Conductive Polymer Composites for Pharmaceutical Applications: A Study
OCT 23, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Conductive Polymer Evolution and Research Objectives
Conductive polymers have undergone significant evolution since their initial discovery in the 1970s when Alan Heeger, Alan MacDiarmid, and Hideki Shirakawa demonstrated that polyacetylene could conduct electricity when doped with iodine. This groundbreaking work, which earned them the Nobel Prize in Chemistry in 2000, opened a new frontier in materials science that bridges the gap between polymers and conductors.
The development trajectory of conductive polymers has seen remarkable advancements through several distinct phases. Initially, research focused primarily on understanding the fundamental mechanisms of conductivity in these organic materials. By the 1980s and 1990s, the focus shifted toward improving processability and stability, addressing the early limitations of poor solubility and environmental degradation.
In recent decades, the field has expanded dramatically with the synthesis of numerous conductive polymer families including polyaniline, polypyrrole, polythiophene, and poly(3,4-ethylenedioxythiophene) (PEDOT). Each of these materials offers unique properties in terms of conductivity, stability, and processability, making them suitable for diverse applications.
The pharmaceutical industry has begun exploring conductive polymers relatively recently, recognizing their potential in drug delivery systems, biosensors, tissue engineering, and medical devices. The biocompatibility of certain conductive polymers, combined with their electrical properties, presents unique opportunities for controlled drug release mechanisms and enhanced therapeutic efficacy.
Current research trends indicate growing interest in conductive polymer composites (CPCs), which combine conductive polymers with other materials to enhance specific properties. These composites often demonstrate superior mechanical strength, improved processability, and tailored electrical conductivity compared to pure conductive polymers.
The primary objectives of this technical research are multifaceted. First, we aim to comprehensively map the current landscape of conductive polymer composites with specific relevance to pharmaceutical applications. Second, we seek to identify the critical challenges limiting broader adoption of these materials in pharmaceutical products, including regulatory hurdles, scalability issues, and performance limitations.
Additionally, this research intends to evaluate emerging synthesis techniques and formulation strategies that could enhance the biocompatibility and functionality of conductive polymer composites in pharmaceutical contexts. Finally, we aim to forecast technological trajectories and potential breakthrough areas that could revolutionize pharmaceutical applications of conductive polymers over the next decade.
The development trajectory of conductive polymers has seen remarkable advancements through several distinct phases. Initially, research focused primarily on understanding the fundamental mechanisms of conductivity in these organic materials. By the 1980s and 1990s, the focus shifted toward improving processability and stability, addressing the early limitations of poor solubility and environmental degradation.
In recent decades, the field has expanded dramatically with the synthesis of numerous conductive polymer families including polyaniline, polypyrrole, polythiophene, and poly(3,4-ethylenedioxythiophene) (PEDOT). Each of these materials offers unique properties in terms of conductivity, stability, and processability, making them suitable for diverse applications.
The pharmaceutical industry has begun exploring conductive polymers relatively recently, recognizing their potential in drug delivery systems, biosensors, tissue engineering, and medical devices. The biocompatibility of certain conductive polymers, combined with their electrical properties, presents unique opportunities for controlled drug release mechanisms and enhanced therapeutic efficacy.
Current research trends indicate growing interest in conductive polymer composites (CPCs), which combine conductive polymers with other materials to enhance specific properties. These composites often demonstrate superior mechanical strength, improved processability, and tailored electrical conductivity compared to pure conductive polymers.
The primary objectives of this technical research are multifaceted. First, we aim to comprehensively map the current landscape of conductive polymer composites with specific relevance to pharmaceutical applications. Second, we seek to identify the critical challenges limiting broader adoption of these materials in pharmaceutical products, including regulatory hurdles, scalability issues, and performance limitations.
Additionally, this research intends to evaluate emerging synthesis techniques and formulation strategies that could enhance the biocompatibility and functionality of conductive polymer composites in pharmaceutical contexts. Finally, we aim to forecast technological trajectories and potential breakthrough areas that could revolutionize pharmaceutical applications of conductive polymers over the next decade.
Pharmaceutical Market Demand for Conductive Polymer Composites
The pharmaceutical industry is witnessing a significant shift towards advanced materials that can enhance drug delivery systems, improve manufacturing processes, and enable innovative therapeutic approaches. Conductive polymer composites (CPCs) represent a rapidly growing segment within this market, driven by their unique electrical, mechanical, and biocompatible properties that address critical challenges in pharmaceutical applications.
Market research indicates that the global pharmaceutical industry, valued at approximately $1.3 trillion in 2022, is increasingly adopting conductive polymer technologies. The specific market segment for conductive materials in pharmaceutical applications is projected to grow at a compound annual growth rate of 8.7% through 2028, significantly outpacing the overall pharmaceutical market growth of 5.2%.
The primary demand drivers for conductive polymer composites in pharmaceuticals include controlled drug delivery systems, which require precise electrical stimulation for targeted release mechanisms. This segment alone represents nearly 40% of the current market for pharmaceutical CPCs, with particular growth in treatments for neurological disorders and chronic pain management.
Smart packaging solutions incorporating conductive polymers for drug stability monitoring and anti-counterfeiting measures constitute another rapidly expanding application area, growing at 12.3% annually as pharmaceutical companies face increasing pressure to ensure supply chain integrity and product authenticity.
Bioelectronic medicine, an emerging field combining electronics with biological systems, is creating substantial new demand for conductive polymer composites. This sector is expected to reach $6.5 billion by 2027, with CPCs playing a crucial role in developing implantable devices and sensors that can monitor health parameters and deliver therapeutic interventions.
Regional analysis reveals that North America currently dominates the pharmaceutical CPC market with a 42% share, followed by Europe (31%) and Asia-Pacific (21%). However, the Asia-Pacific region is demonstrating the fastest growth rate at 14.2% annually, driven by expanding pharmaceutical manufacturing capabilities and increasing healthcare investments in China and India.
Customer segmentation shows that large pharmaceutical corporations account for 65% of current CPC demand, primarily for research and development of next-generation drug delivery systems. However, specialized biotech firms focusing on innovative therapies represent the fastest-growing customer segment, with annual demand increasing by 17.8%.
Market challenges include regulatory hurdles for novel materials in pharmaceutical applications, with approval processes typically taking 2-3 years longer than conventional materials. Additionally, cost considerations remain significant, as specialized conductive polymers can increase production costs by 15-30% compared to traditional materials, necessitating clear demonstration of therapeutic or manufacturing advantages to justify adoption.
Market research indicates that the global pharmaceutical industry, valued at approximately $1.3 trillion in 2022, is increasingly adopting conductive polymer technologies. The specific market segment for conductive materials in pharmaceutical applications is projected to grow at a compound annual growth rate of 8.7% through 2028, significantly outpacing the overall pharmaceutical market growth of 5.2%.
The primary demand drivers for conductive polymer composites in pharmaceuticals include controlled drug delivery systems, which require precise electrical stimulation for targeted release mechanisms. This segment alone represents nearly 40% of the current market for pharmaceutical CPCs, with particular growth in treatments for neurological disorders and chronic pain management.
Smart packaging solutions incorporating conductive polymers for drug stability monitoring and anti-counterfeiting measures constitute another rapidly expanding application area, growing at 12.3% annually as pharmaceutical companies face increasing pressure to ensure supply chain integrity and product authenticity.
Bioelectronic medicine, an emerging field combining electronics with biological systems, is creating substantial new demand for conductive polymer composites. This sector is expected to reach $6.5 billion by 2027, with CPCs playing a crucial role in developing implantable devices and sensors that can monitor health parameters and deliver therapeutic interventions.
Regional analysis reveals that North America currently dominates the pharmaceutical CPC market with a 42% share, followed by Europe (31%) and Asia-Pacific (21%). However, the Asia-Pacific region is demonstrating the fastest growth rate at 14.2% annually, driven by expanding pharmaceutical manufacturing capabilities and increasing healthcare investments in China and India.
Customer segmentation shows that large pharmaceutical corporations account for 65% of current CPC demand, primarily for research and development of next-generation drug delivery systems. However, specialized biotech firms focusing on innovative therapies represent the fastest-growing customer segment, with annual demand increasing by 17.8%.
Market challenges include regulatory hurdles for novel materials in pharmaceutical applications, with approval processes typically taking 2-3 years longer than conventional materials. Additionally, cost considerations remain significant, as specialized conductive polymers can increase production costs by 15-30% compared to traditional materials, necessitating clear demonstration of therapeutic or manufacturing advantages to justify adoption.
Technical Challenges in Pharmaceutical-Grade Conductive Polymers
Despite significant advancements in conductive polymer composites (CPCs), their application in pharmaceutical contexts presents unique technical challenges. The stringent regulatory environment of pharmaceutical manufacturing demands materials with exceptional purity profiles, consistent batch-to-batch properties, and biocompatibility characteristics that many current CPCs struggle to achieve.
A primary challenge lies in the inherent conductivity-toxicity paradox. Many highly conductive polymers incorporate metallic particles or carbon nanotubes that may present biocompatibility concerns when in contact with biological systems or pharmaceutical compounds. This necessitates careful engineering to balance electrical performance with safety requirements for drug delivery systems or pharmaceutical packaging.
Material stability under pharmaceutical processing conditions represents another significant hurdle. Conductive polymers must maintain their electrical properties during sterilization procedures, which often involve high temperatures, radiation exposure, or chemical treatments. Many current formulations show degradation in conductivity or mechanical properties after such processes, limiting their practical implementation.
The interface between conductive polymers and pharmaceutical compounds presents complex interaction challenges. Potential leaching of conductive fillers or polymer additives into drug formulations raises serious regulatory concerns. Additionally, some active pharmaceutical ingredients (APIs) may interact with polymer matrices, potentially altering drug efficacy or polymer conductivity over time.
Scale-up manufacturing of pharmaceutical-grade CPCs encounters reproducibility issues. Laboratory-scale synthesis often yields materials with different properties than those produced at industrial scales. This variability is particularly problematic for pharmaceutical applications where consistent performance is mandatory for regulatory approval.
Surface modification techniques to enhance biocompatibility while preserving conductivity remain underdeveloped. Current approaches often compromise electrical performance when improving biocompatibility, creating a technical trade-off that limits application potential.
Cost considerations further complicate development efforts. Pharmaceutical-grade materials require extensive testing and validation, significantly increasing production expenses compared to industrial-grade alternatives. This economic barrier has slowed research investment and commercial development of specialized pharmaceutical CPCs.
Analytical methodology for characterizing these materials in pharmaceutical contexts is also insufficient. Standard testing protocols for electrical properties often fail to account for the unique environments encountered in pharmaceutical applications, such as exposure to biological fluids or compatibility with specific drug compounds.
Addressing these technical challenges requires interdisciplinary collaboration between polymer scientists, pharmaceutical engineers, and regulatory experts to develop next-generation conductive polymer composites specifically engineered for pharmaceutical applications.
A primary challenge lies in the inherent conductivity-toxicity paradox. Many highly conductive polymers incorporate metallic particles or carbon nanotubes that may present biocompatibility concerns when in contact with biological systems or pharmaceutical compounds. This necessitates careful engineering to balance electrical performance with safety requirements for drug delivery systems or pharmaceutical packaging.
Material stability under pharmaceutical processing conditions represents another significant hurdle. Conductive polymers must maintain their electrical properties during sterilization procedures, which often involve high temperatures, radiation exposure, or chemical treatments. Many current formulations show degradation in conductivity or mechanical properties after such processes, limiting their practical implementation.
The interface between conductive polymers and pharmaceutical compounds presents complex interaction challenges. Potential leaching of conductive fillers or polymer additives into drug formulations raises serious regulatory concerns. Additionally, some active pharmaceutical ingredients (APIs) may interact with polymer matrices, potentially altering drug efficacy or polymer conductivity over time.
Scale-up manufacturing of pharmaceutical-grade CPCs encounters reproducibility issues. Laboratory-scale synthesis often yields materials with different properties than those produced at industrial scales. This variability is particularly problematic for pharmaceutical applications where consistent performance is mandatory for regulatory approval.
Surface modification techniques to enhance biocompatibility while preserving conductivity remain underdeveloped. Current approaches often compromise electrical performance when improving biocompatibility, creating a technical trade-off that limits application potential.
Cost considerations further complicate development efforts. Pharmaceutical-grade materials require extensive testing and validation, significantly increasing production expenses compared to industrial-grade alternatives. This economic barrier has slowed research investment and commercial development of specialized pharmaceutical CPCs.
Analytical methodology for characterizing these materials in pharmaceutical contexts is also insufficient. Standard testing protocols for electrical properties often fail to account for the unique environments encountered in pharmaceutical applications, such as exposure to biological fluids or compatibility with specific drug compounds.
Addressing these technical challenges requires interdisciplinary collaboration between polymer scientists, pharmaceutical engineers, and regulatory experts to develop next-generation conductive polymer composites specifically engineered for pharmaceutical applications.
Current Conductive Polymer Solutions for Drug Delivery
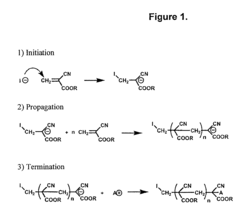
01 Carbon-based conductive polymer composites
Carbon-based materials such as carbon nanotubes, graphene, and carbon black are incorporated into polymer matrices to create conductive composites. These fillers provide excellent electrical conductivity while maintaining the mechanical properties of the polymer. The resulting composites can be used in various applications including electromagnetic shielding, antistatic materials, and flexible electronics. The dispersion method and filler concentration significantly affect the conductivity and performance of these composites.- Carbon-based conductive polymer composites: Carbon-based materials such as carbon nanotubes, graphene, and carbon black are incorporated into polymer matrices to create conductive composites. These materials provide excellent electrical conductivity while maintaining the mechanical properties of the polymer. The carbon fillers create conductive networks within the polymer matrix, allowing for electron transport. These composites are widely used in electromagnetic shielding, antistatic applications, and flexible electronics.
- Metal-polymer conductive composites: Metal particles or nanowires are dispersed within polymer matrices to create conductive composites with enhanced electrical properties. Common metals used include silver, copper, and nickel. These composites combine the processability of polymers with the high conductivity of metals. The metal fillers create conductive pathways through the otherwise insulating polymer matrix. Applications include printed electronics, sensors, and electromagnetic interference shielding materials.
- Intrinsically conductive polymer composites: These composites utilize inherently conductive polymers such as polyaniline, polypyrrole, and PEDOT:PSS as the conductive component. These polymers contain conjugated double bonds that allow for electron movement along the polymer chain. By blending these conductive polymers with conventional polymers, composites with tunable electrical properties can be created. These materials offer advantages in processability and flexibility compared to metal-filled composites.
- Thermal and electrical management applications: Conductive polymer composites are engineered for specific thermal and electrical management applications. These materials combine thermal conductivity with electrical properties for use in heat sinks, thermal interface materials, and electronic packaging. By controlling the type, amount, and distribution of conductive fillers, these composites can be tailored for specific thermal and electrical requirements. They provide solutions for heat dissipation in electronic devices while maintaining electrical functionality.
- Processing methods for conductive polymer composites: Various processing techniques are employed to manufacture conductive polymer composites with optimized properties. These include melt mixing, solution blending, in-situ polymerization, and layer-by-layer assembly. The processing method significantly affects the dispersion of conductive fillers and the resulting electrical properties of the composite. Advanced techniques focus on achieving uniform dispersion of conductive fillers while maintaining the processability of the composite material.
02 Metal-polymer conductive composites
Metal particles or nanowires, such as silver, copper, or aluminum, are incorporated into polymer matrices to create highly conductive composites. These metal-polymer composites offer superior electrical conductivity compared to carbon-based composites and can be formulated to maintain flexibility. Applications include printed electronics, conductive adhesives, and electromagnetic interference shielding. The size, shape, and distribution of metal particles within the polymer matrix significantly influence the electrical properties of the composite.Expand Specific Solutions03 Intrinsically conductive polymers in composites
Intrinsically conductive polymers such as polyaniline, polypyrrole, and PEDOT:PSS are used either as the matrix or as additives in polymer composites to impart electrical conductivity. These polymers contain conjugated double bonds that allow electron movement along the polymer chain. When combined with conventional polymers or inorganic materials, they create versatile composites with tunable electrical properties. Applications include sensors, actuators, and energy storage devices.Expand Specific Solutions04 Thermal management conductive polymer composites
Polymer composites specifically designed for thermal conductivity and heat dissipation incorporate fillers such as boron nitride, aluminum oxide, or metal particles. These composites maintain electrical insulation while providing enhanced thermal conductivity, making them suitable for electronic packaging, LED heat sinks, and thermal interface materials. The orientation and concentration of thermally conductive fillers are optimized to create efficient heat transfer pathways through the polymer matrix.Expand Specific Solutions05 Processing techniques for conductive polymer composites
Various processing techniques are employed to manufacture conductive polymer composites with optimized properties. These include solution blending, melt mixing, in-situ polymerization, and layer-by-layer assembly. Surface modification of fillers is often performed to improve dispersion and interfacial adhesion with the polymer matrix. Advanced techniques such as 3D printing and electrospinning are used to create structured conductive composites with tailored geometries for specific applications.Expand Specific Solutions
Industry Leaders in Pharmaceutical Conductive Materials
The conductive polymer composites (CPCs) market for pharmaceutical applications is in a growth phase, characterized by increasing research activities and expanding applications. The market size is projected to grow significantly due to rising demand for smart drug delivery systems and biomedical devices. Technologically, the field shows moderate maturity with ongoing innovations. Leading academic institutions like Sichuan University, Nanjing University, and University of Alabama are driving fundamental research, while industrial players such as DuPont, SABIC, and Boston Scientific are commercializing applications. Pharmaceutical companies like Allergan Pharmaceuticals are exploring CPCs for drug delivery systems. The collaboration between academic institutions (KIST, Zhengzhou University) and industrial partners (Tosoh Corp., Kumho Petro Chemical) is accelerating technology transfer and application development in this emerging field.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed advanced conductive polymer composites specifically engineered for pharmaceutical applications, focusing on drug delivery systems and packaging solutions. Their proprietary technology combines specialized polymers with conductive fillers like carbon nanotubes and graphene to create materials with precise electrical conductivity profiles. DuPont's pharmaceutical-grade conductive composites feature controlled release mechanisms triggered by electrical stimuli, enabling targeted drug delivery with temporal and spatial precision. Their materials undergo rigorous pharmaceutical validation processes to ensure biocompatibility, stability, and compliance with FDA regulations. DuPont has also pioneered anti-static and EMI-shielding polymer composites for pharmaceutical manufacturing equipment and packaging, reducing contamination risks and protecting sensitive medications from electromagnetic interference.
Strengths: Industry-leading expertise in polymer science with extensive R&D capabilities; established regulatory compliance framework; global manufacturing infrastructure. Weaknesses: Higher cost compared to conventional materials; requires specialized processing equipment; longer development cycles for pharmaceutical approval.
Allergan Pharmaceuticals International Ltd.
Technical Solution: Allergan has developed innovative conductive polymer composite systems specifically for transdermal and implantable drug delivery applications. Their technology platform incorporates biocompatible conductive polymers (primarily PEDOT:PSS and polypyrrole derivatives) with pharmaceutical-grade carriers to create responsive delivery matrices. These smart materials respond to electrical or biochemical triggers to modulate drug release rates. Allergan's approach includes multilayer composite structures where conductive polymer layers interface with drug reservoirs, allowing precise control over release kinetics. Their implantable systems utilize miniaturized electronics integrated with conductive polymer composites to create programmable delivery devices with wireless control capabilities. Allergan has conducted extensive biocompatibility testing and has several formulations in clinical trials for chronic disease management applications.
Strengths: Strong pharmaceutical regulatory expertise; established clinical testing pathways; extensive intellectual property portfolio in drug delivery systems. Weaknesses: Limited manufacturing scale for specialized conductive polymers; higher production costs; longer regulatory approval timelines for implantable devices.
Key Patents in Pharmaceutical Conductive Polymer Technology
Conductive polymeric compositions and applications
PatentInactiveUS20180066132A1
Innovation
- Customizable conductive polymeric compositions with reversible absorption spectra, tailored for various medical applications, including drug delivery and imaging, utilizing conductive polymers and nanogels that can be synthesized to specify desired properties and functions, such as electrochemical reactions and ion transport capabilities.
Polymer composites and methods for producing the same
PatentInactiveUS20100190924A1
Innovation
- The development of electrically conductive and non-conductive polymer composites using cyanoacrylate as a polymeric matrix with conductive fillers like graphite, which undergoes rapid and easy anionic polymerization at room temperature, forming a continuous chain structure and achieving high mechanical properties and adjustable conductance by varying the percentage of conductive fillers.
Biocompatibility and Safety Considerations
Biocompatibility and safety considerations are paramount when developing conductive polymer composites (CPCs) for pharmaceutical applications. The integration of these materials into drug delivery systems, medical devices, and diagnostic tools necessitates rigorous evaluation of their interactions with biological systems. Current regulatory frameworks, including FDA and EMA guidelines, require comprehensive biocompatibility testing according to ISO 10993 standards before any pharmaceutical application can proceed to clinical trials.
The primary biocompatibility concerns for CPCs include potential cytotoxicity, inflammatory responses, and long-term accumulation in tissues. Recent studies have demonstrated that polymer matrix composition significantly influences the biological response, with natural polymers like chitosan and alginate generally exhibiting superior biocompatibility compared to synthetic alternatives. However, the conductive components—often carbon-based nanomaterials or metallic nanoparticles—present more complex safety profiles that require careful assessment.
Leaching of conductive particles represents a critical safety challenge, particularly for implantable or long-term contact applications. Research indicates that strong interfacial bonding between conductive fillers and polymer matrices can substantially reduce leaching potential. Advanced surface functionalization techniques have emerged as effective strategies to enhance this interfacial stability while simultaneously improving biocompatibility through the introduction of bioactive functional groups.
Immunogenicity testing has revealed that particle size and surface characteristics of conductive fillers significantly impact immune system recognition and response. Nanoparticles below 100 nm demonstrate increased cellular uptake and potential for crossing biological barriers, necessitating additional safety considerations. Emerging research suggests that controlled degradation profiles can mitigate long-term accumulation concerns while maintaining therapeutic efficacy throughout the intended treatment duration.
Hemocompatibility represents another critical consideration for CPCs intended for blood-contacting applications. Recent advancements in heparin-mimetic surface modifications have demonstrated promising results in reducing thrombogenicity while maintaining electrical conductivity properties. Additionally, antimicrobial properties inherent to some conductive polymers offer potential secondary benefits for infection prevention, though these must be balanced against potential disruption of beneficial microbiota.
Standardized testing protocols specifically designed for conductive materials in pharmaceutical contexts remain underdeveloped. Current approaches typically combine traditional biocompatibility assessments with specialized electrical safety evaluations. Industry leaders are increasingly adopting risk-based approaches that consider the specific application context, exposure duration, and patient population characteristics when determining appropriate safety testing regimens.
The primary biocompatibility concerns for CPCs include potential cytotoxicity, inflammatory responses, and long-term accumulation in tissues. Recent studies have demonstrated that polymer matrix composition significantly influences the biological response, with natural polymers like chitosan and alginate generally exhibiting superior biocompatibility compared to synthetic alternatives. However, the conductive components—often carbon-based nanomaterials or metallic nanoparticles—present more complex safety profiles that require careful assessment.
Leaching of conductive particles represents a critical safety challenge, particularly for implantable or long-term contact applications. Research indicates that strong interfacial bonding between conductive fillers and polymer matrices can substantially reduce leaching potential. Advanced surface functionalization techniques have emerged as effective strategies to enhance this interfacial stability while simultaneously improving biocompatibility through the introduction of bioactive functional groups.
Immunogenicity testing has revealed that particle size and surface characteristics of conductive fillers significantly impact immune system recognition and response. Nanoparticles below 100 nm demonstrate increased cellular uptake and potential for crossing biological barriers, necessitating additional safety considerations. Emerging research suggests that controlled degradation profiles can mitigate long-term accumulation concerns while maintaining therapeutic efficacy throughout the intended treatment duration.
Hemocompatibility represents another critical consideration for CPCs intended for blood-contacting applications. Recent advancements in heparin-mimetic surface modifications have demonstrated promising results in reducing thrombogenicity while maintaining electrical conductivity properties. Additionally, antimicrobial properties inherent to some conductive polymers offer potential secondary benefits for infection prevention, though these must be balanced against potential disruption of beneficial microbiota.
Standardized testing protocols specifically designed for conductive materials in pharmaceutical contexts remain underdeveloped. Current approaches typically combine traditional biocompatibility assessments with specialized electrical safety evaluations. Industry leaders are increasingly adopting risk-based approaches that consider the specific application context, exposure duration, and patient population characteristics when determining appropriate safety testing regimens.
Regulatory Framework for Pharmaceutical Polymer Materials
The regulatory landscape governing conductive polymer composites in pharmaceutical applications is complex and multifaceted, requiring careful navigation by manufacturers and researchers. At the international level, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) provides guidelines that influence the development and approval of pharmaceutical materials, including polymer composites. These guidelines establish standards for quality, safety, and efficacy that must be met before market authorization.
In the United States, the Food and Drug Administration (FDA) regulates pharmaceutical polymer materials through various pathways. For conductive polymers intended for drug delivery systems, the FDA's Center for Drug Evaluation and Research (CDER) typically oversees approval processes. The 510(k) clearance pathway may apply for certain medical devices incorporating conductive polymers, while novel applications might require the more rigorous Premarket Approval (PMA) process.
The European Medicines Agency (EMA) implements regulations through the European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which include specific provisions for materials used in pharmaceutical applications. Conductive polymer composites must comply with these regulations, particularly regarding biocompatibility and risk assessment requirements outlined in ISO 10993 standards.
Material safety considerations are paramount in regulatory frameworks. Manufacturers must demonstrate that conductive polymers do not leach harmful substances or degrade in ways that could compromise patient safety. The United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) provide specific testing protocols for materials used in pharmaceutical applications, including tests for extractables and leachables.
Recent regulatory trends show increasing scrutiny of nanomaterials in pharmaceutical applications, which affects many conductive polymer composites that incorporate nanoparticles. The FDA's guidance on nanotechnology and the EMA's reflection papers on nanomedicines establish additional considerations for these materials, including specialized toxicological assessments and characterization requirements.
Compliance with Good Manufacturing Practices (GMP) represents another critical regulatory aspect. Manufacturers must implement robust quality management systems that ensure consistent production of conductive polymer composites meeting predetermined specifications. This includes validation of manufacturing processes, supplier qualification, and comprehensive documentation practices.
Emerging regulatory challenges include the classification of combination products that incorporate both drug and device elements, which is common for advanced conductive polymer applications. Regulatory agencies worldwide are still developing frameworks to address these hybrid products, creating potential uncertainty for innovators in this space.
In the United States, the Food and Drug Administration (FDA) regulates pharmaceutical polymer materials through various pathways. For conductive polymers intended for drug delivery systems, the FDA's Center for Drug Evaluation and Research (CDER) typically oversees approval processes. The 510(k) clearance pathway may apply for certain medical devices incorporating conductive polymers, while novel applications might require the more rigorous Premarket Approval (PMA) process.
The European Medicines Agency (EMA) implements regulations through the European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which include specific provisions for materials used in pharmaceutical applications. Conductive polymer composites must comply with these regulations, particularly regarding biocompatibility and risk assessment requirements outlined in ISO 10993 standards.
Material safety considerations are paramount in regulatory frameworks. Manufacturers must demonstrate that conductive polymers do not leach harmful substances or degrade in ways that could compromise patient safety. The United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) provide specific testing protocols for materials used in pharmaceutical applications, including tests for extractables and leachables.
Recent regulatory trends show increasing scrutiny of nanomaterials in pharmaceutical applications, which affects many conductive polymer composites that incorporate nanoparticles. The FDA's guidance on nanotechnology and the EMA's reflection papers on nanomedicines establish additional considerations for these materials, including specialized toxicological assessments and characterization requirements.
Compliance with Good Manufacturing Practices (GMP) represents another critical regulatory aspect. Manufacturers must implement robust quality management systems that ensure consistent production of conductive polymer composites meeting predetermined specifications. This includes validation of manufacturing processes, supplier qualification, and comprehensive documentation practices.
Emerging regulatory challenges include the classification of combination products that incorporate both drug and device elements, which is common for advanced conductive polymer applications. Regulatory agencies worldwide are still developing frameworks to address these hybrid products, creating potential uncertainty for innovators in this space.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!