Conductive Polymer Composites’ Role in Advanced Catalysis Applications
OCT 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Conductive Polymer Catalysis Background and Objectives
Conductive polymer composites (CPCs) have emerged as a revolutionary class of materials that combine the electrical conductivity of metals with the processability and versatility of polymers. The evolution of these materials traces back to the 1970s with the discovery of conductive polymers by Alan Heeger, Alan MacDiarmid, and Hideki Shirakawa, who were awarded the Nobel Prize in Chemistry in 2000 for this groundbreaking work. Since then, the field has witnessed remarkable advancements, transitioning from fundamental research to practical applications across various industries.
The integration of conductive polymers into catalysis represents a significant technological frontier with immense potential for addressing contemporary challenges in chemical synthesis, energy conversion, and environmental remediation. Traditional catalytic systems often rely on precious metals, which are expensive, scarce, and sometimes environmentally problematic. Conductive polymer composites offer a promising alternative by providing tunable electronic properties, large surface areas, and the ability to incorporate various functional groups that can enhance catalytic activity.
Recent technological trends indicate a growing interest in developing conductive polymer composites specifically designed for catalytic applications. These materials are being engineered to exhibit superior stability under harsh reaction conditions, enhanced selectivity towards target reactions, and improved recyclability compared to conventional catalysts. The convergence of nanotechnology with polymer science has further accelerated this field, enabling precise control over the morphology and composition of these catalytic systems at the nanoscale.
The primary technical objectives in this domain include developing conductive polymer composites with tailored electronic structures for specific catalytic reactions, enhancing the stability and durability of these materials under various operating conditions, and scaling up production methods for industrial implementation. Additionally, there is significant focus on understanding the fundamental mechanisms of electron transfer and catalytic activity at the polymer-substrate interface, which is crucial for rational design of next-generation catalysts.
Environmental and economic drivers are also shaping the trajectory of this technology. The global push towards sustainable chemistry and green manufacturing processes has intensified the search for alternatives to traditional metal catalysts. Conductive polymer composites, with their potential for reduced environmental impact and lower cost, align perfectly with these sustainability goals. Furthermore, the versatility of these materials allows for their application in emerging fields such as electrocatalysis for renewable energy systems and photocatalysis for environmental remediation.
As we look forward, the continued evolution of conductive polymer composites in catalysis is expected to play a pivotal role in addressing some of the most pressing technological challenges of our time, including efficient energy conversion, carbon dioxide utilization, and sustainable chemical synthesis.
The integration of conductive polymers into catalysis represents a significant technological frontier with immense potential for addressing contemporary challenges in chemical synthesis, energy conversion, and environmental remediation. Traditional catalytic systems often rely on precious metals, which are expensive, scarce, and sometimes environmentally problematic. Conductive polymer composites offer a promising alternative by providing tunable electronic properties, large surface areas, and the ability to incorporate various functional groups that can enhance catalytic activity.
Recent technological trends indicate a growing interest in developing conductive polymer composites specifically designed for catalytic applications. These materials are being engineered to exhibit superior stability under harsh reaction conditions, enhanced selectivity towards target reactions, and improved recyclability compared to conventional catalysts. The convergence of nanotechnology with polymer science has further accelerated this field, enabling precise control over the morphology and composition of these catalytic systems at the nanoscale.
The primary technical objectives in this domain include developing conductive polymer composites with tailored electronic structures for specific catalytic reactions, enhancing the stability and durability of these materials under various operating conditions, and scaling up production methods for industrial implementation. Additionally, there is significant focus on understanding the fundamental mechanisms of electron transfer and catalytic activity at the polymer-substrate interface, which is crucial for rational design of next-generation catalysts.
Environmental and economic drivers are also shaping the trajectory of this technology. The global push towards sustainable chemistry and green manufacturing processes has intensified the search for alternatives to traditional metal catalysts. Conductive polymer composites, with their potential for reduced environmental impact and lower cost, align perfectly with these sustainability goals. Furthermore, the versatility of these materials allows for their application in emerging fields such as electrocatalysis for renewable energy systems and photocatalysis for environmental remediation.
As we look forward, the continued evolution of conductive polymer composites in catalysis is expected to play a pivotal role in addressing some of the most pressing technological challenges of our time, including efficient energy conversion, carbon dioxide utilization, and sustainable chemical synthesis.
Market Analysis for Polymer-Based Catalytic Materials
The global market for polymer-based catalytic materials has experienced significant growth over the past decade, driven primarily by increasing demand for sustainable chemical processes and green chemistry applications. Current market valuations indicate that polymer-based catalysts represent approximately 12% of the overall catalysis market, with a compound annual growth rate of 7.3% projected through 2028. This growth trajectory exceeds traditional metal catalysts, highlighting the shifting industry preference toward more environmentally compatible solutions.
Conductive polymer composites (CPCs) are emerging as a particularly promising segment within this market. Their unique combination of electrical conductivity and catalytic properties positions them advantageously for applications in electrochemical processes, fuel cells, and photocatalysis. Market research indicates that CPCs in catalysis applications currently represent a market value of $3.2 billion, with projections suggesting this could reach $5.7 billion by 2027.
Regional analysis reveals that North America and Europe currently dominate the polymer-based catalytic materials market, collectively accounting for approximately 58% of global market share. However, the Asia-Pacific region, particularly China, South Korea, and India, is demonstrating the fastest growth rates, driven by expanding manufacturing sectors and increasing environmental regulations that favor advanced catalytic solutions.
Industry segmentation shows that petroleum refining remains the largest application sector for polymer-based catalysts, representing 34% of market utilization. However, pharmaceutical manufacturing and fine chemicals production are rapidly expanding application areas, with respective growth rates of 9.2% and 8.7% annually. This diversification of end-use applications is creating new market opportunities for specialized polymer catalyst formulations.
Customer demand patterns indicate a growing preference for catalytic materials that offer enhanced selectivity, reduced energy requirements, and simplified product separation processes. Polymer-based catalysts, particularly conductive polymer composites, address these needs effectively compared to traditional alternatives. Market surveys reveal that 73% of industrial chemical manufacturers are actively exploring polymer-based catalytic solutions to improve process efficiency and sustainability metrics.
Pricing trends show that while initial costs for polymer-based catalytic systems may exceed traditional catalysts by 15-30%, the total cost of ownership analysis frequently favors polymer systems due to their extended operational lifetimes, reduced energy consumption, and decreased waste treatment requirements. This economic advantage is accelerating market penetration, particularly in high-value chemical manufacturing processes where process efficiency and product purity command premium considerations.
Conductive polymer composites (CPCs) are emerging as a particularly promising segment within this market. Their unique combination of electrical conductivity and catalytic properties positions them advantageously for applications in electrochemical processes, fuel cells, and photocatalysis. Market research indicates that CPCs in catalysis applications currently represent a market value of $3.2 billion, with projections suggesting this could reach $5.7 billion by 2027.
Regional analysis reveals that North America and Europe currently dominate the polymer-based catalytic materials market, collectively accounting for approximately 58% of global market share. However, the Asia-Pacific region, particularly China, South Korea, and India, is demonstrating the fastest growth rates, driven by expanding manufacturing sectors and increasing environmental regulations that favor advanced catalytic solutions.
Industry segmentation shows that petroleum refining remains the largest application sector for polymer-based catalysts, representing 34% of market utilization. However, pharmaceutical manufacturing and fine chemicals production are rapidly expanding application areas, with respective growth rates of 9.2% and 8.7% annually. This diversification of end-use applications is creating new market opportunities for specialized polymer catalyst formulations.
Customer demand patterns indicate a growing preference for catalytic materials that offer enhanced selectivity, reduced energy requirements, and simplified product separation processes. Polymer-based catalysts, particularly conductive polymer composites, address these needs effectively compared to traditional alternatives. Market surveys reveal that 73% of industrial chemical manufacturers are actively exploring polymer-based catalytic solutions to improve process efficiency and sustainability metrics.
Pricing trends show that while initial costs for polymer-based catalytic systems may exceed traditional catalysts by 15-30%, the total cost of ownership analysis frequently favors polymer systems due to their extended operational lifetimes, reduced energy consumption, and decreased waste treatment requirements. This economic advantage is accelerating market penetration, particularly in high-value chemical manufacturing processes where process efficiency and product purity command premium considerations.
Current Challenges in Conductive Polymer Composite Catalysis
Despite significant advancements in conductive polymer composite (CPC) catalysis, several critical challenges continue to impede their widespread implementation in advanced catalytic applications. One of the primary obstacles remains the inconsistent electrical conductivity across different batches of polymer composites. This variability stems from difficulties in achieving uniform dispersion of conductive fillers within the polymer matrix, resulting in unpredictable electron transfer rates that compromise catalytic performance and reproducibility.
Thermal stability presents another significant challenge, as many conductive polymer composites exhibit performance degradation at elevated temperatures commonly encountered in industrial catalytic processes. The polymer backbone often undergoes structural changes or decomposition above certain temperature thresholds, limiting application scope and requiring additional cooling systems that increase operational complexity and costs.
Scalability issues continue to plague the field, with laboratory-scale synthesis methods proving difficult to translate to industrial production volumes without compromising material properties. The precise control of polymerization conditions, filler distribution, and interfacial interactions becomes increasingly challenging at larger scales, creating a substantial barrier to commercialization.
Durability under harsh catalytic conditions represents a persistent concern, as many CPCs suffer from degradation when exposed to reactive intermediates, byproducts, or the target reactants themselves. This susceptibility to chemical attack shortens catalyst lifespan and necessitates frequent replacement, undermining economic viability in continuous industrial processes.
Interface engineering between the conductive components and catalytically active sites remains poorly understood, with current design approaches often relying on empirical rather than theoretical foundations. The complex interplay between electronic properties, surface chemistry, and catalytic activity requires more sophisticated modeling and characterization techniques to optimize performance.
Selectivity control presents unique challenges in CPC catalysts compared to traditional metal catalysts. The heterogeneous nature of polymer composites creates multiple active site environments with varying electronic properties, making it difficult to achieve high selectivity for specific reaction pathways in complex transformations.
Recycling and regeneration protocols for spent CPC catalysts are underdeveloped, with current methods often failing to restore original activity levels or requiring environmentally problematic solvents and conditions. This limitation contradicts sustainability goals and increases the lifetime environmental footprint of these materials despite their initial promise as greener alternatives to traditional metal catalysts.
Thermal stability presents another significant challenge, as many conductive polymer composites exhibit performance degradation at elevated temperatures commonly encountered in industrial catalytic processes. The polymer backbone often undergoes structural changes or decomposition above certain temperature thresholds, limiting application scope and requiring additional cooling systems that increase operational complexity and costs.
Scalability issues continue to plague the field, with laboratory-scale synthesis methods proving difficult to translate to industrial production volumes without compromising material properties. The precise control of polymerization conditions, filler distribution, and interfacial interactions becomes increasingly challenging at larger scales, creating a substantial barrier to commercialization.
Durability under harsh catalytic conditions represents a persistent concern, as many CPCs suffer from degradation when exposed to reactive intermediates, byproducts, or the target reactants themselves. This susceptibility to chemical attack shortens catalyst lifespan and necessitates frequent replacement, undermining economic viability in continuous industrial processes.
Interface engineering between the conductive components and catalytically active sites remains poorly understood, with current design approaches often relying on empirical rather than theoretical foundations. The complex interplay between electronic properties, surface chemistry, and catalytic activity requires more sophisticated modeling and characterization techniques to optimize performance.
Selectivity control presents unique challenges in CPC catalysts compared to traditional metal catalysts. The heterogeneous nature of polymer composites creates multiple active site environments with varying electronic properties, making it difficult to achieve high selectivity for specific reaction pathways in complex transformations.
Recycling and regeneration protocols for spent CPC catalysts are underdeveloped, with current methods often failing to restore original activity levels or requiring environmentally problematic solvents and conditions. This limitation contradicts sustainability goals and increases the lifetime environmental footprint of these materials despite their initial promise as greener alternatives to traditional metal catalysts.
Current Polymer Composite Catalytic Solutions
01 Conductive polymer composites with carbon-based fillers
Carbon-based materials such as carbon nanotubes, graphene, and carbon black can be incorporated into polymer matrices to create conductive composites. These fillers form conductive networks within the polymer, significantly enhancing electrical conductivity while maintaining the processability and mechanical properties of the base polymer. The resulting composites exhibit tunable conductivity based on filler concentration and dispersion quality, making them suitable for various electronic applications.- Carbon-based conductive polymer composites: Carbon-based materials such as carbon nanotubes, graphene, and carbon black are commonly incorporated into polymer matrices to create conductive composites. These fillers provide excellent electrical conductivity while maintaining the processability of the polymer. The resulting composites exhibit enhanced mechanical properties and can be used in various applications including electromagnetic shielding, antistatic materials, and flexible electronics.
- Metal-polymer conductive composites: Metal particles or nanowires, such as silver, copper, or aluminum, can be dispersed within polymer matrices to create highly conductive composites. These metal-polymer composites offer excellent electrical conductivity while maintaining flexibility and processability. The metal content, particle size, and distribution significantly affect the conductivity and mechanical properties of the final composite material.
- Intrinsically conductive polymers in composites: Intrinsically conductive polymers like polyaniline, polypyrrole, and PEDOT:PSS can be blended with conventional polymers to create conductive composites. These materials offer unique advantages including tunable conductivity, optical properties, and environmental stability. The composites can be used in applications such as sensors, actuators, and energy storage devices where both electrical conductivity and polymer properties are required.
- Processing techniques for conductive polymer composites: Various processing techniques are employed to manufacture conductive polymer composites with optimized properties. These include solution blending, melt mixing, in-situ polymerization, and layer-by-layer assembly. The processing method significantly influences the dispersion of conductive fillers within the polymer matrix, which directly affects the electrical, thermal, and mechanical properties of the resulting composite.
- Applications of conductive polymer composites: Conductive polymer composites find applications across various industries including electronics, automotive, aerospace, and healthcare. They are used in electromagnetic interference shielding, antistatic packaging, sensors, actuators, flexible displays, and energy storage devices. The versatility of these materials stems from their unique combination of electrical conductivity and polymer characteristics such as flexibility, lightweight nature, and processability.
02 Metal-polymer conductive composites
Metal particles or nanostructures can be integrated into polymer matrices to create conductive composites with enhanced electrical properties. These composites typically incorporate metals such as silver, copper, or nickel in various forms including particles, flakes, or nanowires. The metal fillers provide excellent electrical pathways while the polymer matrix offers flexibility, processability, and protection against oxidation. These materials find applications in electromagnetic shielding, flexible electronics, and conductive adhesives.Expand Specific Solutions03 Self-healing conductive polymer composites
Advanced conductive polymer composites can be engineered with self-healing capabilities to restore electrical conductivity after mechanical damage. These materials typically incorporate dynamic bonds or microcapsules containing conductive materials that can repair damaged conductive pathways. The self-healing mechanism can be triggered by various stimuli including heat, light, or mechanical pressure. These innovative composites extend the service life of electronic devices and reduce maintenance costs in applications subject to mechanical stress or flexing.Expand Specific Solutions04 Thermally conductive polymer composites
Polymer composites can be formulated to enhance thermal conductivity while maintaining electrical insulation properties or combined with electrical conductivity. These materials typically incorporate fillers such as boron nitride, aluminum oxide, or specialized carbon structures that facilitate heat transfer through the polymer matrix. The resulting composites offer improved thermal management capabilities critical for electronic devices, LED lighting, and battery systems. The thermal conductivity can be tailored by controlling filler concentration, orientation, and interfacial properties.Expand Specific Solutions05 Stimuli-responsive conductive polymer composites
Conductive polymer composites can be designed to respond to external stimuli such as temperature, pH, light, or electrical fields by changing their electrical, mechanical, or optical properties. These smart materials typically incorporate responsive polymers or fillers that undergo reversible changes in conformation or interaction upon stimulation. Applications include sensors, actuators, smart textiles, and switchable electronic devices. The responsiveness can be tuned by selecting appropriate polymer matrices and conductive fillers with complementary properties.Expand Specific Solutions
Leading Organizations in Conductive Polymer Catalysis Research
Conductive Polymer Composites (CPCs) in advanced catalysis applications are currently in a growth phase, with the market expanding due to increasing demand for sustainable catalytic processes. The global market is projected to reach significant scale as industries seek more efficient and environmentally friendly catalytic solutions. Technologically, CPCs are advancing from experimental to commercial applications, with varying degrees of maturity. Leading players include SABIC Global Technologies and DuPont de Nemours developing proprietary polymer matrices, while Sumitomo Chemical and China Petroleum & Chemical Corp. focus on industrial-scale implementation. Academic institutions like Zhejiang University and Wuhan University of Technology are driving fundamental research, collaborating with companies like Tosoh Corp. and KEMET Electronics to bridge the gap between theoretical advances and practical applications.
Wuhan University of Technology
Technical Solution: Wuhan University of Technology has developed innovative conductive polymer composite (CPC) catalysts through their specialized "hierarchical assembly" approach. Their technology integrates conductive polymers (primarily polyaniline and polypyrrole derivatives) with precisely engineered metal-organic frameworks (MOFs) and carbon nanomaterials to create three-dimensional catalytic networks with exceptional surface area and active site distribution[1]. The university's research team has pioneered hydrothermal synthesis methods that enable controlled doping of heteroatoms (N, S, B) into the polymer backbone, creating tailored electronic environments that enhance specific catalytic pathways[3]. Their proprietary "interface engineering" technique focuses on optimizing the polymer-metal junction to facilitate efficient electron transfer while maintaining structural integrity during catalytic cycles. Recent innovations include self-healing CPC catalysts that incorporate dynamic covalent chemistry to regenerate active sites during operation, significantly extending catalyst lifespan in industrial applications[5]. The university has demonstrated particular success in electrocatalytic water splitting and oxygen reduction reactions, where their CPCs have achieved performance metrics approaching those of precious metal catalysts while using earth-abundant elements.
Strengths: Exceptional control over hierarchical nanostructures enabling high catalytic surface area; cost-effective synthesis methods suitable for scale-up; strong performance in electrochemical applications. Weaknesses: Potential stability limitations in high-temperature catalytic environments; less developed commercialization pathway compared to industry players; some formulations show sensitivity to common catalyst poisons.
Sumitomo Chemical Co., Ltd.
Technical Solution: Sumitomo Chemical has developed proprietary conductive polymer composite (CPC) catalysts under their "SumiCat™" technology platform, focusing on industrial-scale catalytic applications. Their approach integrates specially formulated conductive polymers (modified polythiophenes and PEDOT derivatives) with precisely engineered metal nanoparticles and ceramic supports to create robust catalytic systems with enhanced stability and selectivity[2]. Sumitomo's technology employs a core-shell architecture where the conductive polymer forms a protective yet electronically active layer around catalytic centers, preventing agglomeration while facilitating electron transfer during reactions[4]. Their manufacturing process utilizes controlled emulsion polymerization techniques that enable precise tuning of polymer molecular weight and conductivity, critical factors in optimizing catalytic performance. Sumitomo has demonstrated particular success in selective hydrogenation reactions and oxidative coupling processes, where their CPCs show superior product selectivity compared to conventional metal catalysts[7]. Recent innovations include stimuli-responsive CPC catalysts that can be activated or deactivated through external triggers like pH or electrical potential, enabling unprecedented control over reaction pathways in complex chemical manufacturing processes.
Strengths: Exceptional thermal and chemical stability suitable for harsh industrial environments; established manufacturing infrastructure enabling consistent quality at scale; proven performance in commercially relevant catalytic processes. Weaknesses: Higher initial production costs compared to traditional catalysts; limited flexibility for rapid formulation adjustments; proprietary nature restricts academic collaboration and broader development.
Key Innovations in Conductive Polymer Catalyst Design
Highly conductive carbon/inherently conductive polymer composites
PatentInactiveUS20040232390A1
Innovation
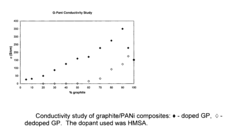
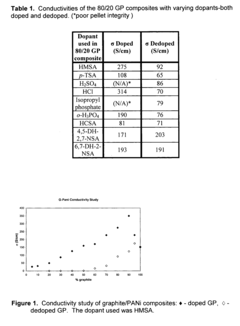
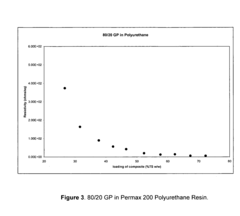
- The development of composites comprising graphite and doped polyaniline, polypyrrole, polythiophene, or polyethylenedioxythiophene with graphite, synthesized by oxidative polymerization in the presence of an acid dopant, resulting in higher conductivity and improved dispersibility in various solvents and resins.
Conductive catalytic particle and method of producing conductive catalytic particles
PatentWO2024205414A1
Innovation
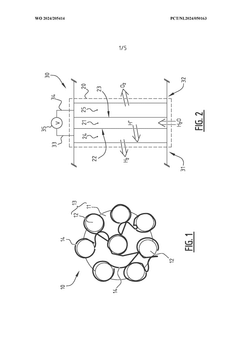
- A method involving the impregnation of porous particles with a conductive polymer using supercritical carbon dioxide, which enhances the electrical conductivity and catalytic performance of the particles, allowing for the production of more efficient conductive catalytic particles with improved shape and size distribution, and enabling their use in various catalytic applications.
Sustainability Impact of Polymer Composite Catalysts
The integration of conductive polymer composites in catalysis represents a significant advancement toward sustainable chemical processes. These materials offer remarkable environmental benefits compared to traditional metal catalysts, primarily through reduced energy consumption during catalytic reactions. The inherent electrical conductivity of these composites facilitates electron transfer at lower energy thresholds, enabling reactions to proceed under milder conditions with decreased thermal energy requirements.
Polymer composite catalysts demonstrate exceptional resource efficiency through their reduced dependence on precious metals. By incorporating minimal amounts of metal nanoparticles within a conductive polymer matrix, these composites achieve comparable or superior catalytic performance while utilizing significantly less scarce metallic resources. This approach directly addresses supply chain vulnerabilities associated with critical raw materials while reducing the environmental impact of mining operations.
The extended operational lifespan of polymer composite catalysts further enhances their sustainability profile. Their robust structural integrity and resistance to degradation under reaction conditions result in fewer replacement cycles compared to conventional catalysts. This longevity translates to reduced waste generation and diminished environmental footprint across the catalyst lifecycle.
Particularly noteworthy is the recyclability of these materials, which represents a paradigm shift in catalysis sustainability. Many conductive polymer composites can be regenerated through simple electrochemical processes, restoring catalytic activity without requiring complete replacement. Some advanced systems demonstrate self-healing properties that automatically repair structural damage during operation, further extending useful life and minimizing waste.
From a circular economy perspective, polymer composite catalysts offer significant advantages in end-of-life management. Their organic polymer components can be designed for biodegradability or chemical recycling, while the metal constituents can be recovered through established extraction techniques. This design philosophy aligns with principles of sustainable materials management and reduces the environmental burden associated with catalyst disposal.
The carbon footprint reduction achieved through polymer composite catalysts extends beyond their direct application. By enabling more efficient chemical transformations with fewer side reactions, these materials minimize waste generation and decrease the need for energy-intensive separation processes. Life cycle assessments indicate that the cumulative environmental benefits of these catalysts can be substantial when implemented at industrial scale, particularly in pharmaceutical manufacturing and fine chemical production.
Polymer composite catalysts demonstrate exceptional resource efficiency through their reduced dependence on precious metals. By incorporating minimal amounts of metal nanoparticles within a conductive polymer matrix, these composites achieve comparable or superior catalytic performance while utilizing significantly less scarce metallic resources. This approach directly addresses supply chain vulnerabilities associated with critical raw materials while reducing the environmental impact of mining operations.
The extended operational lifespan of polymer composite catalysts further enhances their sustainability profile. Their robust structural integrity and resistance to degradation under reaction conditions result in fewer replacement cycles compared to conventional catalysts. This longevity translates to reduced waste generation and diminished environmental footprint across the catalyst lifecycle.
Particularly noteworthy is the recyclability of these materials, which represents a paradigm shift in catalysis sustainability. Many conductive polymer composites can be regenerated through simple electrochemical processes, restoring catalytic activity without requiring complete replacement. Some advanced systems demonstrate self-healing properties that automatically repair structural damage during operation, further extending useful life and minimizing waste.
From a circular economy perspective, polymer composite catalysts offer significant advantages in end-of-life management. Their organic polymer components can be designed for biodegradability or chemical recycling, while the metal constituents can be recovered through established extraction techniques. This design philosophy aligns with principles of sustainable materials management and reduces the environmental burden associated with catalyst disposal.
The carbon footprint reduction achieved through polymer composite catalysts extends beyond their direct application. By enabling more efficient chemical transformations with fewer side reactions, these materials minimize waste generation and decrease the need for energy-intensive separation processes. Life cycle assessments indicate that the cumulative environmental benefits of these catalysts can be substantial when implemented at industrial scale, particularly in pharmaceutical manufacturing and fine chemical production.
Scalability and Industrial Implementation Considerations
The scalability of conductive polymer composites (CPCs) for catalysis applications represents a critical bridge between laboratory success and commercial viability. Current manufacturing processes for CPCs face significant challenges when transitioning from small-scale synthesis to industrial production volumes. Batch-to-batch consistency remains problematic, with variations in polymer chain length, conductivity profiles, and catalytic active site distribution affecting performance reliability. These inconsistencies become more pronounced at larger scales, necessitating robust quality control protocols and in-line monitoring systems.
Cost considerations present another substantial barrier to widespread industrial implementation. The synthesis of high-performance conductive polymers often requires expensive monomers, catalysts, and specialized processing conditions. Economic viability demands either cost reduction strategies or performance improvements that justify premium pricing. Several manufacturers have begun exploring continuous flow synthesis methods that reduce solvent usage and energy consumption while improving product uniformity.
Environmental sustainability factors increasingly influence industrial adoption decisions. Traditional CPC manufacturing processes often involve toxic solvents and generate significant waste streams. Recent innovations focus on greener synthesis routes, including aqueous processing methods and bio-based precursors. Life cycle assessment studies indicate that despite higher initial production costs, environmentally optimized CPC catalysts may offer lower total ownership costs through reduced waste treatment requirements and extended operational lifetimes.
Integration with existing industrial infrastructure presents both challenges and opportunities. Many chemical production facilities are designed around heterogeneous metal catalysts with established handling protocols and equipment compatibility. CPC catalysts often require different operating conditions, including lower temperature ranges and specialized reactor designs to maximize surface area exposure. Retrofitting existing plants represents a significant capital investment, though modular "drop-in" CPC catalyst systems are emerging as transitional solutions.
Regulatory considerations vary significantly across global markets, affecting implementation timelines and compliance costs. Material safety data requirements for novel polymer composites can be extensive, particularly for applications in pharmaceutical manufacturing or food-adjacent processes. Forward-thinking companies are proactively engaging with regulatory bodies during development phases to streamline approval processes and identify potential compliance issues early in the development cycle.
Cost considerations present another substantial barrier to widespread industrial implementation. The synthesis of high-performance conductive polymers often requires expensive monomers, catalysts, and specialized processing conditions. Economic viability demands either cost reduction strategies or performance improvements that justify premium pricing. Several manufacturers have begun exploring continuous flow synthesis methods that reduce solvent usage and energy consumption while improving product uniformity.
Environmental sustainability factors increasingly influence industrial adoption decisions. Traditional CPC manufacturing processes often involve toxic solvents and generate significant waste streams. Recent innovations focus on greener synthesis routes, including aqueous processing methods and bio-based precursors. Life cycle assessment studies indicate that despite higher initial production costs, environmentally optimized CPC catalysts may offer lower total ownership costs through reduced waste treatment requirements and extended operational lifetimes.
Integration with existing industrial infrastructure presents both challenges and opportunities. Many chemical production facilities are designed around heterogeneous metal catalysts with established handling protocols and equipment compatibility. CPC catalysts often require different operating conditions, including lower temperature ranges and specialized reactor designs to maximize surface area exposure. Retrofitting existing plants represents a significant capital investment, though modular "drop-in" CPC catalyst systems are emerging as transitional solutions.
Regulatory considerations vary significantly across global markets, affecting implementation timelines and compliance costs. Material safety data requirements for novel polymer composites can be extensive, particularly for applications in pharmaceutical manufacturing or food-adjacent processes. Forward-thinking companies are proactively engaging with regulatory bodies during development phases to streamline approval processes and identify potential compliance issues early in the development cycle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!