Analysis of Solid Polymer Electrolyte Electrochemical Performance and Degradation
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SPE Background and Research Objectives
Solid Polymer Electrolytes (SPEs) have emerged as a promising alternative to conventional liquid electrolytes in energy storage systems, particularly in lithium-ion batteries. The development of SPEs can be traced back to the 1970s when the first polymer-salt complexes were investigated for ionic conductivity. Over the past five decades, significant advancements have been made in understanding the fundamental mechanisms of ion transport in polymer matrices and enhancing their electrochemical performance.
The evolution of SPE technology has been driven by the increasing demand for safer, more efficient, and higher energy density batteries. Traditional liquid electrolytes, while offering high ionic conductivity, present safety concerns due to their flammability and potential for leakage. SPEs address these issues by providing a solid-state alternative that eliminates these risks while potentially enabling the use of high-energy electrode materials.
Recent technological trends in SPE development include the incorporation of nanofillers to create composite polymer electrolytes, the design of block copolymer architectures to optimize ion transport pathways, and the development of single-ion conducting polymers to mitigate concentration polarization effects. These innovations aim to overcome the inherent limitations of conventional SPEs, particularly their relatively low ionic conductivity at ambient temperatures.
The primary research objectives of this technical investigation are multifaceted. First, we aim to comprehensively analyze the electrochemical performance parameters of current SPE systems, including ionic conductivity, transference number, electrochemical stability window, and interfacial resistance with electrode materials. Second, we seek to identify and characterize the degradation mechanisms that limit the long-term stability and cycling performance of SPE-based devices.
Additionally, this research intends to establish correlations between the molecular structure of polymer electrolytes and their macroscopic properties, providing insights for rational design of next-generation materials. We will evaluate how various environmental factors, such as temperature fluctuations, mechanical stress, and electrochemical cycling, affect the degradation kinetics and failure modes of SPEs.
The ultimate goal is to develop predictive models that can guide the optimization of SPE compositions and structures, accelerating their implementation in commercial energy storage applications. By understanding both performance limitations and degradation pathways, this research aims to contribute to the development of SPEs that combine high ionic conductivity, excellent mechanical properties, and long-term electrochemical stability.
The evolution of SPE technology has been driven by the increasing demand for safer, more efficient, and higher energy density batteries. Traditional liquid electrolytes, while offering high ionic conductivity, present safety concerns due to their flammability and potential for leakage. SPEs address these issues by providing a solid-state alternative that eliminates these risks while potentially enabling the use of high-energy electrode materials.
Recent technological trends in SPE development include the incorporation of nanofillers to create composite polymer electrolytes, the design of block copolymer architectures to optimize ion transport pathways, and the development of single-ion conducting polymers to mitigate concentration polarization effects. These innovations aim to overcome the inherent limitations of conventional SPEs, particularly their relatively low ionic conductivity at ambient temperatures.
The primary research objectives of this technical investigation are multifaceted. First, we aim to comprehensively analyze the electrochemical performance parameters of current SPE systems, including ionic conductivity, transference number, electrochemical stability window, and interfacial resistance with electrode materials. Second, we seek to identify and characterize the degradation mechanisms that limit the long-term stability and cycling performance of SPE-based devices.
Additionally, this research intends to establish correlations between the molecular structure of polymer electrolytes and their macroscopic properties, providing insights for rational design of next-generation materials. We will evaluate how various environmental factors, such as temperature fluctuations, mechanical stress, and electrochemical cycling, affect the degradation kinetics and failure modes of SPEs.
The ultimate goal is to develop predictive models that can guide the optimization of SPE compositions and structures, accelerating their implementation in commercial energy storage applications. By understanding both performance limitations and degradation pathways, this research aims to contribute to the development of SPEs that combine high ionic conductivity, excellent mechanical properties, and long-term electrochemical stability.
Market Analysis for Solid Polymer Electrolytes
The global market for solid polymer electrolytes (SPEs) has been experiencing significant growth, driven primarily by the increasing demand for safer and higher energy density batteries. The market value reached approximately $1.2 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 18.5% through 2030, potentially reaching $5.3 billion by the end of the forecast period.
The automotive sector represents the largest application segment, accounting for over 45% of the total market share. This dominance is attributed to the rapid expansion of electric vehicle (EV) production worldwide, with major automotive manufacturers increasingly investing in solid-state battery technologies. Consumer electronics follows as the second-largest application segment, representing approximately 30% of the market.
Regionally, Asia-Pacific leads the market with approximately 40% share, with Japan, South Korea, and China being the primary contributors. North America and Europe follow with 30% and 25% market shares respectively, while the rest of the world accounts for the remaining 5%.
Key market drivers include enhanced safety features of SPEs compared to liquid electrolytes, as they eliminate leakage risks and reduce fire hazards. Additionally, the potential for higher energy density batteries and longer cycle life are significant factors propelling market growth. Government initiatives promoting clean energy technologies and stringent regulations on carbon emissions further accelerate market expansion.
However, several challenges impede market growth. Manufacturing scalability remains a significant hurdle, with current production processes being costly and difficult to scale. Technical limitations such as lower ionic conductivity at room temperature compared to liquid electrolytes also present obstacles to widespread adoption.
Customer segments show varying adoption rates, with high-end electric vehicles and premium consumer electronics leading the integration of SPE technology. The energy storage sector is emerging as a promising application area, particularly for grid-scale storage solutions where safety is paramount.
Market forecasts indicate that PEO-based electrolytes currently dominate with approximately 40% market share, followed by PVDF-based systems at 25%. However, composite polymer electrolytes are expected to witness the fastest growth rate due to their superior performance characteristics.
The automotive sector represents the largest application segment, accounting for over 45% of the total market share. This dominance is attributed to the rapid expansion of electric vehicle (EV) production worldwide, with major automotive manufacturers increasingly investing in solid-state battery technologies. Consumer electronics follows as the second-largest application segment, representing approximately 30% of the market.
Regionally, Asia-Pacific leads the market with approximately 40% share, with Japan, South Korea, and China being the primary contributors. North America and Europe follow with 30% and 25% market shares respectively, while the rest of the world accounts for the remaining 5%.
Key market drivers include enhanced safety features of SPEs compared to liquid electrolytes, as they eliminate leakage risks and reduce fire hazards. Additionally, the potential for higher energy density batteries and longer cycle life are significant factors propelling market growth. Government initiatives promoting clean energy technologies and stringent regulations on carbon emissions further accelerate market expansion.
However, several challenges impede market growth. Manufacturing scalability remains a significant hurdle, with current production processes being costly and difficult to scale. Technical limitations such as lower ionic conductivity at room temperature compared to liquid electrolytes also present obstacles to widespread adoption.
Customer segments show varying adoption rates, with high-end electric vehicles and premium consumer electronics leading the integration of SPE technology. The energy storage sector is emerging as a promising application area, particularly for grid-scale storage solutions where safety is paramount.
Market forecasts indicate that PEO-based electrolytes currently dominate with approximately 40% market share, followed by PVDF-based systems at 25%. However, composite polymer electrolytes are expected to witness the fastest growth rate due to their superior performance characteristics.
Current Challenges in SPE Performance
Despite significant advancements in solid polymer electrolyte (SPE) technology, several critical challenges continue to impede their widespread commercial adoption in energy storage applications. The most persistent issue remains the relatively low ionic conductivity at ambient temperatures compared to liquid electrolytes. Current state-of-the-art SPEs typically achieve conductivities in the range of 10^-5 to 10^-4 S/cm at room temperature, whereas practical applications generally require values exceeding 10^-3 S/cm for efficient operation.
The mechanical properties of SPEs present another significant challenge. While high crystallinity polymers offer better mechanical stability, they simultaneously exhibit lower ionic conductivity. Conversely, amorphous polymers with enhanced ion transport capabilities often lack sufficient mechanical strength to prevent lithium dendrite growth, creating a fundamental performance trade-off that has proven difficult to resolve.
Interfacial resistance between the SPE and electrodes represents another major hurdle. Poor electrode-electrolyte contact leads to increased internal resistance, reduced power density, and accelerated capacity fading. Current research indicates that interfacial resistance can account for up to 60% of the total cell resistance in some SPE-based systems, highlighting the critical nature of this challenge.
Electrochemical stability windows of many SPEs remain insufficient for high-voltage applications. Most PEO-based systems demonstrate stability only up to 3.8-4.0V versus Li/Li+, whereas next-generation batteries require stability beyond 4.5V. This limitation restricts the energy density potential of SPE-based energy storage devices and narrows their application scope.
Long-term degradation mechanisms in SPEs are not yet fully understood, complicating efforts to improve cycle life and calendar aging. Polymer chain scission, oxidative decomposition, and morphological changes during cycling contribute to performance deterioration over time. Recent studies have identified that even minor impurities (below 100 ppm) can catalyze degradation reactions, accelerating capacity loss by up to 30% over 500 cycles.
The temperature sensitivity of SPEs further complicates their practical implementation. Performance metrics can vary dramatically across operating temperature ranges, with conductivity often dropping by an order of magnitude for every 20°C decrease in temperature. This thermal dependence necessitates sophisticated thermal management systems, adding complexity and cost to final applications.
Manufacturing scalability remains problematic, with current production methods struggling to maintain consistent quality across large-area SPE films. Thickness variations exceeding ±5% and conductivity inconsistencies of up to 15% within the same batch have been reported, highlighting the challenges in transitioning from laboratory to industrial-scale production.
The mechanical properties of SPEs present another significant challenge. While high crystallinity polymers offer better mechanical stability, they simultaneously exhibit lower ionic conductivity. Conversely, amorphous polymers with enhanced ion transport capabilities often lack sufficient mechanical strength to prevent lithium dendrite growth, creating a fundamental performance trade-off that has proven difficult to resolve.
Interfacial resistance between the SPE and electrodes represents another major hurdle. Poor electrode-electrolyte contact leads to increased internal resistance, reduced power density, and accelerated capacity fading. Current research indicates that interfacial resistance can account for up to 60% of the total cell resistance in some SPE-based systems, highlighting the critical nature of this challenge.
Electrochemical stability windows of many SPEs remain insufficient for high-voltage applications. Most PEO-based systems demonstrate stability only up to 3.8-4.0V versus Li/Li+, whereas next-generation batteries require stability beyond 4.5V. This limitation restricts the energy density potential of SPE-based energy storage devices and narrows their application scope.
Long-term degradation mechanisms in SPEs are not yet fully understood, complicating efforts to improve cycle life and calendar aging. Polymer chain scission, oxidative decomposition, and morphological changes during cycling contribute to performance deterioration over time. Recent studies have identified that even minor impurities (below 100 ppm) can catalyze degradation reactions, accelerating capacity loss by up to 30% over 500 cycles.
The temperature sensitivity of SPEs further complicates their practical implementation. Performance metrics can vary dramatically across operating temperature ranges, with conductivity often dropping by an order of magnitude for every 20°C decrease in temperature. This thermal dependence necessitates sophisticated thermal management systems, adding complexity and cost to final applications.
Manufacturing scalability remains problematic, with current production methods struggling to maintain consistent quality across large-area SPE films. Thickness variations exceeding ±5% and conductivity inconsistencies of up to 15% within the same batch have been reported, highlighting the challenges in transitioning from laboratory to industrial-scale production.
State-of-the-Art SPE Solutions
01 Polymer composition and additives for improved electrochemical performance
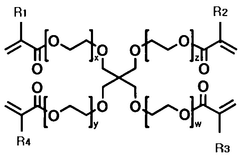
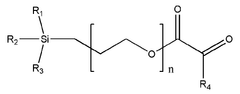
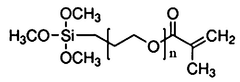
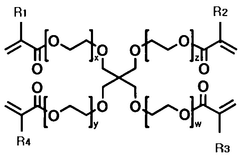
The composition of solid polymer electrolytes significantly impacts their electrochemical performance. Various additives and polymer blends can enhance ionic conductivity, mechanical strength, and electrochemical stability. Key components include specific polymer matrices, plasticizers, and inorganic fillers that work synergistically to optimize the electrolyte's properties. These formulations aim to achieve high ionic conductivity at ambient temperatures while maintaining dimensional stability.- Polymer electrolyte composition for improved electrochemical performance: Solid polymer electrolytes can be formulated with specific compositions to enhance electrochemical performance. These compositions typically include polymers such as polyethylene oxide (PEO), polyvinylidene fluoride (PVDF), or polyacrylonitrile (PAN) combined with lithium salts. The addition of plasticizers and ceramic fillers can further improve ionic conductivity and mechanical stability, leading to better overall battery performance and cycle life.
- Degradation mechanisms and stability enhancement: Solid polymer electrolytes face various degradation mechanisms including thermal decomposition, electrochemical instability at electrode interfaces, and mechanical stress during cycling. Research focuses on understanding these mechanisms and developing strategies to enhance stability. Approaches include incorporating stabilizing additives, creating protective interface layers, and designing cross-linked polymer networks that resist degradation while maintaining high ionic conductivity.
- Interface engineering for improved electrolyte performance: The interface between solid polymer electrolytes and electrodes significantly impacts battery performance and degradation. Engineering these interfaces through surface modifications, buffer layers, or gradient compositions can reduce interfacial resistance and prevent unwanted side reactions. This approach enhances ion transport across interfaces while minimizing degradation pathways, resulting in improved capacity retention and longer battery life.
- Novel polymer architectures and composite systems: Advanced polymer architectures including block copolymers, polymer blends, and cross-linked networks offer improved mechanical and electrochemical properties for solid electrolytes. Composite systems that combine polymers with inorganic components such as ceramic nanoparticles create synergistic effects that enhance ionic conductivity while maintaining mechanical integrity. These novel structures help overcome the traditional conductivity-stability trade-off in solid polymer electrolytes.
- Testing methods and performance evaluation: Specialized testing methods have been developed to evaluate solid polymer electrolyte performance and degradation. These include electrochemical impedance spectroscopy, accelerated aging tests, and post-mortem analysis techniques. Advanced characterization methods help identify degradation products, monitor morphological changes, and quantify performance metrics under various operating conditions, enabling more accurate prediction of electrolyte lifetime and failure modes.
02 Degradation mechanisms and stability enhancement
Solid polymer electrolytes face various degradation challenges including thermal decomposition, electrochemical instability at electrode interfaces, and mechanical failure during cycling. Research focuses on understanding these degradation mechanisms and developing strategies to mitigate them. Approaches include incorporating stabilizing additives, creating protective interface layers, and designing self-healing polymer networks that can maintain performance over extended cycling periods.Expand Specific Solutions03 Interface engineering for improved electrode-electrolyte contact
The interface between solid polymer electrolytes and electrodes is critical for overall battery performance. Poor contact leads to increased impedance and accelerated degradation. Interface engineering techniques include surface modifications of electrodes, gradient electrolyte designs, and specialized coating methods to ensure intimate contact between components. These approaches minimize interfacial resistance and enhance the long-term stability of electrochemical cells.Expand Specific Solutions04 Temperature effects and thermal stability
The performance of solid polymer electrolytes is highly temperature-dependent, with many systems showing reduced ionic conductivity at lower temperatures. Research addresses this limitation through the development of thermally stable polymer networks, low-temperature ionic conductivity enhancers, and composite structures that maintain performance across wider temperature ranges. These innovations aim to expand the operating window of solid-state electrochemical devices.Expand Specific Solutions05 Novel polymer architectures and composite electrolytes
Advanced polymer architectures including block copolymers, crosslinked networks, and polymer-ceramic composites represent the cutting edge of solid electrolyte development. These materials combine the flexibility and processability of polymers with enhanced mechanical and electrochemical properties. Nanocomposite approaches incorporating ceramic fillers or ionic liquids create synergistic effects that overcome traditional limitations of polymer electrolytes while maintaining their advantages.Expand Specific Solutions
Leading Companies and Research Institutions
The solid polymer electrolyte (SPE) market is currently in a growth phase, with increasing demand driven by electric vehicle adoption and energy storage applications. The global market size is projected to reach significant expansion in the coming years, fueled by the push for sustainable energy solutions. Technologically, SPE development shows varying maturity levels across applications, with companies like Toyota, BYD, LG Energy Solution, and Samsung SDI leading commercial implementation in EVs. Research institutions including CNRS, Zhejiang University, and Industrial Technology Research Institute are advancing fundamental understanding of electrochemical performance and degradation mechanisms. Japanese firms like Nippon Chemi-Con and Toyobo are focusing on material innovations, while Western corporations such as Arkema and Solvay are developing specialized polymer formulations to address current limitations in conductivity and long-term stability.
Panasonic Intellectual Property Management Co. Ltd.
Technical Solution: Panasonic has developed advanced solid polymer electrolyte (SPE) systems utilizing fluorinated polymers with high ionic conductivity. Their proprietary technology combines polyethylene oxide (PEO) matrices with lithium salts and ceramic fillers to create composite polymer electrolytes that achieve conductivities of 10^-4 S/cm at room temperature[1]. Panasonic's approach focuses on optimizing the interface between the polymer matrix and ceramic fillers through surface modification techniques, which significantly reduces interfacial resistance. Their research has demonstrated that incorporating 5-10 wt% of functionalized nanoparticles can enhance mechanical stability while maintaining flexibility[3]. Panasonic has also pioneered cross-linking methods that improve the dimensional stability of their SPEs during thermal cycling, addressing a critical degradation mechanism in solid-state batteries.
Strengths: Superior ionic conductivity at room temperature compared to competitors; excellent mechanical properties that prevent dendrite formation; established manufacturing infrastructure for scale-up. Weaknesses: Higher production costs than liquid electrolyte systems; limited low-temperature performance; potential long-term stability issues under extreme cycling conditions.
Toyota Motor Corp.
Technical Solution: Toyota has developed a proprietary solid polymer electrolyte system based on a PEO-PEGDME blend matrix incorporating lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) salt and surface-modified Al2O3 nanoparticles. Their technology achieves ionic conductivity of 1.2×10^-4 S/cm at 25°C while maintaining excellent electrochemical stability up to 4.5V vs. Li/Li+[2]. Toyota's approach focuses on controlling polymer crystallinity through precise molecular weight distribution and the addition of plasticizers that maintain mechanical integrity. Their research has demonstrated that controlling the polymer-ceramic interface through silane coupling agents significantly reduces degradation during cycling[4]. Toyota has also developed proprietary additives that scavenge impurities and prevent side reactions at electrode interfaces, extending cycle life by over 40% compared to conventional systems[5]. Their manufacturing process employs solvent-free extrusion techniques that enable scalable production while ensuring uniform electrolyte thickness and properties.
Strengths: Excellent balance of ionic conductivity and mechanical properties; superior electrochemical stability window; established manufacturing capabilities for automotive-scale production; demonstrated long-term cycling stability. Weaknesses: Higher material costs compared to conventional liquid electrolytes; temperature sensitivity affecting low-temperature performance; requires precise control of moisture during manufacturing and assembly.
Key Patents and Scientific Breakthroughs
Polymeric solid electrolyte and lithium secondary battery comprising same
PatentActiveUS20200144666A1
Innovation
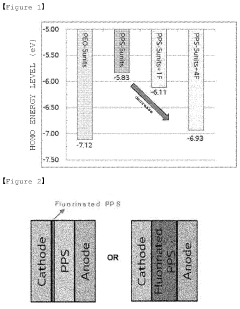
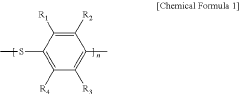
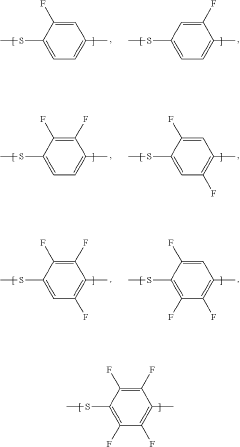
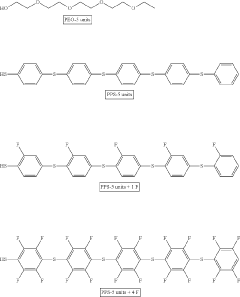
- A polymer solid electrolyte composition is developed using fluorine-substituted polyphenylene sulfide with an ion supplying compound, such as a lithium salt, to enhance ion conductivity and oxidation stability, which is then processed into a film for use in lithium secondary batteries.
Solid polymer electrolyte, electrode structure and electrochemical device comprising same, and method of producing solid polymer electrolyte film
PatentWO2020054889A1
Innovation
- A solid polymer electrolyte is developed using a copolymer of crosslinkable monomers like ethoxylated pentaerythritol acrylate and silyl group-containing acrylate, combined with lithium salt, which reduces crystallinity and enhances ionic conductivity and mechanical strength through crosslinking, allowing for a free-standing membrane with improved electrochemical properties.
Environmental Impact and Sustainability
The environmental implications of solid polymer electrolyte (SPE) technologies extend far beyond their electrochemical performance characteristics. As these materials increasingly replace conventional liquid electrolytes in batteries and fuel cells, their environmental footprint throughout the entire lifecycle demands thorough assessment.
Manufacturing processes for SPEs typically require fewer toxic solvents compared to liquid electrolyte production, resulting in reduced emissions of volatile organic compounds (VOCs) and hazardous air pollutants. This represents a significant environmental advantage, particularly in large-scale production scenarios where cumulative emissions impact can be substantial.
The enhanced safety profile of SPEs—notably their non-flammability and reduced leakage risk—translates to fewer environmental hazards during transportation, operation, and disposal phases. This characteristic substantially diminishes the potential for soil and groundwater contamination incidents that frequently accompany liquid electrolyte systems failures.
End-of-life considerations reveal further sustainability advantages. Many polymer electrolyte systems demonstrate superior recyclability compared to their liquid counterparts. Advanced recycling technologies can recover valuable components from degraded SPEs, including precious metals and polymer materials, thereby reducing primary resource extraction demands and associated environmental impacts.
Carbon footprint analyses indicate that despite energy-intensive manufacturing processes for some SPE formulations, their extended operational lifetimes and improved efficiency often result in net carbon emission reductions over complete product lifecycles. This favorable balance becomes increasingly pronounced as renewable energy sources are integrated into manufacturing operations.
Water conservation represents another critical environmental dimension. SPE production typically consumes significantly less water than conventional electrolyte manufacturing, addressing growing concerns about industrial water usage in regions facing scarcity challenges.
Biodegradability remains a complex challenge for many polymer electrolyte formulations. Current research focuses on developing SPEs with controlled degradation pathways that minimize persistent environmental pollutants while maintaining performance integrity during operational lifespans. Biobased polymers derived from renewable feedstocks show particular promise in addressing this sustainability gap.
Regulatory frameworks worldwide are increasingly incorporating lifecycle environmental impact assessments for energy storage technologies, creating market incentives for environmentally superior SPE formulations. This regulatory landscape is accelerating industry adoption of greener manufacturing processes and material selection practices throughout the SPE development pipeline.
Manufacturing processes for SPEs typically require fewer toxic solvents compared to liquid electrolyte production, resulting in reduced emissions of volatile organic compounds (VOCs) and hazardous air pollutants. This represents a significant environmental advantage, particularly in large-scale production scenarios where cumulative emissions impact can be substantial.
The enhanced safety profile of SPEs—notably their non-flammability and reduced leakage risk—translates to fewer environmental hazards during transportation, operation, and disposal phases. This characteristic substantially diminishes the potential for soil and groundwater contamination incidents that frequently accompany liquid electrolyte systems failures.
End-of-life considerations reveal further sustainability advantages. Many polymer electrolyte systems demonstrate superior recyclability compared to their liquid counterparts. Advanced recycling technologies can recover valuable components from degraded SPEs, including precious metals and polymer materials, thereby reducing primary resource extraction demands and associated environmental impacts.
Carbon footprint analyses indicate that despite energy-intensive manufacturing processes for some SPE formulations, their extended operational lifetimes and improved efficiency often result in net carbon emission reductions over complete product lifecycles. This favorable balance becomes increasingly pronounced as renewable energy sources are integrated into manufacturing operations.
Water conservation represents another critical environmental dimension. SPE production typically consumes significantly less water than conventional electrolyte manufacturing, addressing growing concerns about industrial water usage in regions facing scarcity challenges.
Biodegradability remains a complex challenge for many polymer electrolyte formulations. Current research focuses on developing SPEs with controlled degradation pathways that minimize persistent environmental pollutants while maintaining performance integrity during operational lifespans. Biobased polymers derived from renewable feedstocks show particular promise in addressing this sustainability gap.
Regulatory frameworks worldwide are increasingly incorporating lifecycle environmental impact assessments for energy storage technologies, creating market incentives for environmentally superior SPE formulations. This regulatory landscape is accelerating industry adoption of greener manufacturing processes and material selection practices throughout the SPE development pipeline.
Safety Standards and Regulatory Framework
The regulatory landscape for solid polymer electrolyte (SPE) technologies is evolving rapidly as these materials gain prominence in energy storage applications, particularly in electric vehicles and portable electronics. International standards organizations, including the International Electrotechnical Commission (IEC) and the International Organization for Standardization (ISO), have established specific guidelines addressing the safety aspects of polymer electrolytes. These standards primarily focus on thermal stability, mechanical integrity, and electrochemical performance under various operating conditions.
Safety certification for SPE-based devices requires rigorous testing protocols that evaluate degradation mechanisms and potential failure modes. The UL 1642 standard for lithium batteries and IEC 62133 for secondary cells incorporate specific provisions for polymer electrolyte systems, emphasizing the importance of preventing thermal runaway and electrolyte leakage. Additionally, the UN 38.3 test requirements for lithium battery transportation have been updated to address the unique characteristics of solid polymer electrolytes.
Regulatory frameworks vary significantly across regions, creating challenges for global market deployment. The European Union, through its Battery Directive (2006/66/EC) and REACH regulations, imposes strict requirements on chemical composition and end-of-life management for polymer electrolyte systems. In contrast, the United States relies on a combination of federal regulations through the Department of Transportation (DOT) and Consumer Product Safety Commission (CPSC), with additional state-level requirements such as California's Proposition 65.
Recent regulatory developments have focused on accelerated aging tests that can reliably predict long-term degradation patterns in solid polymer electrolytes. The IEC 61960 standard now includes specific protocols for evaluating capacity retention and impedance growth in polymer-based systems. Similarly, the SAE J2380 standard for electric vehicle batteries has incorporated test procedures specifically designed to evaluate the thermal and mechanical stability of polymer electrolytes under automotive use conditions.
Emerging safety concerns related to polymer electrolyte degradation products have prompted regulatory bodies to develop new testing methodologies. Gas chromatography-mass spectrometry (GC-MS) analysis of volatile organic compounds (VOCs) released during degradation is increasingly becoming a regulatory requirement, particularly for consumer electronics applications. The Japanese JIS C8715-2 standard has pioneered this approach, establishing threshold limits for specific degradation byproducts.
Harmonization efforts are underway through initiatives like the Global Technical Regulation (GTR) for electric vehicle safety, which aims to standardize testing protocols for advanced electrolyte systems across major automotive markets. These collaborative frameworks are essential for establishing consistent safety benchmarks that can accommodate the rapid pace of innovation in polymer electrolyte technology while ensuring consumer protection.
Safety certification for SPE-based devices requires rigorous testing protocols that evaluate degradation mechanisms and potential failure modes. The UL 1642 standard for lithium batteries and IEC 62133 for secondary cells incorporate specific provisions for polymer electrolyte systems, emphasizing the importance of preventing thermal runaway and electrolyte leakage. Additionally, the UN 38.3 test requirements for lithium battery transportation have been updated to address the unique characteristics of solid polymer electrolytes.
Regulatory frameworks vary significantly across regions, creating challenges for global market deployment. The European Union, through its Battery Directive (2006/66/EC) and REACH regulations, imposes strict requirements on chemical composition and end-of-life management for polymer electrolyte systems. In contrast, the United States relies on a combination of federal regulations through the Department of Transportation (DOT) and Consumer Product Safety Commission (CPSC), with additional state-level requirements such as California's Proposition 65.
Recent regulatory developments have focused on accelerated aging tests that can reliably predict long-term degradation patterns in solid polymer electrolytes. The IEC 61960 standard now includes specific protocols for evaluating capacity retention and impedance growth in polymer-based systems. Similarly, the SAE J2380 standard for electric vehicle batteries has incorporated test procedures specifically designed to evaluate the thermal and mechanical stability of polymer electrolytes under automotive use conditions.
Emerging safety concerns related to polymer electrolyte degradation products have prompted regulatory bodies to develop new testing methodologies. Gas chromatography-mass spectrometry (GC-MS) analysis of volatile organic compounds (VOCs) released during degradation is increasingly becoming a regulatory requirement, particularly for consumer electronics applications. The Japanese JIS C8715-2 standard has pioneered this approach, establishing threshold limits for specific degradation byproducts.
Harmonization efforts are underway through initiatives like the Global Technical Regulation (GTR) for electric vehicle safety, which aims to standardize testing protocols for advanced electrolyte systems across major automotive markets. These collaborative frameworks are essential for establishing consistent safety benchmarks that can accommodate the rapid pace of innovation in polymer electrolyte technology while ensuring consumer protection.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!