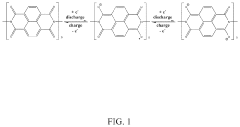
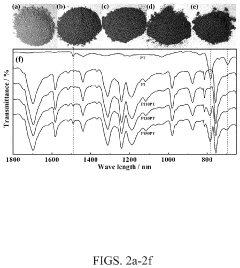
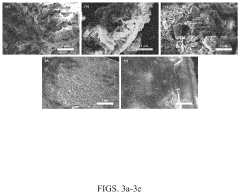
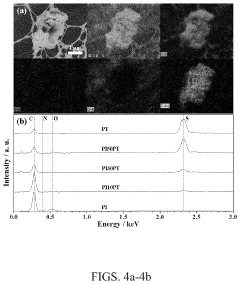
Analyzing Electrode Kinetics in Conductive Polymer Composites
OCT 23, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Conductive Polymer Electrode Kinetics Background and Objectives
Conductive polymer composites (CPCs) have emerged as a significant technological advancement in the field of materials science over the past four decades. These materials combine the electrical properties of conductive polymers with the mechanical and processing advantages of traditional polymers, creating versatile materials with applications spanning from energy storage to biomedical devices. The evolution of CPCs began in the 1970s with the discovery of conductive polymers by Alan Heeger, Alan MacDiarmid, and Hideki Shirakawa, who were later awarded the Nobel Prize in Chemistry in 2000 for this groundbreaking work.
The technological trajectory of CPCs has been characterized by continuous improvements in conductivity, stability, and processability. Early conductive polymers faced significant limitations in terms of environmental stability and mechanical properties, but subsequent research has led to remarkable advancements. The incorporation of various conductive fillers such as carbon nanotubes, graphene, and metal nanoparticles has further expanded the performance envelope of these materials, enabling tailored electrical, thermal, and mechanical properties.
Electrode kinetics—the study of charge transfer processes at electrode-electrolyte interfaces—represents a critical aspect of CPC performance in electrochemical applications. Understanding these kinetics is essential for optimizing energy storage devices, sensors, and electrochemical actuators. The field has progressed from basic empirical observations to sophisticated mechanistic models that account for the complex interplay between polymer structure, filler distribution, and interfacial phenomena.
Current research objectives in CPC electrode kinetics focus on several key areas. First, elucidating the fundamental mechanisms of charge transfer at polymer-electrolyte interfaces remains a priority, as these processes directly impact device performance. Second, researchers aim to develop predictive models that can accurately describe electrode behavior under various operating conditions, enabling more efficient material design. Third, there is significant interest in enhancing the rate capability and cycling stability of CPC electrodes for high-performance energy storage applications.
The technological goals for CPC electrode kinetics include achieving higher power densities in energy storage devices, improving the sensitivity and response time of electrochemical sensors, and developing self-healing electrode materials with extended operational lifetimes. Additionally, there is growing emphasis on environmentally sustainable CPCs that maintain excellent electrochemical performance while reducing reliance on rare or toxic materials.
As we look toward future developments, the integration of artificial intelligence and high-throughput experimentation promises to accelerate the discovery and optimization of novel CPC materials with enhanced electrode kinetics. The convergence of nanotechnology, computational modeling, and advanced characterization techniques is expected to drive breakthroughs in understanding and controlling charge transfer processes at the molecular level, ultimately leading to transformative applications across multiple industries.
The technological trajectory of CPCs has been characterized by continuous improvements in conductivity, stability, and processability. Early conductive polymers faced significant limitations in terms of environmental stability and mechanical properties, but subsequent research has led to remarkable advancements. The incorporation of various conductive fillers such as carbon nanotubes, graphene, and metal nanoparticles has further expanded the performance envelope of these materials, enabling tailored electrical, thermal, and mechanical properties.
Electrode kinetics—the study of charge transfer processes at electrode-electrolyte interfaces—represents a critical aspect of CPC performance in electrochemical applications. Understanding these kinetics is essential for optimizing energy storage devices, sensors, and electrochemical actuators. The field has progressed from basic empirical observations to sophisticated mechanistic models that account for the complex interplay between polymer structure, filler distribution, and interfacial phenomena.
Current research objectives in CPC electrode kinetics focus on several key areas. First, elucidating the fundamental mechanisms of charge transfer at polymer-electrolyte interfaces remains a priority, as these processes directly impact device performance. Second, researchers aim to develop predictive models that can accurately describe electrode behavior under various operating conditions, enabling more efficient material design. Third, there is significant interest in enhancing the rate capability and cycling stability of CPC electrodes for high-performance energy storage applications.
The technological goals for CPC electrode kinetics include achieving higher power densities in energy storage devices, improving the sensitivity and response time of electrochemical sensors, and developing self-healing electrode materials with extended operational lifetimes. Additionally, there is growing emphasis on environmentally sustainable CPCs that maintain excellent electrochemical performance while reducing reliance on rare or toxic materials.
As we look toward future developments, the integration of artificial intelligence and high-throughput experimentation promises to accelerate the discovery and optimization of novel CPC materials with enhanced electrode kinetics. The convergence of nanotechnology, computational modeling, and advanced characterization techniques is expected to drive breakthroughs in understanding and controlling charge transfer processes at the molecular level, ultimately leading to transformative applications across multiple industries.
Market Applications and Demand Analysis for Polymer Composites
The global market for conductive polymer composites has witnessed substantial growth in recent years, driven by increasing demand across multiple industries. The market size was valued at approximately $3.9 billion in 2022 and is projected to reach $8.6 billion by 2030, representing a compound annual growth rate of 10.4%. This growth trajectory is primarily fueled by the expanding applications in electronics, automotive, aerospace, and healthcare sectors.
In the electronics industry, conductive polymer composites are increasingly being utilized in flexible displays, wearable technology, and printed electronics. The miniaturization trend in consumer electronics has created a significant demand for materials that can provide electrical conductivity while maintaining flexibility and lightweight properties. The global wearable technology market alone is expected to exceed $265 billion by 2026, creating substantial opportunities for conductive polymer composites.
The automotive sector represents another major market for these materials, particularly with the rapid growth of electric vehicles (EVs). Conductive polymer composites are essential components in battery systems, sensors, and electromagnetic interference (EMI) shielding applications. With global EV sales projected to reach 26.8 million units by 2030, the demand for high-performance electrode materials with optimized kinetics is set to increase dramatically.
Healthcare applications are emerging as a promising growth area, with conductive polymer composites being utilized in biosensors, drug delivery systems, and tissue engineering. The global biosensors market is expected to reach $42 billion by 2028, with electrochemical biosensors—many utilizing conductive polymer composites—accounting for a significant portion of this market.
Regional analysis indicates that Asia-Pacific currently dominates the market, accounting for approximately 45% of global demand, followed by North America and Europe. China, Japan, and South Korea are the leading consumers in the Asia-Pacific region, driven by their robust electronics manufacturing sectors and growing automotive industries.
Customer requirements are increasingly focused on enhanced electrode kinetics to improve device performance, efficiency, and reliability. End-users are demanding materials with faster electron transfer rates, improved stability under various environmental conditions, and compatibility with existing manufacturing processes. Additionally, there is growing interest in sustainable and environmentally friendly conductive composites, reflecting broader industry trends toward green technology.
Market challenges include price volatility of raw materials, technical limitations in achieving consistent conductivity across large surface areas, and competition from alternative technologies. However, ongoing research in electrode kinetics is addressing many of these challenges, potentially opening new market opportunities and applications for conductive polymer composites.
In the electronics industry, conductive polymer composites are increasingly being utilized in flexible displays, wearable technology, and printed electronics. The miniaturization trend in consumer electronics has created a significant demand for materials that can provide electrical conductivity while maintaining flexibility and lightweight properties. The global wearable technology market alone is expected to exceed $265 billion by 2026, creating substantial opportunities for conductive polymer composites.
The automotive sector represents another major market for these materials, particularly with the rapid growth of electric vehicles (EVs). Conductive polymer composites are essential components in battery systems, sensors, and electromagnetic interference (EMI) shielding applications. With global EV sales projected to reach 26.8 million units by 2030, the demand for high-performance electrode materials with optimized kinetics is set to increase dramatically.
Healthcare applications are emerging as a promising growth area, with conductive polymer composites being utilized in biosensors, drug delivery systems, and tissue engineering. The global biosensors market is expected to reach $42 billion by 2028, with electrochemical biosensors—many utilizing conductive polymer composites—accounting for a significant portion of this market.
Regional analysis indicates that Asia-Pacific currently dominates the market, accounting for approximately 45% of global demand, followed by North America and Europe. China, Japan, and South Korea are the leading consumers in the Asia-Pacific region, driven by their robust electronics manufacturing sectors and growing automotive industries.
Customer requirements are increasingly focused on enhanced electrode kinetics to improve device performance, efficiency, and reliability. End-users are demanding materials with faster electron transfer rates, improved stability under various environmental conditions, and compatibility with existing manufacturing processes. Additionally, there is growing interest in sustainable and environmentally friendly conductive composites, reflecting broader industry trends toward green technology.
Market challenges include price volatility of raw materials, technical limitations in achieving consistent conductivity across large surface areas, and competition from alternative technologies. However, ongoing research in electrode kinetics is addressing many of these challenges, potentially opening new market opportunities and applications for conductive polymer composites.
Current Challenges in Electrode Kinetics Research
Despite significant advancements in conductive polymer composite (CPC) research, electrode kinetics analysis faces several persistent challenges that impede both fundamental understanding and practical applications. The complexity of charge transfer mechanisms at the electrode-electrolyte interface remains insufficiently characterized, particularly regarding the interplay between electronic and ionic conductivity in heterogeneous composite systems.
A primary obstacle is the multi-scale nature of electrode processes in CPCs, which span from molecular interactions to macroscopic phenomena. Current analytical techniques struggle to simultaneously capture events occurring at these different scales, resulting in incomplete mechanistic models. The dynamic evolution of electrode surfaces during operation further complicates analysis, as polymer matrices often undergo structural reorganization, swelling, or degradation that alters kinetic parameters over time.
Reproducibility presents another significant challenge, with electrode performance showing high sensitivity to processing conditions, composite morphology, and interfacial properties. Minor variations in fabrication protocols can lead to substantial differences in electrode behavior, making standardization difficult and hindering comparative studies across research groups.
The integration of multiple components in modern CPCs—including various polymers, conductive fillers, and functional additives—creates complex interfaces whose contributions to overall electrode kinetics remain poorly delineated. Traditional electrochemical methods often provide only averaged responses that mask the distinct roles of individual components and their synergistic effects.
Temperature dependence of electrode kinetics in CPCs represents an underexplored area, with limited systematic studies on activation energies and rate-determining steps across different temperature regimes. This knowledge gap restricts the deployment of CPC electrodes in applications requiring thermal stability or operation across wide temperature ranges.
Advanced in situ and operando characterization techniques, while promising, face limitations when applied to CPCs due to beam damage, limited spatial resolution, or incompatibility with certain polymer systems. The development of non-destructive, high-resolution methods specifically adapted for soft, heterogeneous materials remains an ongoing challenge.
Computational modeling approaches struggle with the inherent complexity of CPCs, particularly in accurately representing the dynamic nature of polymer-filler interfaces and their evolution during electrochemical processes. Current models often rely on simplifications that fail to capture the full complexity of real-world systems, limiting their predictive power for electrode design and optimization.
A primary obstacle is the multi-scale nature of electrode processes in CPCs, which span from molecular interactions to macroscopic phenomena. Current analytical techniques struggle to simultaneously capture events occurring at these different scales, resulting in incomplete mechanistic models. The dynamic evolution of electrode surfaces during operation further complicates analysis, as polymer matrices often undergo structural reorganization, swelling, or degradation that alters kinetic parameters over time.
Reproducibility presents another significant challenge, with electrode performance showing high sensitivity to processing conditions, composite morphology, and interfacial properties. Minor variations in fabrication protocols can lead to substantial differences in electrode behavior, making standardization difficult and hindering comparative studies across research groups.
The integration of multiple components in modern CPCs—including various polymers, conductive fillers, and functional additives—creates complex interfaces whose contributions to overall electrode kinetics remain poorly delineated. Traditional electrochemical methods often provide only averaged responses that mask the distinct roles of individual components and their synergistic effects.
Temperature dependence of electrode kinetics in CPCs represents an underexplored area, with limited systematic studies on activation energies and rate-determining steps across different temperature regimes. This knowledge gap restricts the deployment of CPC electrodes in applications requiring thermal stability or operation across wide temperature ranges.
Advanced in situ and operando characterization techniques, while promising, face limitations when applied to CPCs due to beam damage, limited spatial resolution, or incompatibility with certain polymer systems. The development of non-destructive, high-resolution methods specifically adapted for soft, heterogeneous materials remains an ongoing challenge.
Computational modeling approaches struggle with the inherent complexity of CPCs, particularly in accurately representing the dynamic nature of polymer-filler interfaces and their evolution during electrochemical processes. Current models often rely on simplifications that fail to capture the full complexity of real-world systems, limiting their predictive power for electrode design and optimization.
Methodologies for Analyzing Electrode Kinetics
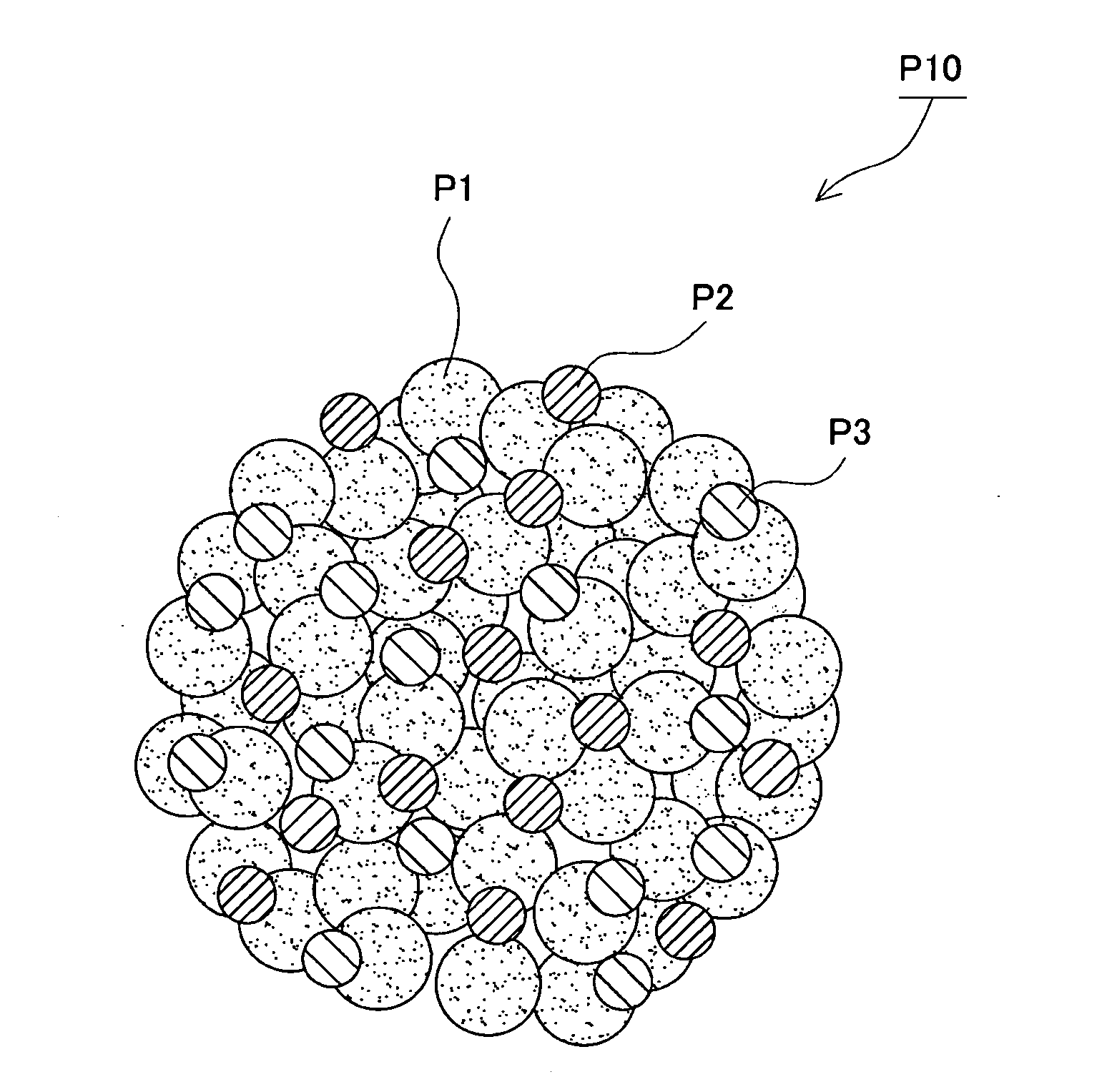
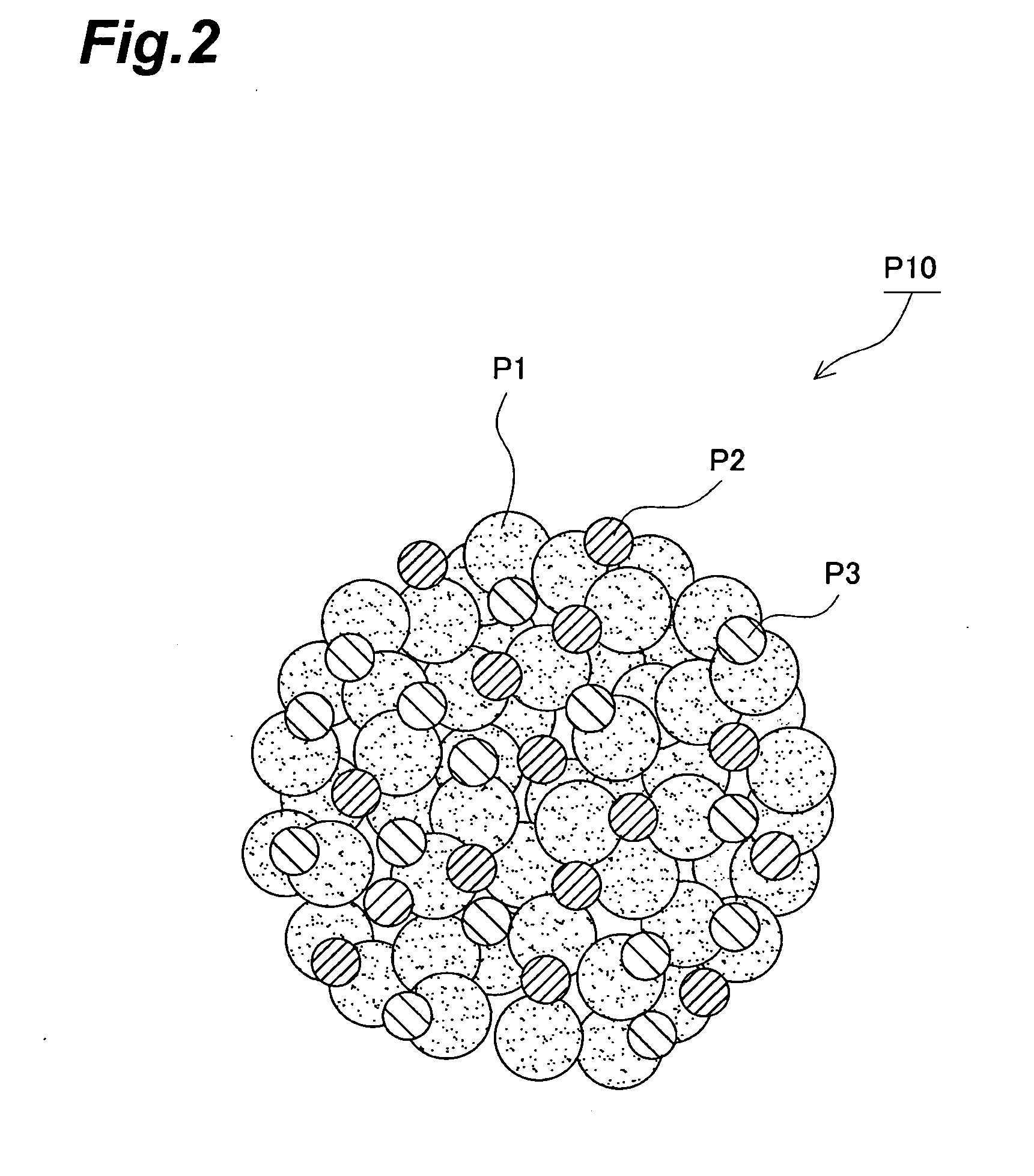
01 Conductive polymer composites for electrode applications
Conductive polymer composites can be formulated specifically for electrode applications, offering improved electrical conductivity and electrochemical performance. These composites typically combine conductive polymers with other materials to enhance electrode kinetics, resulting in better charge transfer and reaction rates. The electrode materials based on these composites demonstrate superior performance in various electrochemical devices including batteries, fuel cells, and sensors.- Conductive polymer composites for electrode applications: Conductive polymer composites can be formulated specifically for electrode applications, offering improved electrical conductivity and electrochemical performance. These composites typically combine conductive polymers with other materials to enhance electrode kinetics, resulting in better charge transfer and reaction rates. The electrode materials based on these composites demonstrate enhanced stability and efficiency in various electrochemical systems.
- Electrode kinetics enhancement through nanostructured composites: Nanostructured conductive polymer composites can significantly improve electrode kinetics through increased surface area and optimized interfacial properties. By incorporating nanomaterials such as carbon nanotubes, graphene, or metal nanoparticles into conductive polymers, the resulting composites exhibit faster electron transfer rates and improved electrochemical response. These nanostructured composites enable more efficient electrode processes with reduced activation barriers.
- Charge transfer mechanisms in polymer composite electrodes: The charge transfer mechanisms in conductive polymer composite electrodes involve complex interactions between polymer chains, conductive fillers, and electrolytes. Understanding these mechanisms is crucial for optimizing electrode kinetics. Factors affecting charge transfer include polymer morphology, filler distribution, interfacial resistance, and ion transport pathways. By controlling these parameters, electrode kinetics can be significantly enhanced, leading to improved performance in electrochemical devices.
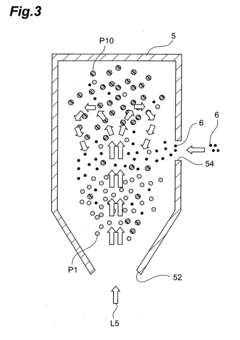
- Fabrication methods for high-performance polymer composite electrodes: Various fabrication techniques can be employed to create conductive polymer composite electrodes with optimized kinetics. These methods include solution processing, electrodeposition, layer-by-layer assembly, and in-situ polymerization. Each technique offers different advantages in terms of controlling composite structure, interfacial properties, and electrochemical performance. Advanced manufacturing approaches can produce electrodes with tailored porosity, thickness, and composition to enhance reaction kinetics for specific applications.
- Applications of polymer composite electrodes with enhanced kinetics: Conductive polymer composite electrodes with enhanced kinetics find applications in various electrochemical devices including batteries, supercapacitors, fuel cells, sensors, and electrocatalytic systems. The improved electrode kinetics translate to better device performance metrics such as power density, energy efficiency, response time, and cycle life. These advanced electrode materials enable the development of next-generation energy storage and conversion technologies with superior performance characteristics.
02 Electrode kinetics enhancement through nanostructured composites
Nanostructured conductive polymer composites can significantly enhance electrode kinetics through increased surface area and improved interfacial properties. By incorporating nanomaterials such as carbon nanotubes, graphene, or metal nanoparticles into conductive polymers, the resulting composites exhibit faster electron transfer rates and reduced charge transfer resistance. These nanostructured composites enable rapid electrochemical reactions at the electrode surface, making them suitable for high-performance energy storage and conversion applications.Expand Specific Solutions03 Polymer composite electrodes with tailored ionic conductivity
Conductive polymer composites can be designed with tailored ionic conductivity properties to optimize electrode kinetics in electrochemical systems. By controlling the composition and morphology of the polymer matrix, ion transport pathways can be engineered to facilitate faster ion movement. These composites often incorporate specific additives or functional groups that enhance ion mobility while maintaining electronic conductivity, resulting in improved overall electrode performance and reaction kinetics.Expand Specific Solutions04 Interface engineering for improved electrode kinetics
Interface engineering in conductive polymer composites is crucial for optimizing electrode kinetics. By modifying the interfaces between different components within the composite or between the composite and electrolyte, charge transfer barriers can be reduced. Various techniques including surface functionalization, incorporation of coupling agents, or creation of hierarchical structures can be employed to enhance the interfacial properties. These engineered interfaces facilitate faster electron and ion transfer, resulting in improved electrochemical performance.Expand Specific Solutions05 Stability and durability of conductive polymer composite electrodes
Enhancing the stability and durability of conductive polymer composite electrodes is essential for maintaining consistent electrode kinetics over extended periods. Various approaches include cross-linking of polymer chains, incorporation of stabilizing additives, or encapsulation techniques to protect against degradation. These methods help preserve the electrochemical properties and structural integrity of the composite electrodes during repeated cycling or under harsh operating conditions, ensuring reliable performance in practical applications.Expand Specific Solutions
Leading Research Groups and Industrial Players
The electrode kinetics in conductive polymer composites market is in a growth phase, with increasing applications in energy storage, electronics, and sensors. The market is expanding rapidly due to rising demand for advanced materials with enhanced electrical properties. Technologically, the field shows varying maturity levels, with companies like Arkema, BASF, and Celanese leading in polymer development, while TDK, LG Energy Solution, and SK Innovation focus on application-specific innovations. Research institutions like MIT, Duke University, and CNRS contribute fundamental breakthroughs. The competitive landscape features established chemical corporations alongside specialized players like Blue Solutions and Conscious Labs, with collaboration between industry and academia accelerating technological advancement and commercialization of conductive polymer composite solutions.
Arkema, Inc.
Technical Solution: Arkema has developed PEBAX® electrostatic discharge (ESD) polymer composites with precisely controlled electrode kinetics. Their approach involves incorporating conductive nanofillers (carbon nanotubes and graphene) into polymer matrices using proprietary dispersion techniques. The company's research focuses on optimizing interfacial interactions between polymer chains and conductive fillers to enhance charge transfer rates. Their KynarFlex® technology utilizes polyvinylidene fluoride (PVDF) copolymers with controlled crystallinity to create electrodes with tunable kinetic properties. Arkema has demonstrated that controlling the microstructure of these composites can significantly reduce charge transfer resistance, with impedance measurements showing up to 40% improvement in electrode kinetics compared to conventional systems.
Strengths: Superior dispersion technology for nanomaterials in polymer matrices; extensive polymer chemistry expertise; established manufacturing infrastructure. Weaknesses: Higher production costs compared to traditional materials; challenges in scaling production while maintaining consistent electrode kinetic properties.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed proprietary conductive polymer composite electrodes optimized for high-performance energy storage applications. Their technology focuses on controlling electrode kinetics through precise engineering of polymer-carbon interfaces and three-dimensional conductive networks. LG's approach involves the use of functionalized polymer binders that form strong chemical bonds with active materials and conductive additives, creating robust electronic pathways throughout the electrode structure. Their research has demonstrated that controlling the spatial distribution of conductive fillers within the polymer matrix can significantly enhance charge transfer rates. Recent developments include gradient-structured electrodes that show up to 45% improvement in rate capability compared to conventional designs. LG Energy Solution has also pioneered advanced manufacturing techniques that enable precise control of composite microstructure during electrode fabrication, resulting in consistent electrochemical performance across large-format cells.
Strengths: Extensive experience in commercial battery production; strong integration between materials research and device manufacturing; established quality control systems. Weaknesses: Proprietary nature limits academic collaboration; primarily focused on lithium-ion battery applications rather than broader electrochemical systems.
Key Scientific Breakthroughs in Polymer Conductivity
Electron conductive polymer composites and their use as electrode materials
PatentInactiveUS20200176762A1
Innovation
- A composite material is developed comprising an ion-permeable, non-electron conductive organic polymer core coated with an electron conductive polymer, specifically aromatic polyimide coated with conductive polythiophene (PI@PT), which enhances electronic conductivity and stability, enabling high reversible capacity and cycling stability in lithium-ion batteries.
Composite particle for electrode and method for producing same, electrode and method for producing same, and electrochemical device and method for producing same
PatentInactiveUS20070003836A1
Innovation
- A composite particle for electrodes is formed through a granulating step where conductive additives and binders are brought into close contact with electrode active materials using a stock solution and spray-drying process, ensuring effective dispersion and contact, thereby establishing a satisfactory electron conduction network.
Environmental Impact and Sustainability Considerations
The environmental implications of conductive polymer composites (CPCs) have become increasingly significant as these materials gain prominence in various technological applications. The production processes for CPCs often involve energy-intensive methods and potentially hazardous chemicals, raising concerns about their ecological footprint. Particularly, the synthesis of conductive polymers frequently requires organic solvents that may contribute to air and water pollution if not properly managed. Additionally, the incorporation of metallic nanoparticles or carbon-based fillers, while enhancing electrode kinetics, introduces challenges related to resource depletion and waste management.
Life cycle assessment (LCA) studies of CPCs reveal that their environmental impact varies significantly depending on the specific materials and manufacturing techniques employed. For instance, polyaniline-based composites generally demonstrate lower environmental burdens compared to those utilizing rare earth elements or precious metals as conductive fillers. The electrode kinetics properties, which are central to the performance of these materials, can be optimized through green chemistry approaches that minimize the use of harmful reagents and reduce energy consumption during synthesis.
Sustainability considerations are driving innovation in CPC development, with researchers increasingly focusing on bio-based polymers and environmentally benign processing methods. The utilization of renewable resources, such as cellulose or lignin derivatives, as polymer matrices represents a promising direction for reducing the carbon footprint of these materials. Furthermore, water-based synthesis routes are being explored as alternatives to traditional solvent-based methods, potentially decreasing the emission of volatile organic compounds during production.
The end-of-life management of CPCs presents both challenges and opportunities for sustainability. While the complex composition of these materials can complicate recycling efforts, advances in separation technologies are enabling more efficient recovery of valuable components. The development of biodegradable conductive polymers offers another pathway to mitigate environmental impact, though balancing degradability with the required electrode kinetics performance remains a technical challenge.
Energy efficiency considerations are paramount when evaluating the sustainability of CPCs in electrochemical applications. Materials that facilitate rapid electrode kinetics at lower energy inputs contribute to reduced operational environmental impacts across various technologies, from batteries to sensors. Research indicates that optimizing the interface between conductive fillers and polymer matrices can significantly enhance charge transfer efficiency, thereby reducing energy requirements during device operation.
Regulatory frameworks worldwide are increasingly addressing the environmental aspects of advanced materials like CPCs. Compliance with regulations such as REACH in Europe and similar initiatives globally necessitates thorough assessment of potential environmental and health risks associated with these composites. Manufacturers are responding by implementing greener production practices and developing more comprehensive material safety data sheets that account for the unique properties of CPCs, including their electrode kinetics behavior under various environmental conditions.
Life cycle assessment (LCA) studies of CPCs reveal that their environmental impact varies significantly depending on the specific materials and manufacturing techniques employed. For instance, polyaniline-based composites generally demonstrate lower environmental burdens compared to those utilizing rare earth elements or precious metals as conductive fillers. The electrode kinetics properties, which are central to the performance of these materials, can be optimized through green chemistry approaches that minimize the use of harmful reagents and reduce energy consumption during synthesis.
Sustainability considerations are driving innovation in CPC development, with researchers increasingly focusing on bio-based polymers and environmentally benign processing methods. The utilization of renewable resources, such as cellulose or lignin derivatives, as polymer matrices represents a promising direction for reducing the carbon footprint of these materials. Furthermore, water-based synthesis routes are being explored as alternatives to traditional solvent-based methods, potentially decreasing the emission of volatile organic compounds during production.
The end-of-life management of CPCs presents both challenges and opportunities for sustainability. While the complex composition of these materials can complicate recycling efforts, advances in separation technologies are enabling more efficient recovery of valuable components. The development of biodegradable conductive polymers offers another pathway to mitigate environmental impact, though balancing degradability with the required electrode kinetics performance remains a technical challenge.
Energy efficiency considerations are paramount when evaluating the sustainability of CPCs in electrochemical applications. Materials that facilitate rapid electrode kinetics at lower energy inputs contribute to reduced operational environmental impacts across various technologies, from batteries to sensors. Research indicates that optimizing the interface between conductive fillers and polymer matrices can significantly enhance charge transfer efficiency, thereby reducing energy requirements during device operation.
Regulatory frameworks worldwide are increasingly addressing the environmental aspects of advanced materials like CPCs. Compliance with regulations such as REACH in Europe and similar initiatives globally necessitates thorough assessment of potential environmental and health risks associated with these composites. Manufacturers are responding by implementing greener production practices and developing more comprehensive material safety data sheets that account for the unique properties of CPCs, including their electrode kinetics behavior under various environmental conditions.
Scalability and Manufacturing Challenges
The scaling of conductive polymer composite (CPC) electrode production from laboratory to industrial scale presents significant challenges that impact electrode kinetics performance. Current manufacturing processes often struggle with maintaining consistent dispersion of conductive fillers throughout the polymer matrix when production volumes increase. This inconsistency directly affects electron transfer rates and electrochemical performance, creating variability in product quality that is unacceptable for commercial applications.
Material processing techniques that work effectively at small scales frequently encounter complications during scale-up. For instance, solution processing methods that achieve excellent nanoscale dispersion in laboratory settings may become economically prohibitive or technically challenging when implemented in large-scale production environments. The increased mixing volumes can lead to agglomeration of conductive particles, creating "dead zones" with poor conductivity and compromised electrode kinetics.
Temperature control represents another critical manufacturing challenge. The curing or annealing processes that establish optimal polymer-filler interfaces require precise thermal management across larger production batches. Even minor temperature gradients can result in varying degrees of crystallinity within the polymer matrix, directly affecting charge transfer characteristics at the electrode interface.
Equipment design for large-scale production introduces additional complexities. Conventional manufacturing equipment may not adequately address the unique rheological properties of polymer composites, particularly those with high filler content necessary for enhanced conductivity. Specialized equipment development often requires substantial capital investment, creating barriers to market entry for smaller manufacturers.
Environmental considerations further complicate scaling efforts. Many high-performance conductive polymers require processing with volatile organic solvents that pose health and environmental risks when used at industrial scales. Transitioning to greener manufacturing approaches while maintaining electrode kinetic performance represents a significant challenge that researchers continue to address through alternative solvent systems and solvent-free processing methods.
Quality control methodologies must evolve alongside production scaling. Traditional sampling approaches may prove insufficient for detecting localized defects or inconsistencies that impact electrode performance. Advanced in-line monitoring techniques are needed to evaluate critical parameters such as filler distribution, interface quality, and electrochemical response characteristics during manufacturing rather than through post-production testing.
Cost-effectiveness ultimately determines commercial viability. While laboratory-scale production can utilize expensive materials and time-intensive processes to achieve optimal electrode kinetics, industrial applications demand economically sustainable approaches. Finding the balance between performance optimization and manufacturing efficiency remains a central challenge in translating promising CPC electrode technologies from research to commercial reality.
Material processing techniques that work effectively at small scales frequently encounter complications during scale-up. For instance, solution processing methods that achieve excellent nanoscale dispersion in laboratory settings may become economically prohibitive or technically challenging when implemented in large-scale production environments. The increased mixing volumes can lead to agglomeration of conductive particles, creating "dead zones" with poor conductivity and compromised electrode kinetics.
Temperature control represents another critical manufacturing challenge. The curing or annealing processes that establish optimal polymer-filler interfaces require precise thermal management across larger production batches. Even minor temperature gradients can result in varying degrees of crystallinity within the polymer matrix, directly affecting charge transfer characteristics at the electrode interface.
Equipment design for large-scale production introduces additional complexities. Conventional manufacturing equipment may not adequately address the unique rheological properties of polymer composites, particularly those with high filler content necessary for enhanced conductivity. Specialized equipment development often requires substantial capital investment, creating barriers to market entry for smaller manufacturers.
Environmental considerations further complicate scaling efforts. Many high-performance conductive polymers require processing with volatile organic solvents that pose health and environmental risks when used at industrial scales. Transitioning to greener manufacturing approaches while maintaining electrode kinetic performance represents a significant challenge that researchers continue to address through alternative solvent systems and solvent-free processing methods.
Quality control methodologies must evolve alongside production scaling. Traditional sampling approaches may prove insufficient for detecting localized defects or inconsistencies that impact electrode performance. Advanced in-line monitoring techniques are needed to evaluate critical parameters such as filler distribution, interface quality, and electrochemical response characteristics during manufacturing rather than through post-production testing.
Cost-effectiveness ultimately determines commercial viability. While laboratory-scale production can utilize expensive materials and time-intensive processes to achieve optimal electrode kinetics, industrial applications demand economically sustainable approaches. Finding the balance between performance optimization and manufacturing efficiency remains a central challenge in translating promising CPC electrode technologies from research to commercial reality.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!