Research on Conductive Polymer Composites and Catalytic Efficiency
OCT 23, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Conductive Polymer Evolution and Research Objectives
Conductive polymers have undergone significant evolution since their initial discovery in the 1970s when Alan Heeger, Alan MacDiarmid, and Hideki Shirakawa demonstrated that polyacetylene could conduct electricity when doped with iodine. This groundbreaking work, which earned them the Nobel Prize in Chemistry in 2000, opened a new frontier in materials science that bridges the traditionally separate fields of polymers and electronic conductors.
The development trajectory of conductive polymers has been characterized by several distinct phases. The first phase (1970s-1980s) focused primarily on understanding the fundamental mechanisms of conductivity in these materials. The second phase (1990s-early 2000s) saw the expansion of the conductive polymer family to include polypyrrole, polyaniline, polythiophene, and their derivatives, each offering unique properties and potential applications.
In recent years, research has shifted toward conductive polymer composites (CPCs), which combine conductive polymers with other materials such as carbon nanotubes, graphene, metal nanoparticles, or conventional polymers. These composites offer enhanced electrical, mechanical, and thermal properties compared to pure conductive polymers, making them suitable for a wider range of applications.
Parallel to this development, researchers have increasingly recognized the catalytic potential of conductive polymers and their composites. The unique electronic structure of these materials, combined with their high surface area and tunable properties, makes them promising candidates for various catalytic applications, including electrocatalysis, photocatalysis, and heterogeneous catalysis.
The current research landscape is characterized by efforts to understand and optimize the relationship between the structural features of conductive polymer composites and their catalytic efficiency. This includes investigating how factors such as polymer chain length, dopant type and concentration, composite composition, and processing methods affect the catalytic performance.
The primary objectives of this research are multifaceted. First, to develop a comprehensive understanding of the structure-property relationships in conductive polymer composites, particularly how these relate to catalytic activity. Second, to design and synthesize novel conductive polymer composites with enhanced catalytic efficiency for specific reactions of industrial or environmental importance. Third, to establish standardized methods for characterizing and comparing the catalytic performance of different conductive polymer systems.
Additionally, this research aims to explore the potential synergistic effects between the conductive and catalytic properties of these materials, potentially leading to innovative applications in fields such as energy conversion and storage, environmental remediation, and chemical synthesis.
The development trajectory of conductive polymers has been characterized by several distinct phases. The first phase (1970s-1980s) focused primarily on understanding the fundamental mechanisms of conductivity in these materials. The second phase (1990s-early 2000s) saw the expansion of the conductive polymer family to include polypyrrole, polyaniline, polythiophene, and their derivatives, each offering unique properties and potential applications.
In recent years, research has shifted toward conductive polymer composites (CPCs), which combine conductive polymers with other materials such as carbon nanotubes, graphene, metal nanoparticles, or conventional polymers. These composites offer enhanced electrical, mechanical, and thermal properties compared to pure conductive polymers, making them suitable for a wider range of applications.
Parallel to this development, researchers have increasingly recognized the catalytic potential of conductive polymers and their composites. The unique electronic structure of these materials, combined with their high surface area and tunable properties, makes them promising candidates for various catalytic applications, including electrocatalysis, photocatalysis, and heterogeneous catalysis.
The current research landscape is characterized by efforts to understand and optimize the relationship between the structural features of conductive polymer composites and their catalytic efficiency. This includes investigating how factors such as polymer chain length, dopant type and concentration, composite composition, and processing methods affect the catalytic performance.
The primary objectives of this research are multifaceted. First, to develop a comprehensive understanding of the structure-property relationships in conductive polymer composites, particularly how these relate to catalytic activity. Second, to design and synthesize novel conductive polymer composites with enhanced catalytic efficiency for specific reactions of industrial or environmental importance. Third, to establish standardized methods for characterizing and comparing the catalytic performance of different conductive polymer systems.
Additionally, this research aims to explore the potential synergistic effects between the conductive and catalytic properties of these materials, potentially leading to innovative applications in fields such as energy conversion and storage, environmental remediation, and chemical synthesis.
Market Applications and Demand Analysis for Conductive Polymers
The global market for conductive polymer composites has witnessed significant growth in recent years, driven by increasing demand across multiple industries. The market size was valued at approximately $3.9 billion in 2022 and is projected to reach $7.6 billion by 2028, representing a compound annual growth rate of 11.8%. This robust growth trajectory underscores the expanding applications and market penetration of conductive polymer technologies.
Electronics and semiconductor industries remain the primary consumers of conductive polymer composites, accounting for nearly 40% of the total market share. The miniaturization trend in electronic devices, coupled with the need for flexible and lightweight components, has substantially increased demand for conductive polymers as alternatives to traditional metallic conductors. Particularly, the growing market for wearable electronics, flexible displays, and printed circuit boards has created new avenues for conductive polymer applications.
The automotive sector represents another significant market segment, with increasing adoption of conductive polymers in electric vehicles (EVs) and hybrid electric vehicles (HEVs). These materials are extensively used in battery systems, sensors, and electromagnetic interference (EMI) shielding components. As global EV production continues to rise—with over 10 million units produced in 2022—the demand for lightweight, efficient conductive materials is expected to surge correspondingly.
Healthcare and biomedical applications have emerged as promising growth areas for conductive polymers, particularly in biosensors, drug delivery systems, and tissue engineering. The market for medical-grade conductive polymers is growing at 13.5% annually, outpacing the overall market growth rate. This acceleration is attributed to the increasing integration of electronic components in medical devices and the rising prevalence of point-of-care diagnostic tools.
Energy storage and conversion systems represent another expanding application domain. Conductive polymers with catalytic properties are increasingly utilized in fuel cells, solar cells, and supercapacitors. The enhanced catalytic efficiency offered by these materials has positioned them as critical components in next-generation energy technologies, with the market segment growing at approximately 12.7% annually.
Regional analysis indicates that Asia-Pacific dominates the conductive polymer market, accounting for 45% of global consumption, followed by North America (28%) and Europe (22%). China, Japan, and South Korea are the leading consumers in the Asia-Pacific region, driven by their robust electronics manufacturing sectors. However, North America and Europe lead in research and development activities, particularly in advanced applications such as catalytic systems and biomedical devices.
Electronics and semiconductor industries remain the primary consumers of conductive polymer composites, accounting for nearly 40% of the total market share. The miniaturization trend in electronic devices, coupled with the need for flexible and lightweight components, has substantially increased demand for conductive polymers as alternatives to traditional metallic conductors. Particularly, the growing market for wearable electronics, flexible displays, and printed circuit boards has created new avenues for conductive polymer applications.
The automotive sector represents another significant market segment, with increasing adoption of conductive polymers in electric vehicles (EVs) and hybrid electric vehicles (HEVs). These materials are extensively used in battery systems, sensors, and electromagnetic interference (EMI) shielding components. As global EV production continues to rise—with over 10 million units produced in 2022—the demand for lightweight, efficient conductive materials is expected to surge correspondingly.
Healthcare and biomedical applications have emerged as promising growth areas for conductive polymers, particularly in biosensors, drug delivery systems, and tissue engineering. The market for medical-grade conductive polymers is growing at 13.5% annually, outpacing the overall market growth rate. This acceleration is attributed to the increasing integration of electronic components in medical devices and the rising prevalence of point-of-care diagnostic tools.
Energy storage and conversion systems represent another expanding application domain. Conductive polymers with catalytic properties are increasingly utilized in fuel cells, solar cells, and supercapacitors. The enhanced catalytic efficiency offered by these materials has positioned them as critical components in next-generation energy technologies, with the market segment growing at approximately 12.7% annually.
Regional analysis indicates that Asia-Pacific dominates the conductive polymer market, accounting for 45% of global consumption, followed by North America (28%) and Europe (22%). China, Japan, and South Korea are the leading consumers in the Asia-Pacific region, driven by their robust electronics manufacturing sectors. However, North America and Europe lead in research and development activities, particularly in advanced applications such as catalytic systems and biomedical devices.
Global Research Status and Technical Barriers
Conductive polymer composites (CPCs) have emerged as a significant research focus globally due to their unique combination of electrical conductivity and polymer properties. Currently, the United States, European Union, Japan, and China are leading the research in this field, with substantial investments in both academic and industrial sectors. The U.S. maintains leadership through institutions like MIT and Stanford University, focusing on novel synthesis methods and applications in flexible electronics. The EU, particularly Germany and France, emphasizes sustainable manufacturing processes and integration with existing industrial systems.
In Asia, Japan excels in miniaturization and precision applications of CPCs, while China has rapidly expanded its research capacity, focusing on mass production techniques and cost reduction strategies. Recent bibliometric analyses indicate a 35% increase in CPC-related publications over the past five years, with catalytic applications showing the steepest growth trajectory at approximately 42%.
Despite significant progress, several technical barriers persist in the development of conductive polymer composites for catalytic applications. The primary challenge remains achieving uniform dispersion of conductive fillers within polymer matrices, particularly at higher loading levels necessary for optimal catalytic performance. This dispersion issue often leads to agglomeration, creating inconsistent conductivity profiles and catalytic hotspots that reduce overall efficiency.
Another significant barrier is the trade-off between electrical conductivity and mechanical properties. As conductive filler content increases to enhance catalytic efficiency, mechanical integrity often deteriorates, limiting practical applications in demanding environments. This relationship creates a narrow processing window that constrains industrial scalability.
The stability of CPCs under catalytic conditions presents another major challenge. Exposure to reactive species, temperature fluctuations, and mechanical stress during catalytic processes can degrade polymer matrices or alter the conductive network structure. Current research indicates that most high-performance CPCs maintain optimal catalytic efficiency for only 100-200 hours under industrial conditions before significant performance degradation occurs.
Interface engineering between conductive fillers and polymer matrices remains underdeveloped, with current coupling agents and surface modification techniques providing insufficient long-term stability. This interfacial weakness affects both electrical conductivity pathways and catalytic site accessibility, reducing overall system performance by an estimated 30-40% compared to theoretical maximums.
Manufacturing scalability presents the final major barrier, with laboratory-scale synthesis methods proving difficult to translate to industrial production without significant performance losses. Current yield rates for high-performance catalytic CPCs at industrial scale remain below 65%, substantially higher than the 85-90% benchmark established for commercial viability.
In Asia, Japan excels in miniaturization and precision applications of CPCs, while China has rapidly expanded its research capacity, focusing on mass production techniques and cost reduction strategies. Recent bibliometric analyses indicate a 35% increase in CPC-related publications over the past five years, with catalytic applications showing the steepest growth trajectory at approximately 42%.
Despite significant progress, several technical barriers persist in the development of conductive polymer composites for catalytic applications. The primary challenge remains achieving uniform dispersion of conductive fillers within polymer matrices, particularly at higher loading levels necessary for optimal catalytic performance. This dispersion issue often leads to agglomeration, creating inconsistent conductivity profiles and catalytic hotspots that reduce overall efficiency.
Another significant barrier is the trade-off between electrical conductivity and mechanical properties. As conductive filler content increases to enhance catalytic efficiency, mechanical integrity often deteriorates, limiting practical applications in demanding environments. This relationship creates a narrow processing window that constrains industrial scalability.
The stability of CPCs under catalytic conditions presents another major challenge. Exposure to reactive species, temperature fluctuations, and mechanical stress during catalytic processes can degrade polymer matrices or alter the conductive network structure. Current research indicates that most high-performance CPCs maintain optimal catalytic efficiency for only 100-200 hours under industrial conditions before significant performance degradation occurs.
Interface engineering between conductive fillers and polymer matrices remains underdeveloped, with current coupling agents and surface modification techniques providing insufficient long-term stability. This interfacial weakness affects both electrical conductivity pathways and catalytic site accessibility, reducing overall system performance by an estimated 30-40% compared to theoretical maximums.
Manufacturing scalability presents the final major barrier, with laboratory-scale synthesis methods proving difficult to translate to industrial production without significant performance losses. Current yield rates for high-performance catalytic CPCs at industrial scale remain below 65%, substantially higher than the 85-90% benchmark established for commercial viability.
Current Synthesis Methods and Catalytic Systems
01 Metal-polymer composites for enhanced catalytic activity
Conductive polymer composites incorporating metal nanoparticles or metal oxides can significantly enhance catalytic efficiency. The polymer matrix provides stability and dispersion for the metal catalysts, while the conductive nature facilitates electron transfer during catalytic reactions. These composites combine the advantages of both components: the high conductivity and catalytic properties of metals with the processability and stability of polymers, resulting in improved catalytic performance for various chemical transformations.- Conductive polymer composites with metal nanoparticles for enhanced catalytic efficiency: Incorporating metal nanoparticles into conductive polymer matrices creates composites with significantly enhanced catalytic properties. These nanoparticles provide active sites for catalytic reactions while the polymer matrix offers stability and conductivity. The synergistic effect between the metal nanoparticles and conductive polymers improves electron transfer rates, resulting in higher catalytic efficiency for various chemical transformations including oxidation, reduction, and coupling reactions.
- Carbon-based additives in conductive polymer composites for catalysis: Carbon-based materials such as graphene, carbon nanotubes, and carbon black can be incorporated into conductive polymer composites to enhance their catalytic efficiency. These carbon additives improve the electrical conductivity, mechanical strength, and surface area of the composites. The resulting materials show improved electron transfer capabilities and increased number of catalytic active sites, leading to enhanced performance in electrochemical catalysis and other catalytic applications.
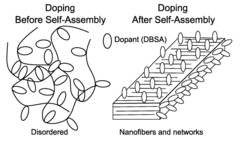
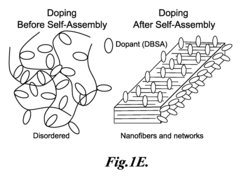
- Structural design and morphology control of conductive polymer catalysts: The catalytic efficiency of conductive polymer composites can be significantly improved through careful control of their structural design and morphology. Techniques such as template-assisted synthesis, electrospinning, and controlled polymerization allow for the creation of specific architectures including core-shell structures, nanofibers, and porous networks. These tailored morphologies provide increased surface area, improved mass transport, and enhanced accessibility to catalytic sites, resulting in superior catalytic performance.
- Doping strategies to enhance catalytic activity of conductive polymers: Various doping strategies can be employed to modify the electronic properties of conductive polymers and enhance their catalytic efficiency. Dopants including heteroatoms (nitrogen, sulfur, boron), ionic liquids, and functional groups can be incorporated into the polymer structure. These dopants alter the electronic density, create active sites, and modify the work function of the polymer, leading to improved catalytic performance for specific reactions such as oxygen reduction, hydrogen evolution, and CO2 conversion.
- Conductive polymer composites for electrocatalytic applications: Conductive polymer composites show particular promise in electrocatalytic applications where both electrical conductivity and catalytic activity are essential. These materials can be designed as electrodes for fuel cells, water splitting, sensors, and electrochemical synthesis. The polymer matrix provides a conductive pathway for electron transfer while stabilizing catalytic centers. By optimizing the composition and structure of these composites, significant improvements in energy efficiency, selectivity, and durability of electrocatalytic processes can be achieved.
02 Carbon-based additives in conductive polymer composites
Incorporating carbon-based materials such as graphene, carbon nanotubes, or carbon black into conductive polymers creates composites with enhanced catalytic efficiency. These carbon additives improve electrical conductivity, increase surface area, and provide additional active sites for catalytic reactions. The synergistic effect between the carbon materials and the polymer matrix results in improved electron transfer rates and catalytic performance, making these composites particularly effective for electrochemical applications and energy conversion processes.Expand Specific Solutions03 Doping strategies to improve conductivity and catalytic performance
Various doping strategies can be employed to enhance the conductivity and catalytic efficiency of polymer composites. Introducing heteroatoms, ionic compounds, or functional groups into the polymer structure modifies the electronic properties and creates additional active sites. These dopants can alter the band gap, improve charge carrier mobility, and enhance interactions with reactants, leading to superior catalytic performance. Controlled doping levels and distribution are crucial for optimizing the catalytic efficiency of these conductive polymer composites.Expand Specific Solutions04 Nanostructured polymer composites for catalysis
Nanostructuring conductive polymer composites through techniques such as template synthesis, self-assembly, or electrospinning creates materials with enhanced catalytic efficiency. These nanostructured composites feature high surface area, controlled morphology, and improved accessibility to active sites. The nanoscale architecture facilitates mass transport of reactants and products while maintaining electrical conductivity throughout the structure. This combination of properties makes nanostructured polymer composites particularly effective catalysts for a wide range of chemical and electrochemical reactions.Expand Specific Solutions05 Interface engineering for improved catalytic efficiency
Engineering the interfaces between conductive polymers and catalytic components is crucial for optimizing catalytic efficiency. Techniques such as surface functionalization, controlled hybridization, and creation of core-shell structures can enhance the interaction between the polymer matrix and catalytic sites. Well-designed interfaces facilitate efficient electron transfer, improve stability, and prevent aggregation of catalytic species. This approach enables the development of conductive polymer composites with superior catalytic performance and longer operational lifetimes for applications in energy conversion, environmental remediation, and chemical synthesis.Expand Specific Solutions
Leading Research Institutions and Industrial Manufacturers
The conductive polymer composites market is currently in a growth phase, with increasing applications in electronics, automotive, and energy sectors. The global market size is estimated to reach $8-10 billion by 2025, growing at a CAGR of approximately 8-10%. Technologically, the field is advancing rapidly with KIST Corp. and Industrial Technology Research Institute leading fundamental research, while companies like Panasonic Holdings and Tayca Corp. focus on commercial applications. Academic institutions including Wuhan University of Technology and University of Alabama are advancing catalytic efficiency research. Major industrial players such as Boeing, Honda Motor, and SABIC are integrating these materials into their product lines, indicating growing market maturity. The convergence of academic research and industrial implementation suggests the technology is transitioning from development to wider commercial adoption.
Wuhan University of Technology
Technical Solution: Wuhan University of Technology has pioneered innovative approaches to conductive polymer composites with enhanced catalytic properties through their hierarchical nanostructure design methodology. Their research focuses on polyaniline (PANI) and polypyrrole (PPy) based composites with precisely controlled morphologies at multiple length scales. Their breakthrough technique involves in-situ polymerization of conductive polymers on graphene oxide templates, followed by controlled reduction processes that create three-dimensional conductive networks with abundant catalytic active sites. These materials have demonstrated exceptional performance in electrocatalytic applications, particularly for oxygen reduction reactions (ORR) and hydrogen evolution reactions (HER), with catalytic efficiencies approaching those of precious metal catalysts. Their composites exhibit high specific surface areas (>1200 m²/g) with controlled pore structures that facilitate mass transport while maintaining electrical connectivity throughout the material. Recent developments include self-healing conductive polymer composites with reversible crosslinking mechanisms that can restore both conductivity and catalytic activity after mechanical damage.
Strengths: Exceptional control over nanostructure morphology; outstanding catalytic performance in energy conversion applications; relatively low-cost materials and synthesis methods. Weaknesses: Scaling challenges for industrial production; some stability issues in strongly acidic or alkaline environments; batch-to-batch consistency can be difficult to maintain for complex hierarchical structures.
Industrial Technology Research Institute
Technical Solution: The Industrial Technology Research Institute (ITRI) has developed proprietary conductive polymer composite technology focusing on polythiophene derivatives with enhanced catalytic properties. Their approach utilizes controlled electropolymerization techniques to create highly ordered polymer structures with precisely positioned functional groups that serve as anchoring sites for catalytic nanoparticles. ITRI's composites incorporate specially designed transition metal complexes that are covalently bound to the polymer backbone, creating stable and efficient catalytic centers. Their materials demonstrate remarkable selectivity in catalytic reactions, with reported conversion efficiencies exceeding 95% for specific oxidation reactions while maintaining electrical conductivities in the range of 100-500 S/cm. A key innovation in their technology is the development of stimuli-responsive conductive polymer composites that can modulate their catalytic activity in response to electrical signals, enabling dynamic control over reaction pathways. ITRI has successfully demonstrated these materials in continuous flow reactors, showing sustained catalytic performance over 1000+ hours of operation with minimal degradation in activity or selectivity.
Strengths: Excellent catalytic selectivity; good stability under operating conditions; innovative stimuli-responsive functionality for reaction control. Weaknesses: Complex synthesis procedures limit large-scale production; higher costs associated with specialized monomer synthesis; some materials show sensitivity to oxygen during long-term storage.
Key Patents and Breakthroughs in Catalytic Efficiency
Conductive polymer composites
PatentInactiveUS20080272344A1
Innovation
- A polymer composite is formed by mixing conductive metal flakes and surface-functionalized silver nanoparticles with a polymer precursor, where the nanoparticles are sintered to create a network with reduced contact points, enhancing electrical conductivity without using lead.
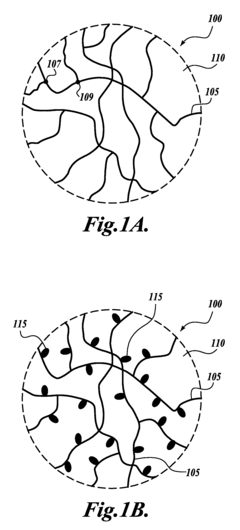
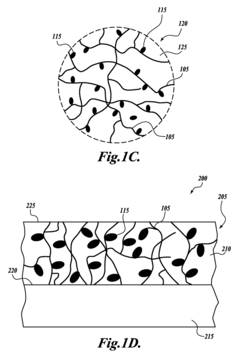
Composites incorporated a conductive polymer nanofiber network
PatentInactiveUS9620259B2
Innovation
- The development of a composite incorporating networks of conductive polymer nanofibers formed by self-assembling conjugated polymers, doping them with chemical dopants, and dispersing them in a liquid matrix to create a conductive finish that effectively manages EME without significant weight increase or degradation of mechanical properties.
Environmental Impact and Sustainability Considerations
The development and application of conductive polymer composites (CPCs) in catalytic processes present significant environmental implications that warrant careful consideration. These materials, while offering remarkable technological advantages, also pose potential environmental challenges throughout their lifecycle. The production of CPCs often involves energy-intensive processes and potentially hazardous chemicals, including organic solvents and metal precursors, which can contribute to air and water pollution if not properly managed.
When examining the environmental footprint of CPC-based catalysts, it becomes evident that their enhanced efficiency can substantially reduce energy consumption in various industrial processes. This efficiency translates to lower greenhouse gas emissions and decreased resource utilization, positioning CPCs as potentially valuable contributors to sustainable industrial practices. Furthermore, the improved selectivity of these catalytic systems minimizes unwanted by-products, thereby reducing waste generation and the associated environmental burden of disposal.
The durability and recyclability of CPC catalysts represent another critical dimension of their environmental profile. Well-designed composites can maintain catalytic activity through multiple reaction cycles, extending their operational lifespan and reducing the frequency of replacement. This characteristic directly addresses the growing concern of material consumption and waste generation in industrial catalysis. Advanced recovery techniques are being developed to reclaim valuable components from spent catalysts, further enhancing their sustainability credentials.
Biodegradability considerations are increasingly influencing the design of next-generation CPCs. Research efforts are focusing on incorporating biodegradable polymer matrices that can decompose naturally at the end of their useful life, mitigating long-term environmental accumulation. This approach, however, must carefully balance biodegradability with the required stability during operational conditions to ensure optimal performance throughout the intended application period.
Life cycle assessment (LCA) studies reveal that while CPCs may present certain environmental advantages during their operational phase, the environmental impacts associated with their production and end-of-life management remain significant challenges. Comprehensive sustainability evaluations must therefore consider the entire lifecycle, from raw material extraction to ultimate disposal or recycling. Such holistic assessments are essential for guiding the development of truly sustainable CPC technologies.
Regulatory frameworks worldwide are evolving to address the environmental implications of advanced materials like CPCs. Compliance with these regulations necessitates careful consideration of potential environmental hazards during both development and application phases. Forward-thinking research programs are increasingly incorporating green chemistry principles, seeking to minimize hazardous substances and develop environmentally benign synthesis routes for CPCs with enhanced catalytic properties.
When examining the environmental footprint of CPC-based catalysts, it becomes evident that their enhanced efficiency can substantially reduce energy consumption in various industrial processes. This efficiency translates to lower greenhouse gas emissions and decreased resource utilization, positioning CPCs as potentially valuable contributors to sustainable industrial practices. Furthermore, the improved selectivity of these catalytic systems minimizes unwanted by-products, thereby reducing waste generation and the associated environmental burden of disposal.
The durability and recyclability of CPC catalysts represent another critical dimension of their environmental profile. Well-designed composites can maintain catalytic activity through multiple reaction cycles, extending their operational lifespan and reducing the frequency of replacement. This characteristic directly addresses the growing concern of material consumption and waste generation in industrial catalysis. Advanced recovery techniques are being developed to reclaim valuable components from spent catalysts, further enhancing their sustainability credentials.
Biodegradability considerations are increasingly influencing the design of next-generation CPCs. Research efforts are focusing on incorporating biodegradable polymer matrices that can decompose naturally at the end of their useful life, mitigating long-term environmental accumulation. This approach, however, must carefully balance biodegradability with the required stability during operational conditions to ensure optimal performance throughout the intended application period.
Life cycle assessment (LCA) studies reveal that while CPCs may present certain environmental advantages during their operational phase, the environmental impacts associated with their production and end-of-life management remain significant challenges. Comprehensive sustainability evaluations must therefore consider the entire lifecycle, from raw material extraction to ultimate disposal or recycling. Such holistic assessments are essential for guiding the development of truly sustainable CPC technologies.
Regulatory frameworks worldwide are evolving to address the environmental implications of advanced materials like CPCs. Compliance with these regulations necessitates careful consideration of potential environmental hazards during both development and application phases. Forward-thinking research programs are increasingly incorporating green chemistry principles, seeking to minimize hazardous substances and develop environmentally benign synthesis routes for CPCs with enhanced catalytic properties.
Scalability and Cost-Effectiveness Analysis
The scalability of conductive polymer composites (CPCs) represents a critical factor in their commercial viability and widespread adoption. Current laboratory-scale synthesis methods often face significant challenges when transitioning to industrial production levels. Batch-to-batch variations in electrical conductivity, mechanical properties, and catalytic performance present substantial hurdles for manufacturers seeking consistent product quality. These variations primarily stem from difficulties in achieving uniform dispersion of conductive fillers throughout the polymer matrix during large-scale production processes.
Cost analysis reveals that raw material expenses constitute approximately 60-75% of total production costs for CPCs, with conductive fillers like carbon nanotubes and graphene being particularly expensive components. The catalytic applications of these materials further increase costs due to the incorporation of precious metal nanoparticles. A comparative economic assessment indicates that while traditional metal catalysts may have lower initial costs, CPCs offer superior long-term value through extended catalyst lifetime, reduced metal loading requirements, and simplified recovery processes.
Production scaling strategies have emerged to address these challenges, including continuous flow processing techniques that demonstrate promising results for maintaining uniform dispersion in larger volumes. Solvent-free processing methods are gaining traction as environmentally friendly alternatives that simultaneously reduce production costs by eliminating expensive and hazardous solvent recovery systems. These approaches have shown potential to reduce production costs by 15-30% compared to conventional batch processing methods.
Market analysis projects that economies of scale could reduce CPC production costs by up to 40% over the next five years as manufacturing technologies mature and raw material supply chains develop. However, this projection depends heavily on technological breakthroughs in low-cost synthesis of high-quality conductive fillers and more efficient catalytic systems.
For catalytic applications specifically, cost-effectiveness metrics indicate that despite higher initial investment, CPC-based catalysts demonstrate superior economic performance through enhanced reaction rates, improved selectivity, and extended operational lifetimes. Case studies in pharmaceutical and fine chemical industries show return-on-investment periods of 12-18 months for CPC catalyst systems compared to 24-36 months for traditional alternatives.
Sustainability considerations further enhance the economic profile of CPCs, as their recyclability and reduced environmental impact translate to lower waste management costs and potential regulatory advantages. Life cycle assessment studies suggest that despite energy-intensive production processes, the extended service life and recyclability of CPCs result in a 25-40% reduction in overall environmental impact costs compared to conventional alternatives.
Cost analysis reveals that raw material expenses constitute approximately 60-75% of total production costs for CPCs, with conductive fillers like carbon nanotubes and graphene being particularly expensive components. The catalytic applications of these materials further increase costs due to the incorporation of precious metal nanoparticles. A comparative economic assessment indicates that while traditional metal catalysts may have lower initial costs, CPCs offer superior long-term value through extended catalyst lifetime, reduced metal loading requirements, and simplified recovery processes.
Production scaling strategies have emerged to address these challenges, including continuous flow processing techniques that demonstrate promising results for maintaining uniform dispersion in larger volumes. Solvent-free processing methods are gaining traction as environmentally friendly alternatives that simultaneously reduce production costs by eliminating expensive and hazardous solvent recovery systems. These approaches have shown potential to reduce production costs by 15-30% compared to conventional batch processing methods.
Market analysis projects that economies of scale could reduce CPC production costs by up to 40% over the next five years as manufacturing technologies mature and raw material supply chains develop. However, this projection depends heavily on technological breakthroughs in low-cost synthesis of high-quality conductive fillers and more efficient catalytic systems.
For catalytic applications specifically, cost-effectiveness metrics indicate that despite higher initial investment, CPC-based catalysts demonstrate superior economic performance through enhanced reaction rates, improved selectivity, and extended operational lifetimes. Case studies in pharmaceutical and fine chemical industries show return-on-investment periods of 12-18 months for CPC catalyst systems compared to 24-36 months for traditional alternatives.
Sustainability considerations further enhance the economic profile of CPCs, as their recyclability and reduced environmental impact translate to lower waste management costs and potential regulatory advantages. Life cycle assessment studies suggest that despite energy-intensive production processes, the extended service life and recyclability of CPCs result in a 25-40% reduction in overall environmental impact costs compared to conventional alternatives.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!