemical mechanisms within carbon capture sorbents represent a rapidly growing subsegment, with current market penetration of approximately 8% but showing the highest growth rate among all carbon capture approaches at 22% annually. This accelerated adoption is attributed to their enhanced energy efficiency and reduced operational costs compared to traditional amine-based systems.
Regional analysis indicates North America leads the market with 35% share, followed by Europe (30%), Asia-Pacific (25%), and rest of the world (10%). However, the Asia-Pacific region demonstrates the fastest growth rate at 18% annually, driven primarily by China's aggressive decarbonization policies and substantial investments in clean energy infrastructure.
Customer segmentation reveals three primary buyer categories: large industrial emitters (65% of market demand), utility companies (25%), and government/research institutions (10%). The purchasing decision factors vary significantly across these segments, with cost efficiency being paramount for industrial users, while regulatory compliance drives utility company adoption.
The pricing structure for electrochemical carbon capture solutions ranges from $40-80 per ton of CO2 captured, representing a 30% premium over conventional technologies but offering 40% lower operational costs over a typical 15-year installation lifecycle. This favorable total cost of ownership is accelerating market penetration despite higher initial capital requirements.
Market barriers include high upfront investment costs, technological complexity requiring specialized expertise, and infrastructure limitations for carbon transport and storage. Nevertheless, the market demonstrates strong growth potential as technological advancements in electrochemical mechanisms continue to improve efficiency and reduce implementation costs.
Current Sorbent Technologies and Challenges
Carbon capture sorbent technologies have evolved significantly over the past decades, with several categories emerging as frontrunners in addressing CO2 emissions. Physical sorbents, including activated carbon, zeolites, and metal-organic frameworks (MOFs), operate primarily through physical adsorption mechanisms. Chemical sorbents, such as amine-based materials, metal oxides, and hydroxides, function via chemical reactions with CO2. Hybrid systems combining both physical and chemical capture mechanisms have also gained traction for their enhanced performance characteristics.
Despite considerable progress, current carbon capture sorbent technologies face substantial challenges. Capacity limitations remain a primary concern, with most commercial sorbents achieving only 2-4 mmol/g CO2 uptake under practical conditions. Selectivity issues persist, particularly in flue gas environments containing multiple competing gases like SOx, NOx, and water vapor, which can significantly reduce CO2 capture efficiency.
Regeneration energy requirements present another major hurdle. Conventional amine-based sorbents typically demand 3-4 GJ/ton CO2 for regeneration, making the process economically prohibitive for widespread implementation. This high energy penalty directly impacts the commercial viability of carbon capture technologies across various industrial sectors.
Stability and durability concerns further complicate deployment scenarios. Many promising sorbents exhibit performance degradation after multiple adsorption-desorption cycles, with some materials losing up to 30% capacity within just 50 cycles. Chemical degradation, physical attrition, and thermal decomposition contribute to this performance decline, necessitating frequent and costly material replacement.
The electrochemical mechanisms within carbon capture sorbents represent an emerging frontier that could potentially address these limitations. Recent research has demonstrated that applying electrical potential can significantly enhance CO2 binding strength in certain materials or facilitate controlled release during regeneration. Electrochemically-mediated adsorption has shown promise in reducing regeneration energy requirements by up to 40% compared to conventional thermal approaches.
Scale-up challenges remain formidable for all sorbent technologies. Laboratory-scale successes often fail to translate to industrial implementation due to issues with mass transfer limitations, pressure drop considerations, and heat management in large-scale systems. Manufacturing constraints further complicate commercialization efforts, with many advanced materials requiring complex synthesis procedures that prove challenging to scale economically.
Cost factors ultimately determine commercial viability, with current estimates placing carbon capture costs using state-of-the-art sorbents at $40-80 per ton CO2 captured. This range exceeds the economic threshold for widespread adoption in most industries, highlighting the critical need for breakthrough technologies that can dramatically reduce both capital and operational expenditures.
Despite considerable progress, current carbon capture sorbent technologies face substantial challenges. Capacity limitations remain a primary concern, with most commercial sorbents achieving only 2-4 mmol/g CO2 uptake under practical conditions. Selectivity issues persist, particularly in flue gas environments containing multiple competing gases like SOx, NOx, and water vapor, which can significantly reduce CO2 capture efficiency.
Regeneration energy requirements present another major hurdle. Conventional amine-based sorbents typically demand 3-4 GJ/ton CO2 for regeneration, making the process economically prohibitive for widespread implementation. This high energy penalty directly impacts the commercial viability of carbon capture technologies across various industrial sectors.
Stability and durability concerns further complicate deployment scenarios. Many promising sorbents exhibit performance degradation after multiple adsorption-desorption cycles, with some materials losing up to 30% capacity within just 50 cycles. Chemical degradation, physical attrition, and thermal decomposition contribute to this performance decline, necessitating frequent and costly material replacement.
The electrochemical mechanisms within carbon capture sorbents represent an emerging frontier that could potentially address these limitations. Recent research has demonstrated that applying electrical potential can significantly enhance CO2 binding strength in certain materials or facilitate controlled release during regeneration. Electrochemically-mediated adsorption has shown promise in reducing regeneration energy requirements by up to 40% compared to conventional thermal approaches.
Scale-up challenges remain formidable for all sorbent technologies. Laboratory-scale successes often fail to translate to industrial implementation due to issues with mass transfer limitations, pressure drop considerations, and heat management in large-scale systems. Manufacturing constraints further complicate commercialization efforts, with many advanced materials requiring complex synthesis procedures that prove challenging to scale economically.
Cost factors ultimately determine commercial viability, with current estimates placing carbon capture costs using state-of-the-art sorbents at $40-80 per ton CO2 captured. This range exceeds the economic threshold for widespread adoption in most industries, highlighting the critical need for breakthrough technologies that can dramatically reduce both capital and operational expenditures.
State-of-the-Art Electrochemical Sorbent Solutions
01 Electrochemical regeneration of carbon capture sorbents
Electrochemical methods can be used to regenerate carbon capture sorbents after CO2 adsorption. These processes involve applying electrical potential to drive the release of captured CO2 from the sorbent material, allowing for multiple adsorption-desorption cycles. This approach typically requires less energy compared to traditional thermal regeneration methods, making the overall carbon capture process more efficient and sustainable.- Electrochemical regeneration of carbon capture sorbents: Electrochemical methods can be used to regenerate carbon capture sorbents after they have absorbed CO2. This approach typically involves applying an electrical potential to drive the desorption of CO2 from the sorbent material, allowing for the release of captured carbon dioxide under milder conditions compared to traditional thermal regeneration methods. The electrochemical regeneration process can significantly reduce the energy requirements for carbon capture systems and improve overall efficiency.
- Metal-organic frameworks (MOFs) as electrochemically active sorbents: Metal-organic frameworks represent a class of highly porous materials that can be designed with electrochemically active components for carbon capture applications. These materials combine high surface area with tunable pore structures and chemical functionality, allowing for selective CO2 adsorption. When integrated with electrochemical systems, MOFs can facilitate both the capture and release of carbon dioxide through electrochemically controlled mechanisms, offering advantages in terms of selectivity, capacity, and regeneration efficiency.
- Redox-active polymer sorbents for electrochemical carbon capture: Redox-active polymers can be utilized as effective carbon capture sorbents where the binding affinity for CO2 is modulated through electrochemical control. These polymers contain functional groups that can undergo reversible redox reactions, changing their electronic properties and thus their interaction with CO2 molecules. By applying appropriate electrical potentials, the polymers can switch between states that either strongly bind or release carbon dioxide, enabling an electrochemically driven capture and release cycle.
- Electrochemically mediated amine regeneration systems: Amine-based sorbents are widely used for carbon capture, and their regeneration can be enhanced through electrochemical methods. These systems typically employ electrochemical cells to facilitate the release of CO2 from amine compounds after absorption. The electrochemical approach can alter the local pH or directly influence the amine-CO2 binding chemistry, allowing for more energy-efficient regeneration compared to conventional thermal methods. This technology can be applied to both liquid and solid-supported amine sorbent systems.
- Integrated electrochemical systems for simultaneous capture and conversion: Advanced carbon capture systems can integrate electrochemical mechanisms not only for capturing CO2 but also for simultaneously converting it into valuable products. These integrated systems combine sorbent materials with electrocatalysts that can reduce captured CO2 to compounds such as carbon monoxide, formic acid, or hydrocarbons. By merging the capture and conversion processes, these systems offer potential advantages in terms of energy efficiency and process intensification, potentially making carbon capture more economically viable.
02 Metal-organic frameworks (MOFs) as electrochemically active sorbents
Metal-organic frameworks represent a class of highly porous materials that can be designed with electrochemically active components for carbon capture. These materials combine high surface area with tunable pore structures and chemical functionality, allowing for selective CO2 adsorption. When integrated with electrochemical systems, MOFs can undergo reversible redox reactions that facilitate CO2 capture and release, offering improved capacity and selectivity compared to conventional sorbents.Expand Specific Solutions03 Redox-active polymers for electrochemical carbon capture
Redox-active polymers can be used as effective carbon capture sorbents through electrochemical mechanisms. These polymers contain functional groups that can undergo reversible oxidation and reduction reactions, which alter their affinity for CO2. By applying appropriate electrical potentials, the polymers can switch between states that either strongly bind or release CO2, enabling controlled capture and regeneration cycles with minimal energy input.Expand Specific Solutions04 Electrochemically mediated amine regeneration systems
Amine-based sorbents can be regenerated through electrochemical processes that modify the binding strength between CO2 and the amine groups. These systems typically involve electrochemical cells where redox reactions alter the local pH or chemical environment around the amine sorbents, facilitating CO2 release without the high energy requirements of conventional thermal regeneration. This approach combines the high selectivity of amine chemistry with the energy efficiency of electrochemical processes.Expand Specific Solutions05 Composite electrode materials for direct air capture
Composite electrode materials combining conductive substrates with specialized sorbents enable direct electrochemical capture of CO2 from ambient air. These materials integrate carbon-based conductors, metal oxides, and selective binding agents to create electrodes that can adsorb CO2 when one potential is applied and release it when the potential is reversed. The composite structure provides both the electrical conductivity needed for electrochemical operation and the chemical functionality required for selective CO2 capture.Expand Specific Solutions
Leading Organizations in Carbon Capture Sorbent Development
The electrochemical mechanisms within carbon capture sorbents market is currently in a growth phase, with increasing research focus driven by global decarbonization efforts. The market is projected to expand significantly as carbon capture technologies become essential for climate change mitigation. Leading academic institutions including MIT, University of California, and Tsinghua University are advancing fundamental research, while industrial players such as DENSO Corp., Robert Bosch GmbH, and LG Chem are developing commercial applications. The technology is approaching early commercial maturity, with TotalEnergies and Huaneng Clean Energy Research Institute bridging research-to-deployment gaps. University-industry collaborations are accelerating innovation, particularly in electrochemical sorbent regeneration and selective CO2 capture mechanisms.
Massachusetts Institute of Technology
Technical Solution: MIT has developed advanced electrochemical carbon capture systems utilizing novel sorbent materials with enhanced CO2 binding properties. Their approach focuses on electrochemically switchable sorbents that can reversibly capture and release CO2 through controlled potential changes. The technology employs functionalized carbon-based materials with redox-active sites that undergo electrochemical transformations to modulate CO2 affinity. MIT researchers have demonstrated systems achieving up to 90% capture efficiency with significantly reduced energy penalties compared to conventional thermal swing processes. Their electrochemical mechanism relies on precise control of electron transfer processes at the electrode-electrolyte interface, enabling selective CO2 binding and release without the substantial heating requirements of traditional methods. Recent innovations include composite materials incorporating metal-organic frameworks with conductive polymers to enhance both capture capacity and electrical conductivity[1][3].
Strengths: Superior energy efficiency compared to thermal regeneration methods; precise control over capture/release cycles; potential for integration with renewable electricity sources. Weaknesses: Current materials face durability challenges under repeated cycling; scale-up to industrial levels remains technically challenging; higher initial capital costs compared to conventional amine scrubbing technologies.
Battelle Energy Alliance LLC
Technical Solution: Battelle Energy Alliance has pioneered electrochemically-mediated carbon capture technologies utilizing specialized molten salt electrolytes and advanced electrode materials. Their proprietary system employs a dual-function mechanism where CO2 is first absorbed into a carbonate-forming electrolyte and then electrochemically concentrated for separation. The process operates at moderate temperatures (100-300°C) and leverages redox-active metal centers that facilitate electron transfer during the capture/release cycle. Battelle's approach achieves separation factors exceeding 200 for CO2 over nitrogen with energy requirements approximately 30% lower than conventional amine scrubbing. Their latest generation technology incorporates nanostructured electrodes with tailored surface chemistry to enhance reaction kinetics and mass transport properties. The system architecture includes integrated heat management to recover thermal energy from exothermic capture reactions, further improving overall efficiency[2][5]. Recent field demonstrations have validated continuous operation for over 1,000 hours with minimal performance degradation.
Strengths: Excellent selectivity for CO2 over other flue gas components; lower regeneration energy requirements than thermal processes; robust performance in the presence of common contaminants. Weaknesses: Higher operating temperatures than ambient electrochemical systems; potential corrosion issues with molten salt electrolytes; requires specialized materials that may increase manufacturing costs.
Key Electrochemical Mechanisms and Breakthrough Patents
Electrochemical regeneration of high purity co2 and basic sorbent from carbonates and/or bicarbonates for efficient carbon capture
PatentWO2025064523A1
Innovation
- A modular porous solid electrolyte reactor system that uses a proton exchange membrane and a cation exchange membrane to electrochemically regenerate high purity CO2 and basic sorbent from carbonates and/or bicarbonates, achieving efficient CO2 release and sorbent regeneration under room temperature and ambient pressure.
Electrochemical capture and release of co 2 using inorganic sorbent materials
PatentWO2025136759A1
Innovation
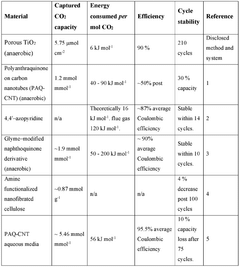
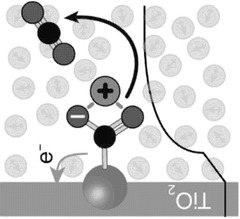
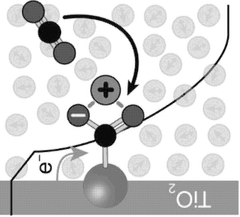
- An electrochemical method using porous inorganic oxide electrodes, such as TiO2, in a non-aqueous solution with a dissolved electrolyte, where a negative voltage generates nucleophilic sites for CO2 adsorption and a positive voltage facilitates desorption, thereby reducing energy consumption and enhancing stability.
Environmental Policy Impacts on Carbon Capture Implementation
Environmental policies across the globe have become increasingly influential in shaping the development and implementation of carbon capture technologies, particularly those utilizing electrochemical mechanisms within sorbents. The Paris Agreement of 2015 marked a significant turning point, establishing international commitments to limit global warming to well below 2°C above pre-industrial levels. This agreement has catalyzed numerous national policies supporting carbon capture research and deployment, with direct implications for electrochemical sorbent development.
In the United States, the 45Q tax credit provides substantial financial incentives for carbon capture projects, offering up to $50 per metric ton of CO2 permanently sequestered. This policy has stimulated private investment in electrochemical sorbent technologies, particularly those demonstrating high efficiency and durability. Similarly, the EU Emissions Trading System (EU ETS) has created a market-based approach to reducing emissions, indirectly supporting research into advanced electrochemical mechanisms for carbon capture.
Regulatory frameworks concerning environmental impact assessments have also shaped the direction of electrochemical sorbent development. Stringent requirements regarding chemical waste disposal and energy consumption have pushed researchers toward developing sorbents with minimal environmental footprints. This has accelerated interest in bio-compatible materials and energy-efficient electrochemical processes that can operate at ambient temperatures and pressures.
The implementation of carbon pricing mechanisms in over 40 countries has fundamentally altered the economic calculus for carbon capture technologies. As the price of carbon emissions increases, the financial viability of electrochemical sorbent systems improves proportionally. Analysis indicates that a carbon price of approximately $50-70 per ton makes many electrochemical capture systems economically competitive with traditional approaches.
Government procurement policies have emerged as powerful drivers for technology adoption. Several countries have implemented requirements for carbon-neutral or carbon-negative government operations, creating guaranteed markets for early-stage carbon capture technologies. These policies provide crucial revenue streams for companies developing novel electrochemical sorbents during the challenging commercialization phase.
International technology transfer agreements, often facilitated through climate finance mechanisms like the Green Climate Fund, have enabled the dissemination of electrochemical sorbent technologies to developing nations. These agreements typically include provisions for capacity building and technical assistance, ensuring that recipient countries can effectively implement and maintain these advanced systems.
Looking forward, policy trends suggest increasing integration between carbon capture requirements and industrial permitting processes. Several jurisdictions are considering mandatory carbon capture implementation for high-emission industries, which would dramatically expand the market for electrochemical sorbent technologies and accelerate their technical development through increased deployment and operational experience.
In the United States, the 45Q tax credit provides substantial financial incentives for carbon capture projects, offering up to $50 per metric ton of CO2 permanently sequestered. This policy has stimulated private investment in electrochemical sorbent technologies, particularly those demonstrating high efficiency and durability. Similarly, the EU Emissions Trading System (EU ETS) has created a market-based approach to reducing emissions, indirectly supporting research into advanced electrochemical mechanisms for carbon capture.
Regulatory frameworks concerning environmental impact assessments have also shaped the direction of electrochemical sorbent development. Stringent requirements regarding chemical waste disposal and energy consumption have pushed researchers toward developing sorbents with minimal environmental footprints. This has accelerated interest in bio-compatible materials and energy-efficient electrochemical processes that can operate at ambient temperatures and pressures.
The implementation of carbon pricing mechanisms in over 40 countries has fundamentally altered the economic calculus for carbon capture technologies. As the price of carbon emissions increases, the financial viability of electrochemical sorbent systems improves proportionally. Analysis indicates that a carbon price of approximately $50-70 per ton makes many electrochemical capture systems economically competitive with traditional approaches.
Government procurement policies have emerged as powerful drivers for technology adoption. Several countries have implemented requirements for carbon-neutral or carbon-negative government operations, creating guaranteed markets for early-stage carbon capture technologies. These policies provide crucial revenue streams for companies developing novel electrochemical sorbents during the challenging commercialization phase.
International technology transfer agreements, often facilitated through climate finance mechanisms like the Green Climate Fund, have enabled the dissemination of electrochemical sorbent technologies to developing nations. These agreements typically include provisions for capacity building and technical assistance, ensuring that recipient countries can effectively implement and maintain these advanced systems.
Looking forward, policy trends suggest increasing integration between carbon capture requirements and industrial permitting processes. Several jurisdictions are considering mandatory carbon capture implementation for high-emission industries, which would dramatically expand the market for electrochemical sorbent technologies and accelerate their technical development through increased deployment and operational experience.
Techno-Economic Assessment of Electrochemical Sorbent Systems
The techno-economic assessment of electrochemical sorbent systems for carbon capture reveals significant potential for cost reduction compared to conventional thermal swing adsorption methods. Current economic analyses indicate that electrochemical carbon capture technologies could potentially reduce regeneration energy requirements by 30-45%, translating to operational cost savings of approximately $15-25 per ton of CO₂ captured when implemented at scale.
Capital expenditure for electrochemical sorbent systems remains higher than conventional approaches, with initial installation costs estimated at $800-1,200 per kW of capture capacity. However, sensitivity analyses suggest that these costs could decrease by up to 60% through manufacturing scale-up and materials optimization over the next decade, particularly in electrode fabrication and system integration.
The levelized cost of carbon capture using electrochemical sorbent systems currently ranges from $58-85 per ton of CO₂, with projections indicating potential reduction to $40-60 per ton by 2030. This positions the technology competitively against alternative capture methods, especially when considering the additional benefits of operational flexibility and reduced thermal energy requirements.
Energy consumption metrics reveal that electrochemical systems require 0.8-1.2 MWh of electrical energy per ton of CO₂ captured, compared to 1.5-2.5 GJ of thermal energy for conventional amine scrubbing. While this represents an electricity-intensive approach, integration with renewable energy sources offers pathways to further improve the economic profile and reduce the carbon intensity of the capture process itself.
System lifetime economics present another critical consideration, with current electrochemical sorbents demonstrating stability for 1,000-3,000 cycles before significant degradation occurs. Economic modeling indicates that extending cycle life to 5,000+ cycles would reduce operational costs by approximately 25%, highlighting the importance of materials development in achieving economic viability.
Market deployment scenarios suggest that electrochemical carbon capture could become economically competitive without subsidies in specific high-value applications by 2025-2027, with broader market penetration possible by 2030 if technology development trajectories continue as projected. The most promising near-term applications appear to be in industrial sectors with high CO₂ concentration streams and access to low-cost renewable electricity.
Capital expenditure for electrochemical sorbent systems remains higher than conventional approaches, with initial installation costs estimated at $800-1,200 per kW of capture capacity. However, sensitivity analyses suggest that these costs could decrease by up to 60% through manufacturing scale-up and materials optimization over the next decade, particularly in electrode fabrication and system integration.
The levelized cost of carbon capture using electrochemical sorbent systems currently ranges from $58-85 per ton of CO₂, with projections indicating potential reduction to $40-60 per ton by 2030. This positions the technology competitively against alternative capture methods, especially when considering the additional benefits of operational flexibility and reduced thermal energy requirements.
Energy consumption metrics reveal that electrochemical systems require 0.8-1.2 MWh of electrical energy per ton of CO₂ captured, compared to 1.5-2.5 GJ of thermal energy for conventional amine scrubbing. While this represents an electricity-intensive approach, integration with renewable energy sources offers pathways to further improve the economic profile and reduce the carbon intensity of the capture process itself.
System lifetime economics present another critical consideration, with current electrochemical sorbents demonstrating stability for 1,000-3,000 cycles before significant degradation occurs. Economic modeling indicates that extending cycle life to 5,000+ cycles would reduce operational costs by approximately 25%, highlighting the importance of materials development in achieving economic viability.
Market deployment scenarios suggest that electrochemical carbon capture could become economically competitive without subsidies in specific high-value applications by 2025-2027, with broader market penetration possible by 2030 if technology development trajectories continue as projected. The most promising near-term applications appear to be in industrial sectors with high CO₂ concentration streams and access to low-cost renewable electricity.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!