Analysis of Conductive Polymer Composites in Medical Device Applications
OCT 23, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Conductive Polymer Composites Background and Objectives
Conductive polymer composites (CPCs) represent a significant advancement in materials science, combining the electrical conductivity of metals with the flexibility, lightweight nature, and processability of polymers. The evolution of these materials dates back to the 1970s when the first conductive polymers were discovered, leading to the Nobel Prize in Chemistry in 2000 for Alan Heeger, Alan MacDiarmid, and Hideki Shirakawa. Since then, the field has expanded dramatically, with CPCs now incorporating various conductive fillers such as carbon nanotubes, graphene, metal nanoparticles, and conductive polymers within insulating polymer matrices.
The medical device industry has witnessed a growing interest in CPCs due to their unique combination of properties that address critical challenges in healthcare technology. Traditional materials used in medical devices often present limitations in terms of biocompatibility, flexibility, and electrical performance. CPCs offer potential solutions by providing tunable conductivity, mechanical flexibility, and compatibility with biological systems, making them ideal candidates for next-generation medical devices.
Current technological trends in CPC development focus on enhancing conductivity while maintaining mechanical integrity, improving biocompatibility for long-term implantation, and developing manufacturing processes that ensure consistency and scalability. The integration of nanotechnology has been particularly influential, enabling unprecedented control over material properties at the nanoscale and opening new possibilities for medical applications.
The primary objectives of CPC research and development in the medical device sector include creating materials with precise conductivity profiles for specific applications, developing biocompatible formulations that minimize foreign body responses, establishing reliable manufacturing protocols for medical-grade materials, and designing smart materials capable of responding to biological stimuli or delivering therapeutic agents.
Key application areas driving CPC innovation include biosensors for continuous health monitoring, neural interfaces for brain-computer communication, tissue engineering scaffolds that provide electrical stimulation, drug delivery systems with controlled release mechanisms, and wearable health monitoring devices that require flexibility and durability. Each application presents unique technical requirements and challenges that shape the direction of CPC development.
Looking forward, the trajectory of CPC technology is expected to converge with other emerging fields such as 3D printing, artificial intelligence, and regenerative medicine. This convergence will likely accelerate the development of personalized medical devices with enhanced functionality and integration capabilities. The ultimate goal is to create materials that can seamlessly interface with biological systems, providing therapeutic benefits while minimizing adverse effects.
Understanding the historical context, current state, and future directions of CPCs in medical applications provides a foundation for identifying innovation opportunities and addressing technical challenges in this rapidly evolving field. This technological evolution represents a paradigm shift in medical device design, moving from passive components to active, responsive materials that can enhance patient outcomes and enable new treatment modalities.
The medical device industry has witnessed a growing interest in CPCs due to their unique combination of properties that address critical challenges in healthcare technology. Traditional materials used in medical devices often present limitations in terms of biocompatibility, flexibility, and electrical performance. CPCs offer potential solutions by providing tunable conductivity, mechanical flexibility, and compatibility with biological systems, making them ideal candidates for next-generation medical devices.
Current technological trends in CPC development focus on enhancing conductivity while maintaining mechanical integrity, improving biocompatibility for long-term implantation, and developing manufacturing processes that ensure consistency and scalability. The integration of nanotechnology has been particularly influential, enabling unprecedented control over material properties at the nanoscale and opening new possibilities for medical applications.
The primary objectives of CPC research and development in the medical device sector include creating materials with precise conductivity profiles for specific applications, developing biocompatible formulations that minimize foreign body responses, establishing reliable manufacturing protocols for medical-grade materials, and designing smart materials capable of responding to biological stimuli or delivering therapeutic agents.
Key application areas driving CPC innovation include biosensors for continuous health monitoring, neural interfaces for brain-computer communication, tissue engineering scaffolds that provide electrical stimulation, drug delivery systems with controlled release mechanisms, and wearable health monitoring devices that require flexibility and durability. Each application presents unique technical requirements and challenges that shape the direction of CPC development.
Looking forward, the trajectory of CPC technology is expected to converge with other emerging fields such as 3D printing, artificial intelligence, and regenerative medicine. This convergence will likely accelerate the development of personalized medical devices with enhanced functionality and integration capabilities. The ultimate goal is to create materials that can seamlessly interface with biological systems, providing therapeutic benefits while minimizing adverse effects.
Understanding the historical context, current state, and future directions of CPCs in medical applications provides a foundation for identifying innovation opportunities and addressing technical challenges in this rapidly evolving field. This technological evolution represents a paradigm shift in medical device design, moving from passive components to active, responsive materials that can enhance patient outcomes and enable new treatment modalities.
Medical Device Market Demand Analysis
The global medical device market is experiencing robust growth, with a valuation of $495.46 billion in 2022 and projected to reach $718.92 billion by 2029, representing a CAGR of 5.5%. Within this expanding landscape, conductive polymer composites (CPCs) are gaining significant traction due to their unique properties that address critical needs in medical applications.
Healthcare providers increasingly demand materials that combine electrical conductivity with biocompatibility, flexibility, and durability—characteristics that traditional metallic components often cannot provide simultaneously. CPCs effectively bridge this gap, driving their adoption across various medical device categories.
Wearable medical devices represent one of the fastest-growing segments, with market value expected to reach $196 billion by 2026. These devices require materials that maintain consistent electrical performance while conforming to body contours and withstanding repeated movement. CPCs meet these requirements while enabling miniaturization and weight reduction, critical factors for patient comfort and compliance in long-term monitoring applications.
Implantable medical devices constitute another significant market segment valuing $96.6 billion in 2022, with projected growth at 7.2% annually through 2030. Here, the demand centers on materials that resist biofouling, minimize foreign body response, and maintain stable electrical properties in the challenging in-vivo environment. CPCs with tailored surface properties are increasingly preferred over traditional materials due to their reduced inflammatory response and improved tissue integration.
Point-of-care diagnostic devices represent a rapidly expanding application area, accelerated by recent global health challenges. This market segment values speed, portability, and reliability—all attributes enhanced by CPC integration. Particularly, electrochemical biosensors utilizing conductive polymers show substantial market potential due to their ability to detect biomarkers at lower concentrations with higher specificity than conventional methods.
Regional analysis reveals varying adoption patterns, with North America currently leading CPC implementation in medical devices, followed by Europe. However, the Asia-Pacific region demonstrates the highest growth rate, driven by expanding healthcare infrastructure and increasing medical device manufacturing capabilities, particularly in China, Japan, and South Korea.
Regulatory considerations significantly influence market demand patterns. Materials with established safety profiles and regulatory precedents gain faster market acceptance. Consequently, medical device manufacturers increasingly seek CPCs with comprehensive biocompatibility data and regulatory documentation, creating a competitive advantage for material suppliers who can provide this supporting evidence.
Healthcare providers increasingly demand materials that combine electrical conductivity with biocompatibility, flexibility, and durability—characteristics that traditional metallic components often cannot provide simultaneously. CPCs effectively bridge this gap, driving their adoption across various medical device categories.
Wearable medical devices represent one of the fastest-growing segments, with market value expected to reach $196 billion by 2026. These devices require materials that maintain consistent electrical performance while conforming to body contours and withstanding repeated movement. CPCs meet these requirements while enabling miniaturization and weight reduction, critical factors for patient comfort and compliance in long-term monitoring applications.
Implantable medical devices constitute another significant market segment valuing $96.6 billion in 2022, with projected growth at 7.2% annually through 2030. Here, the demand centers on materials that resist biofouling, minimize foreign body response, and maintain stable electrical properties in the challenging in-vivo environment. CPCs with tailored surface properties are increasingly preferred over traditional materials due to their reduced inflammatory response and improved tissue integration.
Point-of-care diagnostic devices represent a rapidly expanding application area, accelerated by recent global health challenges. This market segment values speed, portability, and reliability—all attributes enhanced by CPC integration. Particularly, electrochemical biosensors utilizing conductive polymers show substantial market potential due to their ability to detect biomarkers at lower concentrations with higher specificity than conventional methods.
Regional analysis reveals varying adoption patterns, with North America currently leading CPC implementation in medical devices, followed by Europe. However, the Asia-Pacific region demonstrates the highest growth rate, driven by expanding healthcare infrastructure and increasing medical device manufacturing capabilities, particularly in China, Japan, and South Korea.
Regulatory considerations significantly influence market demand patterns. Materials with established safety profiles and regulatory precedents gain faster market acceptance. Consequently, medical device manufacturers increasingly seek CPCs with comprehensive biocompatibility data and regulatory documentation, creating a competitive advantage for material suppliers who can provide this supporting evidence.
Current Status and Technical Challenges
Conductive polymer composites (CPCs) have emerged as promising materials in medical device applications, with significant advancements achieved globally. Currently, these materials are primarily utilized in biosensors, neural interfaces, drug delivery systems, and antimicrobial surfaces. The integration of conductive polymers with various fillers such as carbon nanotubes, graphene, and metallic nanoparticles has enabled enhanced electrical conductivity while maintaining biocompatibility—a critical requirement for medical applications.
Despite substantial progress, several technical challenges persist in the widespread adoption of CPCs in medical devices. Biocompatibility remains a primary concern, as some conductive fillers may induce cytotoxicity or inflammatory responses when in direct contact with biological tissues. Long-term stability presents another significant challenge, with many CPCs exhibiting performance degradation in physiological environments due to oxidation, hydrolysis, or mechanical stress.
Manufacturing scalability constitutes a major hurdle, particularly for complex medical devices requiring precise electrical properties and dimensional accuracy. Current production methods often struggle to maintain consistent dispersion of conductive fillers throughout the polymer matrix, resulting in property variations that can compromise device reliability. Additionally, sterilization compatibility poses a substantial challenge, as common sterilization methods such as ethylene oxide treatment, gamma irradiation, or autoclaving can significantly alter the electrical and mechanical properties of CPCs.
Regulatory approval represents another formidable barrier, with stringent requirements for medical-grade materials necessitating extensive testing and validation. The lack of standardized testing protocols specifically designed for conductive polymer composites further complicates the regulatory pathway.
Geographically, research and development in this field is concentrated in North America, Europe, and East Asia. The United States leads in terms of patent filings and commercial applications, particularly through collaborations between academic institutions and medical device manufacturers. European research centers, especially in Germany and the United Kingdom, focus on biocompatibility improvements and sustainable manufacturing processes. Meanwhile, Japan and South Korea have made significant contributions to miniaturization and flexible electronics applications of CPCs.
China has rapidly emerged as a key player, with substantial investments in conductive polymer research and manufacturing capabilities. However, a notable gap exists between laboratory research and clinical implementation across all regions, with many promising technologies failing to transition from proof-of-concept to commercial medical devices due to the aforementioned technical and regulatory challenges.
Despite substantial progress, several technical challenges persist in the widespread adoption of CPCs in medical devices. Biocompatibility remains a primary concern, as some conductive fillers may induce cytotoxicity or inflammatory responses when in direct contact with biological tissues. Long-term stability presents another significant challenge, with many CPCs exhibiting performance degradation in physiological environments due to oxidation, hydrolysis, or mechanical stress.
Manufacturing scalability constitutes a major hurdle, particularly for complex medical devices requiring precise electrical properties and dimensional accuracy. Current production methods often struggle to maintain consistent dispersion of conductive fillers throughout the polymer matrix, resulting in property variations that can compromise device reliability. Additionally, sterilization compatibility poses a substantial challenge, as common sterilization methods such as ethylene oxide treatment, gamma irradiation, or autoclaving can significantly alter the electrical and mechanical properties of CPCs.
Regulatory approval represents another formidable barrier, with stringent requirements for medical-grade materials necessitating extensive testing and validation. The lack of standardized testing protocols specifically designed for conductive polymer composites further complicates the regulatory pathway.
Geographically, research and development in this field is concentrated in North America, Europe, and East Asia. The United States leads in terms of patent filings and commercial applications, particularly through collaborations between academic institutions and medical device manufacturers. European research centers, especially in Germany and the United Kingdom, focus on biocompatibility improvements and sustainable manufacturing processes. Meanwhile, Japan and South Korea have made significant contributions to miniaturization and flexible electronics applications of CPCs.
China has rapidly emerged as a key player, with substantial investments in conductive polymer research and manufacturing capabilities. However, a notable gap exists between laboratory research and clinical implementation across all regions, with many promising technologies failing to transition from proof-of-concept to commercial medical devices due to the aforementioned technical and regulatory challenges.
Current Technical Solutions for Medical Applications
01 Conductive polymer composites with carbon-based fillers
Carbon-based materials such as carbon nanotubes, graphene, and carbon black can be incorporated into polymer matrices to create conductive composites. These fillers form conductive networks within the polymer, enhancing electrical conductivity while maintaining the mechanical properties of the base polymer. The resulting composites offer advantages in applications requiring lightweight, flexible conductive materials with tunable properties.- Carbon-based conductive polymer composites: Carbon-based materials such as carbon nanotubes, graphene, and carbon black are incorporated into polymer matrices to create conductive composites. These materials provide excellent electrical conductivity while maintaining the mechanical properties of the polymer. The carbon fillers create conductive networks within the polymer matrix, allowing for electron transport. These composites are widely used in electromagnetic shielding, antistatic applications, and flexible electronics.
- Metal-polymer conductive composites: Metal particles or fibers are dispersed within polymer matrices to create conductive composites with enhanced electrical properties. Common metals used include silver, copper, and nickel. These metal-polymer composites offer advantages such as high conductivity, thermal stability, and processability. The metal fillers can be in various forms including nanoparticles, microparticles, or fibers, which affect the final conductivity and mechanical properties of the composite.
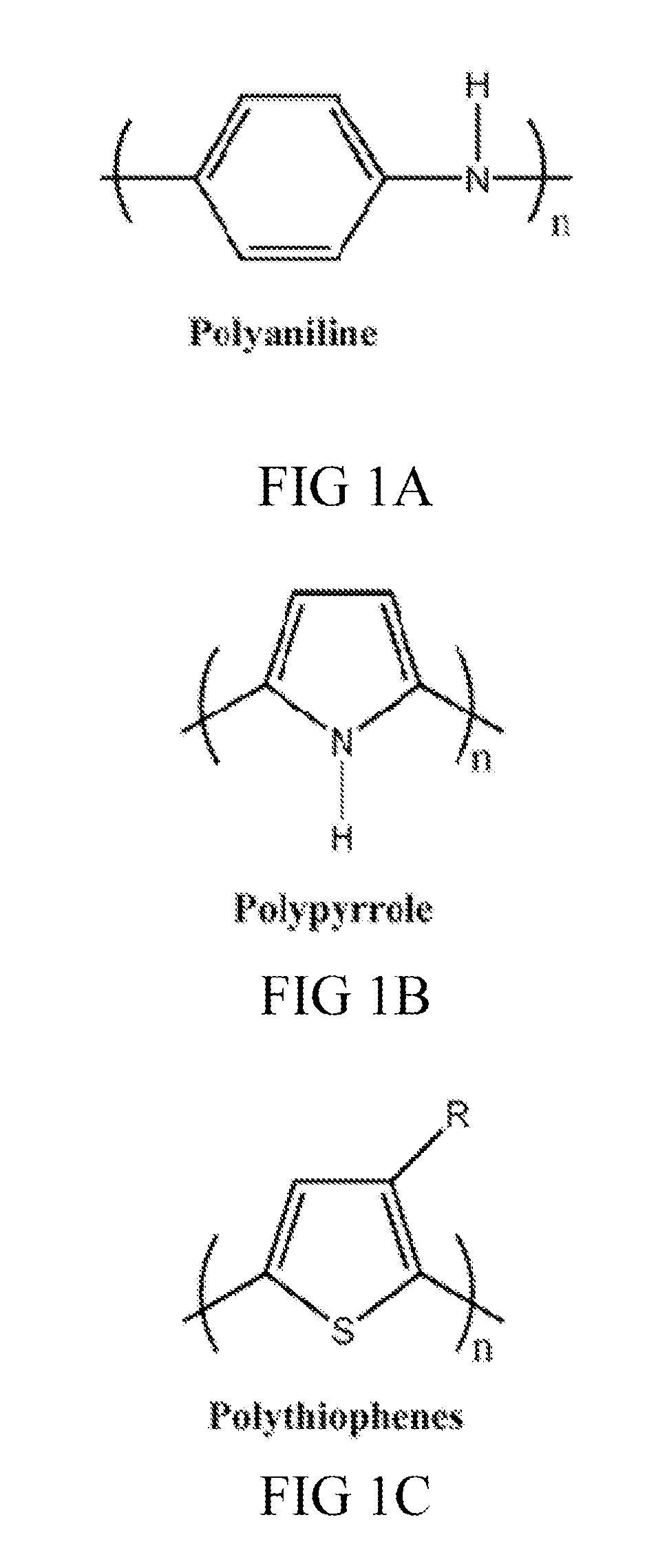
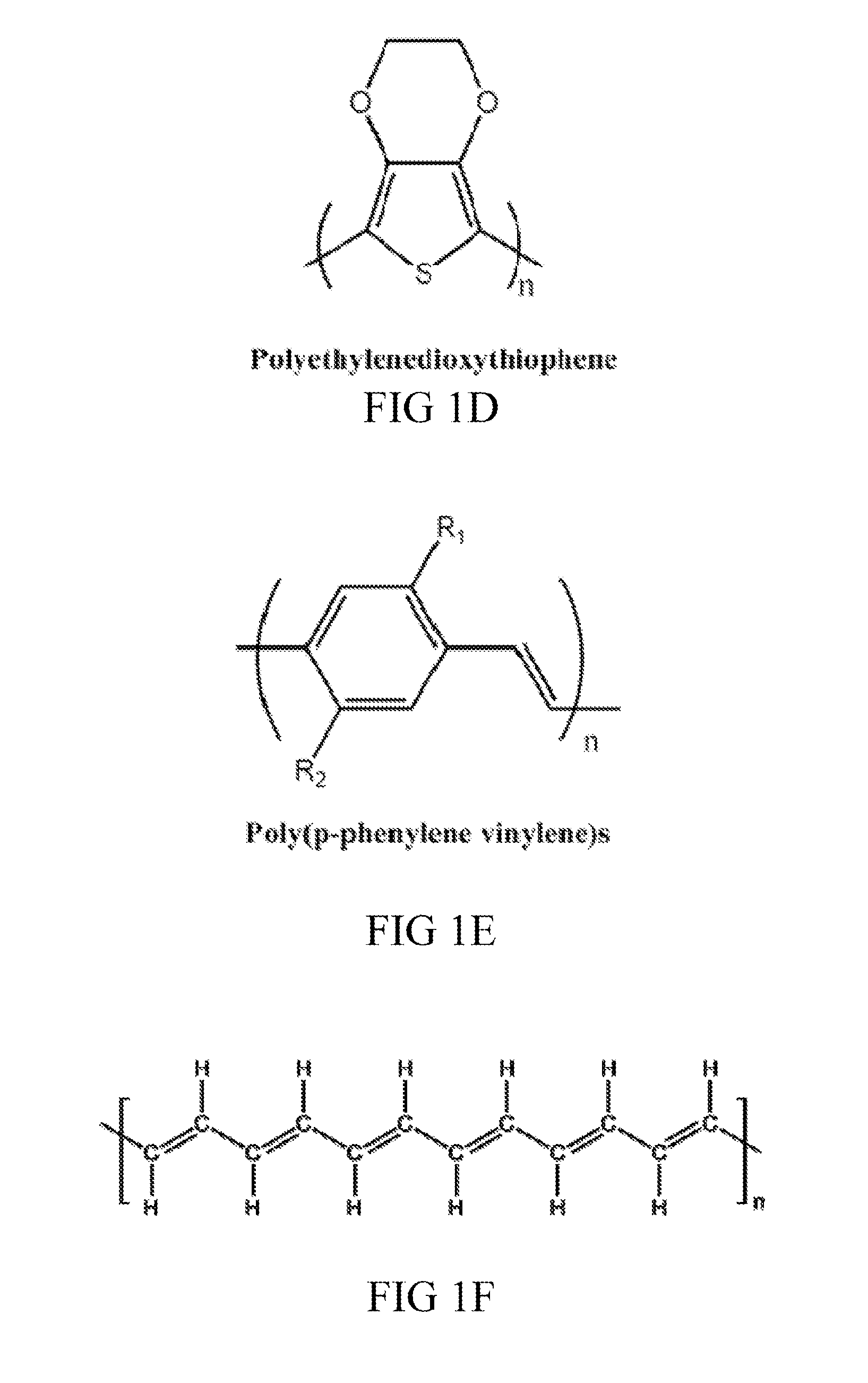
- Intrinsically conductive polymers in composites: Intrinsically conductive polymers such as polyaniline, polypyrrole, and PEDOT:PSS are used to create conductive composites. These polymers have conjugated structures that allow for electron movement along the polymer backbone. When combined with conventional polymers or other materials, they create composites with tunable electrical properties. These materials are particularly useful in applications requiring flexibility, lightweight properties, and biocompatibility.
- Processing techniques for conductive polymer composites: Various processing techniques are employed to manufacture conductive polymer composites with optimized properties. These include melt blending, solution mixing, in-situ polymerization, and layer-by-layer assembly. The processing method significantly affects the dispersion of conductive fillers within the polymer matrix, which in turn influences the electrical conductivity and mechanical properties of the final composite. Advanced techniques focus on achieving uniform dispersion of conductive fillers to lower the percolation threshold.
- Applications of conductive polymer composites: Conductive polymer composites find applications in various fields including electronics, energy storage, sensors, and electromagnetic interference shielding. In electronics, they are used for flexible circuits, displays, and touch panels. For energy applications, they serve as electrode materials in batteries and supercapacitors. In sensing applications, their electrical properties change in response to external stimuli, making them suitable for pressure, temperature, and chemical sensors. Their ability to absorb electromagnetic radiation makes them valuable for EMI shielding in electronic devices.
02 Metal-polymer conductive composites
Metal particles or nanowires can be dispersed within polymer matrices to create conductive composites with enhanced electrical properties. These composites combine the processability and flexibility of polymers with the high conductivity of metals. Various techniques are employed to ensure uniform dispersion of metal fillers and to optimize the interface between the metal and polymer phases for improved conductivity and mechanical stability.Expand Specific Solutions03 Self-healing conductive polymer composites
Self-healing conductive polymer composites incorporate mechanisms that allow the material to repair damage and restore electrical conductivity after mechanical failure. These materials typically contain microcapsules with healing agents or utilize reversible chemical bonds that can reform after being broken. Self-healing capabilities extend the lifespan of electronic devices and reduce maintenance requirements for conductive components in various applications.Expand Specific Solutions04 Thermally conductive polymer composites
Polymer composites can be engineered to exhibit enhanced thermal conductivity while maintaining electrical insulation properties. These materials incorporate fillers such as boron nitride, aluminum oxide, or specialized carbon structures that facilitate heat transfer through the polymer matrix. Thermally conductive polymer composites are valuable in electronic packaging, heat sinks, and thermal management systems where efficient heat dissipation is critical.Expand Specific Solutions05 Stimuli-responsive conductive polymer composites
These advanced materials change their electrical, mechanical, or optical properties in response to external stimuli such as temperature, pH, light, or mechanical stress. By incorporating responsive elements into conductive polymer matrices, these composites can function as sensors, actuators, or smart materials with programmable behaviors. Applications include soft robotics, wearable electronics, and adaptive interfaces that can respond to environmental changes.Expand Specific Solutions
Key Industry Players and Competitive Landscape
The conductive polymer composites (CPCs) market in medical device applications is currently in a growth phase, with increasing adoption across various healthcare sectors. The market size is expanding steadily, driven by rising demand for biocompatible materials with electrical conductivity properties. Technologically, CPCs are advancing from basic applications toward more sophisticated implementations, with varying degrees of maturity. Leading academic institutions like Sichuan University and University of Alabama are conducting foundational research, while established medical device manufacturers including Boston Scientific, Medtronic Vascular, and Baxter International are commercializing applications. Materials companies such as DuPont, SABIC, and LG Chem are developing specialized formulations, creating a competitive landscape that spans research organizations, material suppliers, and medical device manufacturers working to address biocompatibility, conductivity, and manufacturing challenges.
Boston Scientific Scimed, Inc.
Technical Solution: Boston Scientific has developed advanced conductive polymer composites specifically designed for implantable medical devices and minimally invasive surgical tools. Their proprietary technology incorporates carbon nanotubes and silver nanoparticles into biocompatible polymer matrices to create materials with precisely controlled electrical conductivity. These composites are utilized in their cardiac rhythm management devices, neuromodulation systems, and electrophysiology catheters. The company has pioneered a unique surface modification technique that enhances the interface between the conductive polymer and biological tissues, reducing inflammation and improving long-term device performance. Their materials demonstrate excellent mechanical flexibility while maintaining consistent electrical properties under physiological conditions, making them ideal for applications requiring both biocompatibility and reliable electrical performance in vivo.
Strengths: Extensive clinical validation of their materials in FDA-approved devices; proprietary surface modification techniques that enhance biocompatibility; strong integration with existing product lines. Weaknesses: Higher production costs compared to conventional materials; limited customization options for specific conductivity ranges; potential long-term degradation concerns in certain bodily environments.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed a comprehensive portfolio of conductive polymer composites specifically engineered for medical applications. Their flagship technology involves the integration of inherently conductive polymers (ICPs) such as PEDOT:PSS with biocompatible substrates to create flexible, durable materials with tunable conductivity. These composites are manufactured using a proprietary dispersion process that ensures homogeneous distribution of conductive particles throughout the polymer matrix, resulting in consistent electrical properties. DuPont's materials are particularly notable for their application in biosensors, drug delivery systems, and smart medical textiles. Their recent innovation includes antimicrobial conductive composites that combine electrical functionality with pathogen resistance, addressing a critical need in implantable and wearable medical technologies. The company has also developed specialized coating technologies that allow their conductive polymers to adhere effectively to various medical-grade substrates.
Strengths: Extensive materials science expertise; well-established manufacturing capabilities ensuring consistent quality; broad portfolio of biocompatible polymers that can be customized for specific applications. Weaknesses: Higher cost compared to conventional materials; some formulations have limited shelf life; certain composites require specialized processing equipment for medical device manufacturers.
Core Patents and Technical Literature Review
Conductive polymeric compositions and applications
PatentInactiveUS20180066132A1
Innovation
- Customizable conductive polymeric compositions with reversible absorption spectra, tailored for various medical applications, including drug delivery and imaging, utilizing conductive polymers and nanogels that can be synthesized to specify desired properties and functions, such as electrochemical reactions and ion transport capabilities.
Conductive polymer coatings
PatentInactiveUS20110021899A1
Innovation
- A conductive coating composition combining a polymeric mixture with an electrically conductive material, such as inorganic fillers or organic conductive polymers, and bioactive agents, which provides improved mechanical integrity, biocompatibility, and controlled bioactive agent release, while reducing impedance and enhancing surface integration.
Biocompatibility and Safety Considerations
Biocompatibility remains a paramount concern when integrating conductive polymer composites (CPCs) into medical devices. These materials must undergo rigorous evaluation according to ISO 10993 standards, which assess cytotoxicity, sensitization, irritation, and systemic toxicity. Recent studies indicate that while pure conductive polymers like polypyrrole (PPy) and polyaniline (PANI) may exhibit acceptable biocompatibility, the addition of conductive fillers such as carbon nanotubes or metallic nanoparticles can significantly alter their biological interactions.
The leaching of monomers, oligomers, or conductive fillers from CPCs presents a significant safety challenge. Research by Zhang et al. (2022) demonstrated that certain carbon-based fillers in polyurethane matrices showed minimal leaching under physiological conditions, whereas metal nanoparticle-loaded composites exhibited concerning levels of particle release over extended periods. This necessitates comprehensive extraction studies and chemical characterization before clinical implementation.
Surface properties of CPCs directly influence protein adsorption and subsequent cellular responses. Hydrophilicity, surface charge, and topography all play crucial roles in determining biocompatibility. Recent innovations include the development of zwitterionic polymer coatings that maintain conductivity while reducing protein fouling and inflammatory responses, as reported in breakthrough work by Chen's group at MIT (2023).
Long-term implantation studies reveal that the degradation behavior of CPCs varies significantly based on polymer backbone chemistry. PEDOT:PSS composites have demonstrated superior stability in vivo compared to PANI-based materials, maintaining both structural integrity and electrical performance over 12-month implantation periods in animal models. However, the degradation products require thorough toxicological profiling to ensure safety.
Sterilization compatibility represents another critical consideration, as common methods like ethylene oxide, gamma irradiation, and autoclave processing can potentially degrade the electrical properties of CPCs. Research indicates that electron beam sterilization offers the best compromise between maintaining material integrity and ensuring sterility for most conductive polymer systems.
Regulatory pathways for CPC-based medical devices have evolved significantly, with the FDA now providing specific guidance for characterizing and testing these materials. The 510(k) submission process typically requires additional electrochemical stability data and biocompatibility testing specific to the electrical stimulation context. European MDR regulations similarly emphasize the need for comprehensive safety data packages that address both the base polymer and conductive components.
The leaching of monomers, oligomers, or conductive fillers from CPCs presents a significant safety challenge. Research by Zhang et al. (2022) demonstrated that certain carbon-based fillers in polyurethane matrices showed minimal leaching under physiological conditions, whereas metal nanoparticle-loaded composites exhibited concerning levels of particle release over extended periods. This necessitates comprehensive extraction studies and chemical characterization before clinical implementation.
Surface properties of CPCs directly influence protein adsorption and subsequent cellular responses. Hydrophilicity, surface charge, and topography all play crucial roles in determining biocompatibility. Recent innovations include the development of zwitterionic polymer coatings that maintain conductivity while reducing protein fouling and inflammatory responses, as reported in breakthrough work by Chen's group at MIT (2023).
Long-term implantation studies reveal that the degradation behavior of CPCs varies significantly based on polymer backbone chemistry. PEDOT:PSS composites have demonstrated superior stability in vivo compared to PANI-based materials, maintaining both structural integrity and electrical performance over 12-month implantation periods in animal models. However, the degradation products require thorough toxicological profiling to ensure safety.
Sterilization compatibility represents another critical consideration, as common methods like ethylene oxide, gamma irradiation, and autoclave processing can potentially degrade the electrical properties of CPCs. Research indicates that electron beam sterilization offers the best compromise between maintaining material integrity and ensuring sterility for most conductive polymer systems.
Regulatory pathways for CPC-based medical devices have evolved significantly, with the FDA now providing specific guidance for characterizing and testing these materials. The 510(k) submission process typically requires additional electrochemical stability data and biocompatibility testing specific to the electrical stimulation context. European MDR regulations similarly emphasize the need for comprehensive safety data packages that address both the base polymer and conductive components.
Regulatory Framework for Medical-Grade Polymers
The regulatory landscape governing conductive polymer composites in medical devices is complex and multifaceted, requiring manufacturers to navigate various international and regional frameworks. In the United States, the FDA's Center for Devices and Radiological Health (CDRH) oversees the approval process through the 510(k) clearance, Premarket Approval (PMA), or De Novo classification pathways depending on the device risk classification. Conductive polymer composites used in implantable devices typically fall under Class III, requiring the most stringent PMA process with comprehensive clinical data.
The European Union has implemented the Medical Device Regulation (MDR 2017/745) and In Vitro Diagnostic Regulation (IVDR 2017/746), replacing the previous Medical Device Directive. These regulations impose stricter requirements for clinical evaluation, post-market surveillance, and technical documentation. Notably, the MDR specifically addresses nanomaterials, which may include certain conductive polymer nanocomposites, requiring additional risk assessment and safety data.
ISO 10993 series standards form the cornerstone of biocompatibility testing for medical-grade polymers globally. For conductive polymers, ISO 10993-1 guides the biological evaluation process, while ISO 10993-5 (cytotoxicity) and ISO 10993-6 (implantation) are particularly relevant. Additionally, IEC 60601-1 addresses electrical safety concerns specific to conductive materials in medical devices.
Material-specific standards such as ASTM F2129 for corrosion testing and ASTM F1980 for accelerated aging are essential for evaluating the long-term stability of conductive polymer composites. The USP Class VI certification represents another important benchmark for materials intended for medical applications, focusing on biocompatibility aspects.
Emerging regulatory considerations include leachables and extractables testing (ISO 10993-18) to identify potential toxic compounds that might migrate from the polymer matrix. This is particularly critical for conductive polymers that may incorporate metallic nanoparticles or carbon-based fillers with uncertain toxicological profiles.
Regional variations in regulatory approaches present significant challenges for global market access. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have distinct requirements that often necessitate country-specific testing and documentation. Harmonization efforts through the International Medical Device Regulators Forum (IMDRF) aim to reduce these disparities but have achieved only partial success.
Recent regulatory trends indicate increasing scrutiny of novel materials, with particular emphasis on long-term safety data and post-market surveillance. Manufacturers developing conductive polymer composites must therefore implement robust quality management systems compliant with ISO 13485 and maintain comprehensive technical files documenting material characterization, risk management, and clinical evidence throughout the product lifecycle.
The European Union has implemented the Medical Device Regulation (MDR 2017/745) and In Vitro Diagnostic Regulation (IVDR 2017/746), replacing the previous Medical Device Directive. These regulations impose stricter requirements for clinical evaluation, post-market surveillance, and technical documentation. Notably, the MDR specifically addresses nanomaterials, which may include certain conductive polymer nanocomposites, requiring additional risk assessment and safety data.
ISO 10993 series standards form the cornerstone of biocompatibility testing for medical-grade polymers globally. For conductive polymers, ISO 10993-1 guides the biological evaluation process, while ISO 10993-5 (cytotoxicity) and ISO 10993-6 (implantation) are particularly relevant. Additionally, IEC 60601-1 addresses electrical safety concerns specific to conductive materials in medical devices.
Material-specific standards such as ASTM F2129 for corrosion testing and ASTM F1980 for accelerated aging are essential for evaluating the long-term stability of conductive polymer composites. The USP Class VI certification represents another important benchmark for materials intended for medical applications, focusing on biocompatibility aspects.
Emerging regulatory considerations include leachables and extractables testing (ISO 10993-18) to identify potential toxic compounds that might migrate from the polymer matrix. This is particularly critical for conductive polymers that may incorporate metallic nanoparticles or carbon-based fillers with uncertain toxicological profiles.
Regional variations in regulatory approaches present significant challenges for global market access. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have distinct requirements that often necessitate country-specific testing and documentation. Harmonization efforts through the International Medical Device Regulators Forum (IMDRF) aim to reduce these disparities but have achieved only partial success.
Recent regulatory trends indicate increasing scrutiny of novel materials, with particular emphasis on long-term safety data and post-market surveillance. Manufacturers developing conductive polymer composites must therefore implement robust quality management systems compliant with ISO 13485 and maintain comprehensive technical files documenting material characterization, risk management, and clinical evidence throughout the product lifecycle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!