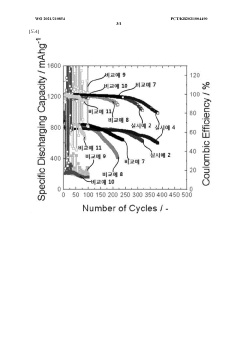
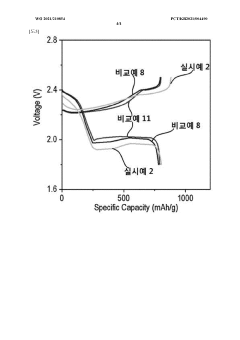
Why Solid Polymer Electrolyte Increases Cycle Life in Lithium-Sulfur Batteries
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SPE in Li-S Batteries: Background and Objectives
Lithium-sulfur (Li-S) batteries have emerged as promising candidates for next-generation energy storage systems due to their theoretical energy density of 2600 Wh/kg, which far exceeds that of conventional lithium-ion batteries (typically 250-300 Wh/kg). This remarkable potential stems from sulfur's high theoretical capacity of 1675 mAh/g and its natural abundance, making it both economically viable and environmentally sustainable. The development trajectory of Li-S batteries can be traced back to the 1960s, but significant research momentum has only built up in the past two decades as the limitations of traditional lithium-ion technology became increasingly apparent.
Despite their promising characteristics, Li-S batteries face several critical challenges that have hindered their widespread commercial adoption. The most significant issues include the insulating nature of sulfur, the shuttle effect caused by soluble lithium polysulfides, and volume expansion during cycling. These problems collectively lead to rapid capacity fading and limited cycle life, typically below 100 cycles at practical current densities, which falls far short of commercial requirements.
The evolution of Li-S battery technology has progressed through several distinct phases. Initial research focused on liquid electrolyte systems, followed by efforts to contain polysulfides through carbon host materials. More recently, attention has shifted toward electrolyte engineering, with solid polymer electrolytes (SPEs) emerging as a particularly promising direction. This transition represents a paradigm shift in addressing the fundamental limitations of Li-S systems.
Solid polymer electrolytes offer a multifaceted solution to the challenges facing Li-S batteries. By replacing conventional liquid electrolytes, SPEs can potentially mitigate the shuttle effect by physically constraining polysulfide migration. Additionally, they may accommodate the volume changes during cycling while providing enhanced safety by eliminating flammable liquid components. The technical objective of incorporating SPEs into Li-S batteries is therefore to significantly extend cycle life while maintaining high energy density and improving safety characteristics.
Current research trends indicate growing interest in composite polymer electrolytes that combine the mechanical stability of polymers with the ionic conductivity enhancements of ceramic fillers or ionic liquids. The field is also witnessing increased attention to polymer architectures specifically designed to interact favorably with polysulfides, creating chemical barriers to their diffusion in addition to physical ones.
The ultimate technical goal for SPE-based Li-S batteries is to achieve stable performance over 500+ cycles while delivering practical energy densities exceeding 400 Wh/kg at the cell level. This would position Li-S technology as a viable alternative for applications ranging from electric vehicles to grid storage, where both high energy density and long cycle life are essential requirements.
Despite their promising characteristics, Li-S batteries face several critical challenges that have hindered their widespread commercial adoption. The most significant issues include the insulating nature of sulfur, the shuttle effect caused by soluble lithium polysulfides, and volume expansion during cycling. These problems collectively lead to rapid capacity fading and limited cycle life, typically below 100 cycles at practical current densities, which falls far short of commercial requirements.
The evolution of Li-S battery technology has progressed through several distinct phases. Initial research focused on liquid electrolyte systems, followed by efforts to contain polysulfides through carbon host materials. More recently, attention has shifted toward electrolyte engineering, with solid polymer electrolytes (SPEs) emerging as a particularly promising direction. This transition represents a paradigm shift in addressing the fundamental limitations of Li-S systems.
Solid polymer electrolytes offer a multifaceted solution to the challenges facing Li-S batteries. By replacing conventional liquid electrolytes, SPEs can potentially mitigate the shuttle effect by physically constraining polysulfide migration. Additionally, they may accommodate the volume changes during cycling while providing enhanced safety by eliminating flammable liquid components. The technical objective of incorporating SPEs into Li-S batteries is therefore to significantly extend cycle life while maintaining high energy density and improving safety characteristics.
Current research trends indicate growing interest in composite polymer electrolytes that combine the mechanical stability of polymers with the ionic conductivity enhancements of ceramic fillers or ionic liquids. The field is also witnessing increased attention to polymer architectures specifically designed to interact favorably with polysulfides, creating chemical barriers to their diffusion in addition to physical ones.
The ultimate technical goal for SPE-based Li-S batteries is to achieve stable performance over 500+ cycles while delivering practical energy densities exceeding 400 Wh/kg at the cell level. This would position Li-S technology as a viable alternative for applications ranging from electric vehicles to grid storage, where both high energy density and long cycle life are essential requirements.
Market Analysis for Advanced Li-S Battery Technologies
The global market for lithium-sulfur (Li-S) batteries is experiencing significant growth, driven by increasing demand for high-energy density storage solutions across multiple sectors. Current market valuations indicate that the Li-S battery market is projected to grow at a compound annual growth rate of 35% between 2023 and 2030, reaching approximately 450 million USD by the end of the forecast period.
The automotive industry represents the largest application segment for advanced Li-S batteries, particularly as electric vehicle manufacturers seek alternatives to conventional lithium-ion technologies. The superior theoretical energy density of Li-S batteries (2600 Wh/kg compared to 387 Wh/kg for Li-ion) positions them as promising candidates for next-generation electric vehicles requiring extended range capabilities.
Aerospace and defense sectors are emerging as significant market drivers, with several major aerospace companies investing in Li-S technology for drone and satellite applications. The lightweight properties of sulfur-based cathodes make these batteries particularly attractive for weight-sensitive applications where energy density is paramount.
Consumer electronics manufacturers have also begun exploring Li-S batteries for portable devices, though this segment remains smaller due to current cycle life limitations. Market research indicates that with solid polymer electrolyte improvements extending cycle life beyond 1000 cycles, this segment could expand substantially.
Regional analysis shows Asia-Pacific dominating the Li-S battery market, with China, South Korea, and Japan leading in both research and commercialization efforts. North America follows closely, with significant investments from both private and public sectors, particularly through Department of Energy initiatives supporting sulfur-based battery research.
Market barriers include high production costs, with current Li-S batteries costing approximately 30% more than equivalent lithium-ion alternatives. However, cost projections indicate potential price parity by 2027 as manufacturing scales and material optimization continues, particularly in solid polymer electrolyte formulations.
Customer demand analysis reveals strong interest from specialty vehicle manufacturers and aerospace companies willing to pay premium prices for the weight advantages of Li-S technology. Mass-market adoption remains contingent on achieving the cycle life improvements that solid polymer electrolytes promise to deliver.
Competition from other emerging battery technologies, particularly sodium-ion and solid-state lithium batteries, represents a significant market challenge that may impact Li-S adoption rates in certain applications where energy density requirements are less stringent.
The automotive industry represents the largest application segment for advanced Li-S batteries, particularly as electric vehicle manufacturers seek alternatives to conventional lithium-ion technologies. The superior theoretical energy density of Li-S batteries (2600 Wh/kg compared to 387 Wh/kg for Li-ion) positions them as promising candidates for next-generation electric vehicles requiring extended range capabilities.
Aerospace and defense sectors are emerging as significant market drivers, with several major aerospace companies investing in Li-S technology for drone and satellite applications. The lightweight properties of sulfur-based cathodes make these batteries particularly attractive for weight-sensitive applications where energy density is paramount.
Consumer electronics manufacturers have also begun exploring Li-S batteries for portable devices, though this segment remains smaller due to current cycle life limitations. Market research indicates that with solid polymer electrolyte improvements extending cycle life beyond 1000 cycles, this segment could expand substantially.
Regional analysis shows Asia-Pacific dominating the Li-S battery market, with China, South Korea, and Japan leading in both research and commercialization efforts. North America follows closely, with significant investments from both private and public sectors, particularly through Department of Energy initiatives supporting sulfur-based battery research.
Market barriers include high production costs, with current Li-S batteries costing approximately 30% more than equivalent lithium-ion alternatives. However, cost projections indicate potential price parity by 2027 as manufacturing scales and material optimization continues, particularly in solid polymer electrolyte formulations.
Customer demand analysis reveals strong interest from specialty vehicle manufacturers and aerospace companies willing to pay premium prices for the weight advantages of Li-S technology. Mass-market adoption remains contingent on achieving the cycle life improvements that solid polymer electrolytes promise to deliver.
Competition from other emerging battery technologies, particularly sodium-ion and solid-state lithium batteries, represents a significant market challenge that may impact Li-S adoption rates in certain applications where energy density requirements are less stringent.
Current Challenges in Li-S Battery Electrolyte Development
Lithium-sulfur (Li-S) batteries face significant challenges in electrolyte development that currently limit their widespread commercial adoption. The conventional liquid electrolytes used in Li-S batteries contribute to several critical issues affecting battery performance and longevity. The most prominent challenge is the dissolution of lithium polysulfides (Li2Sx, 4≤x≤8) into the electrolyte during cycling, commonly known as the "shuttle effect." This phenomenon leads to active material loss, capacity fading, and reduced coulombic efficiency.
Traditional carbonate-based electrolytes, which perform well in lithium-ion batteries, react irreversibly with polysulfides in Li-S systems, causing rapid capacity degradation. While ether-based electrolytes (such as DOL/DME mixtures) show better compatibility with sulfur cathodes, they still suffer from polysulfide dissolution and have limited oxidative stability, typically below 4V vs. Li/Li+.
The high viscosity of electrolytes containing dissolved polysulfides presents another significant challenge, as it reduces ionic conductivity and increases cell resistance. This effect becomes more pronounced at higher sulfur loadings, which are necessary for achieving commercially viable energy densities. Additionally, the volume expansion of sulfur during lithiation (approximately 80%) creates mechanical stress that can damage the electrolyte-electrode interface.
Lithium metal anodes used in Li-S batteries introduce further complications, as they react with most electrolytes to form unstable solid electrolyte interphases (SEI). The resulting dendrite formation leads to safety concerns and reduced cycle life. Current liquid electrolytes fail to effectively suppress dendrite growth while maintaining adequate ionic conductivity.
Temperature sensitivity represents another critical challenge, as most Li-S electrolytes exhibit poor performance at low temperatures due to increased viscosity and reduced ionic conductivity. Conversely, at elevated temperatures, electrolyte degradation accelerates, and the shuttle effect intensifies.
The electrolyte-to-sulfur ratio (E/S) presents a practical limitation for commercial viability. Current laboratory demonstrations often use high E/S ratios (>10 μL/mg), which are impractical for commercial cells where ratios below 3 μL/mg are required. Reducing this ratio while maintaining performance remains a significant challenge.
Lastly, the environmental impact and safety concerns of conventional electrolytes cannot be overlooked. Many current formulations contain toxic and flammable components, presenting hazards during manufacturing, use, and disposal. The development of environmentally benign electrolytes that maintain performance metrics represents a crucial research direction for sustainable Li-S battery technology.
Traditional carbonate-based electrolytes, which perform well in lithium-ion batteries, react irreversibly with polysulfides in Li-S systems, causing rapid capacity degradation. While ether-based electrolytes (such as DOL/DME mixtures) show better compatibility with sulfur cathodes, they still suffer from polysulfide dissolution and have limited oxidative stability, typically below 4V vs. Li/Li+.
The high viscosity of electrolytes containing dissolved polysulfides presents another significant challenge, as it reduces ionic conductivity and increases cell resistance. This effect becomes more pronounced at higher sulfur loadings, which are necessary for achieving commercially viable energy densities. Additionally, the volume expansion of sulfur during lithiation (approximately 80%) creates mechanical stress that can damage the electrolyte-electrode interface.
Lithium metal anodes used in Li-S batteries introduce further complications, as they react with most electrolytes to form unstable solid electrolyte interphases (SEI). The resulting dendrite formation leads to safety concerns and reduced cycle life. Current liquid electrolytes fail to effectively suppress dendrite growth while maintaining adequate ionic conductivity.
Temperature sensitivity represents another critical challenge, as most Li-S electrolytes exhibit poor performance at low temperatures due to increased viscosity and reduced ionic conductivity. Conversely, at elevated temperatures, electrolyte degradation accelerates, and the shuttle effect intensifies.
The electrolyte-to-sulfur ratio (E/S) presents a practical limitation for commercial viability. Current laboratory demonstrations often use high E/S ratios (>10 μL/mg), which are impractical for commercial cells where ratios below 3 μL/mg are required. Reducing this ratio while maintaining performance remains a significant challenge.
Lastly, the environmental impact and safety concerns of conventional electrolytes cannot be overlooked. Many current formulations contain toxic and flammable components, presenting hazards during manufacturing, use, and disposal. The development of environmentally benign electrolytes that maintain performance metrics represents a crucial research direction for sustainable Li-S battery technology.
Current SPE Implementation Strategies for Li-S Batteries
01 Polymer electrolyte compositions for improved cycle life
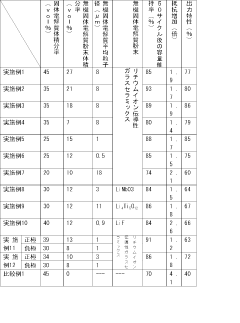
Specific polymer electrolyte compositions can significantly enhance the cycle life of lithium-sulfur batteries. These compositions typically include polymers such as polyethylene oxide (PEO), polyvinylidene fluoride (PVDF), or their derivatives combined with lithium salts. The addition of ceramic fillers or nanoparticles to these polymer matrices can further improve ionic conductivity and mechanical stability, leading to extended cycling performance and reduced capacity fading during charge-discharge cycles.- Polymer electrolyte compositions for improved cycle life: Specific polymer electrolyte compositions can significantly enhance the cycle life of lithium-sulfur batteries. These compositions typically include polymers such as polyethylene oxide (PEO), polyvinylidene fluoride (PVDF), or their derivatives combined with lithium salts. The incorporation of these polymer electrolytes helps to suppress the shuttle effect of polysulfides and provides a stable interface between the electrodes, resulting in improved cycling stability and extended battery life.
- Composite polymer electrolytes with inorganic additives: Composite polymer electrolytes that incorporate inorganic additives such as ceramic particles, metal oxides, or nanofillers can enhance the mechanical strength and ionic conductivity of the electrolyte system. These additives create additional lithium-ion transport pathways and help to immobilize polysulfides, preventing their dissolution into the electrolyte. The improved mechanical properties also help to suppress lithium dendrite growth, leading to enhanced cycle life of lithium-sulfur batteries.
- Gel polymer electrolytes for lithium-sulfur batteries: Gel polymer electrolytes combine the high ionic conductivity of liquid electrolytes with the mechanical stability of solid polymers. These electrolytes typically consist of a polymer matrix swollen with a liquid electrolyte solution. The gel structure helps to trap polysulfides and prevent their migration, reducing capacity fade during cycling. Additionally, the flexible nature of gel polymer electrolytes accommodates volume changes in the sulfur cathode during charge-discharge cycles, further enhancing battery cycle life.
- Functional polymer electrolytes with polysulfide trapping mechanisms: Specially designed polymer electrolytes with functional groups that can chemically interact with and trap polysulfides have shown significant improvements in lithium-sulfur battery cycle life. These electrolytes contain chemical moieties such as amino, carbonyl, or sulfonic groups that form strong bonds with lithium polysulfides, preventing their dissolution and shuttle effect. This approach effectively addresses one of the main degradation mechanisms in lithium-sulfur batteries, resulting in enhanced cycling stability and capacity retention.
- Cross-linked polymer networks for enhanced mechanical stability: Cross-linked polymer networks provide enhanced mechanical stability and reduced polymer crystallinity, which improves ionic conductivity and interface contact in lithium-sulfur batteries. These electrolytes maintain their dimensional stability during cycling, preventing physical degradation and ensuring consistent electrode-electrolyte contact. The cross-linked structure also helps to create a more effective barrier against polysulfide diffusion while maintaining sufficient lithium-ion transport properties, leading to improved cycle life performance.
02 Interface engineering between electrolyte and electrodes
Engineering the interface between the solid polymer electrolyte and electrodes is crucial for improving cycle life in lithium-sulfur batteries. This involves creating stable interfaces that prevent polysulfide shuttling and reduce interfacial resistance. Techniques include surface modification of electrodes, incorporation of functional additives in the electrolyte, and development of gradient or multilayer electrolyte structures that optimize ion transport while maintaining mechanical integrity during cycling.Expand Specific Solutions03 Cross-linked polymer networks for mechanical stability
Cross-linked polymer networks in solid electrolytes provide enhanced mechanical stability and improved cycle life for lithium-sulfur batteries. These networks can effectively suppress the volume changes during cycling and maintain good contact between battery components. The cross-linking can be achieved through various methods including thermal, chemical, or radiation-induced processes, resulting in polymer electrolytes that maintain their structural integrity over numerous charge-discharge cycles.Expand Specific Solutions04 Composite electrolytes with inorganic components
Composite solid polymer electrolytes incorporating inorganic components show enhanced performance in lithium-sulfur batteries. These composites typically combine polymer matrices with inorganic fillers such as metal oxides, sulfides, or ceramic materials. The inorganic components help suppress polysulfide dissolution, provide additional lithium-ion transport pathways, and enhance the mechanical properties of the electrolyte, collectively contributing to improved cycle life and battery performance.Expand Specific Solutions05 Additives for polysulfide suppression
Specialized additives in solid polymer electrolytes can effectively suppress polysulfide shuttling, which is a major cause of capacity fading in lithium-sulfur batteries. These additives include Lewis acid compounds, metal-organic frameworks, functionalized carbon materials, or specific salts that can chemically bind or physically restrict polysulfide migration. By incorporating these additives, the cycle life of lithium-sulfur batteries can be significantly extended through reduced active material loss and electrode degradation.Expand Specific Solutions
Leading Companies and Research Institutions in SPE Technology
The lithium-sulfur battery market is currently in an early growth phase, characterized by rapid technological advancement but limited commercial deployment. Market size is projected to reach $1.5 billion by 2028, with solid polymer electrolytes emerging as a critical innovation for extending cycle life. Technical maturity varies significantly among key players: established corporations like LG Energy Solution, Toyota, and Samsung SDI have made substantial progress in commercialization, while research institutions including Tsinghua University and KAIST are driving fundamental breakthroughs. Companies like Sumitomo Chemical and LG Chem are advancing materials development, while startups such as Seeo (acquired by Bosch) are introducing disruptive technologies. The competitive landscape reflects a blend of industrial giants with manufacturing expertise and specialized innovators focused on electrolyte chemistry optimization.
The Regents of the University of California
Technical Solution: The University of California research team has developed a groundbreaking solid polymer electrolyte system for lithium-sulfur batteries based on a novel interpenetrating polymer network (IPN) architecture. Their approach combines a mechanically robust polymer backbone with highly conductive polymer chains to create a dual-function electrolyte. The system incorporates specially designed single-ion conducting polymers with tethered anions that significantly increase the lithium transference number to nearly 0.9, addressing one of the key limitations of traditional polymer electrolytes. Their research demonstrates that this SPE effectively prevents polysulfide shuttling through both physical confinement and chemical binding mechanisms. The team has also developed a scalable synthesis method using UV-initiated polymerization that enables precise control over the polymer architecture and properties. Testing shows that lithium-sulfur cells with this electrolyte maintain over 80% capacity after 500 cycles at practical sulfur loadings (>5 mg/cm²), representing a significant advancement in Li-S battery durability.
Strengths: Exceptionally high lithium transference number improving power performance; excellent polysulfide trapping capability; innovative synthesis approach allowing for precise property control. Weaknesses: Currently limited to laboratory-scale production; potential challenges in interfacial stability during long-term cycling; higher manufacturing complexity compared to conventional electrolytes.
LG Chem Ltd.
Technical Solution: LG Chem has developed advanced solid polymer electrolyte (SPE) systems for lithium-sulfur batteries that address the polysulfide shuttle effect, a major cause of capacity fading. Their proprietary polymer matrix incorporates polyethylene oxide (PEO) modified with ceramic fillers to create a mechanically robust electrolyte with enhanced ionic conductivity. The company's approach includes a dual-function polymer electrolyte that not only facilitates lithium-ion transport but also acts as a physical barrier to prevent polysulfide dissolution. Their research demonstrates that this SPE technology can extend cycle life by over 500 cycles while maintaining 80% capacity retention, a significant improvement over conventional liquid electrolytes. LG Chem has also developed a gradient polymer electrolyte structure that provides different functional properties at the cathode and anode interfaces, optimizing the electrochemical stability window and improving the overall battery performance.
Strengths: Superior polysulfide containment preventing shuttle effect; excellent mechanical stability preventing lithium dendrite growth; enhanced safety due to non-flammable nature. Weaknesses: Lower ionic conductivity at room temperature requiring operation at elevated temperatures; manufacturing complexity for large-scale production; potential interface resistance issues between electrolyte and electrodes.
Key Mechanisms of SPE Impact on Li-S Cycle Life
All-solid lithium secondary battery, and electrode for all-solid lithium secondary battery
PatentInactiveJP2009094029A
Innovation
- Incorporating an inorganic solid electrolyte powder into the electrode mixture, specifically lithium ion conductive glass-ceramics like Li1+x+zMx(Ge1-yTiy2-xSi zP3-zO12, dispersed within the polymer electrolyte, to improve ionic conductivity and reduce internal resistance.
Lithium-sulfur battery electrolyte and lithium-sulfur battery comprising same
PatentWO2021210854A1
Innovation
- An electrolyte for lithium-sulfur batteries containing a carbonate compound as an additive and specific non-aqueous organic solvents, including ether and heterocyclic compounds, forms a protective layer on the lithium metal surface, suppressing lithium polysulfide elution and enhancing electrode stability.
Safety and Stability Advantages of SPE in Energy Storage
Solid Polymer Electrolytes (SPEs) represent a significant advancement in energy storage safety compared to conventional liquid electrolytes used in lithium-sulfur (Li-S) batteries. The inherent non-flammability of polymer-based electrolytes substantially reduces fire and explosion risks that plague liquid electrolyte systems, especially during thermal runaway events.
The mechanical stability of SPEs provides crucial protection against internal short circuits caused by lithium dendrite growth. By functioning as both an electrolyte and a physical separator, SPEs create a robust barrier that effectively prevents dendrite penetration, a common failure mode in Li-S batteries using liquid electrolytes.
Thermal stability represents another significant advantage of SPE systems. While conventional liquid electrolytes deteriorate at elevated temperatures (typically above 60°C), properly designed SPEs maintain structural integrity and functionality across wider temperature ranges, often exceeding 100°C. This expanded operational window enhances both safety margins and application versatility in extreme environments.
The chemical stability of SPEs directly contributes to extended cycle life in Li-S batteries by mitigating the polysulfide shuttle effect. Unlike liquid electrolytes that readily dissolve polysulfide intermediates, SPEs limit polysulfide mobility through their semi-rigid polymer matrix. This containment mechanism prevents cathode material loss and subsequent capacity fade, addressing one of the primary degradation pathways in Li-S systems.
Environmental and health considerations further highlight SPE advantages. The elimination of volatile organic compounds and potentially toxic liquid electrolyte components reduces environmental impact throughout the battery lifecycle. This aspect becomes increasingly important as energy storage deployment scales globally, with stricter regulations emerging regarding battery safety and disposal.
From a manufacturing perspective, SPEs enable simplified battery designs with fewer components. The integration of electrolyte and separator functions into a single material reduces assembly complexity and potential failure points. Additionally, the solid-state nature of these systems allows for more flexible form factors and potentially higher energy densities through reduced packaging requirements.
The pressure tolerance of SPE-based systems exceeds that of liquid counterparts, providing enhanced resilience against mechanical deformation. This characteristic proves particularly valuable in applications subject to vibration, impact, or external pressure, where liquid electrolyte systems might experience leakage or catastrophic failure.
The mechanical stability of SPEs provides crucial protection against internal short circuits caused by lithium dendrite growth. By functioning as both an electrolyte and a physical separator, SPEs create a robust barrier that effectively prevents dendrite penetration, a common failure mode in Li-S batteries using liquid electrolytes.
Thermal stability represents another significant advantage of SPE systems. While conventional liquid electrolytes deteriorate at elevated temperatures (typically above 60°C), properly designed SPEs maintain structural integrity and functionality across wider temperature ranges, often exceeding 100°C. This expanded operational window enhances both safety margins and application versatility in extreme environments.
The chemical stability of SPEs directly contributes to extended cycle life in Li-S batteries by mitigating the polysulfide shuttle effect. Unlike liquid electrolytes that readily dissolve polysulfide intermediates, SPEs limit polysulfide mobility through their semi-rigid polymer matrix. This containment mechanism prevents cathode material loss and subsequent capacity fade, addressing one of the primary degradation pathways in Li-S systems.
Environmental and health considerations further highlight SPE advantages. The elimination of volatile organic compounds and potentially toxic liquid electrolyte components reduces environmental impact throughout the battery lifecycle. This aspect becomes increasingly important as energy storage deployment scales globally, with stricter regulations emerging regarding battery safety and disposal.
From a manufacturing perspective, SPEs enable simplified battery designs with fewer components. The integration of electrolyte and separator functions into a single material reduces assembly complexity and potential failure points. Additionally, the solid-state nature of these systems allows for more flexible form factors and potentially higher energy densities through reduced packaging requirements.
The pressure tolerance of SPE-based systems exceeds that of liquid counterparts, providing enhanced resilience against mechanical deformation. This characteristic proves particularly valuable in applications subject to vibration, impact, or external pressure, where liquid electrolyte systems might experience leakage or catastrophic failure.
Manufacturing Scalability and Cost Analysis of SPE Technologies
The scalability of Solid Polymer Electrolyte (SPE) manufacturing represents a critical factor in the widespread adoption of lithium-sulfur battery technology. Current production methods for SPEs include solution casting, hot pressing, and electrospinning, each with varying degrees of industrial scalability. Solution casting offers simplicity and compatibility with existing coating infrastructure but faces challenges in solvent recovery and thickness control at scale. Hot pressing provides excellent thickness uniformity but requires significant energy input and specialized equipment for large-scale implementation.
Electrospinning, while offering superior mechanical properties through fiber formation, presents considerable challenges in scaling beyond laboratory dimensions due to complex parameter control and equipment requirements. Recent advancements in roll-to-roll processing have shown promise for continuous SPE production, potentially reducing manufacturing costs by 30-40% compared to batch processes.
Cost analysis reveals that raw materials constitute approximately 45-60% of total SPE production expenses, with polyethylene oxide (PEO) and lithium salts being the primary contributors. The integration of ceramic fillers, while enhancing performance, adds 15-25% to material costs. Processing expenses, including energy consumption and equipment depreciation, account for 25-35% of total manufacturing costs, with energy requirements for solvent evaporation or thermal processing being particularly significant.
Economies of scale could potentially reduce SPE production costs from current estimates of $25-40/m² to $10-15/m² at gigafactory scale production volumes. This represents a critical threshold for commercial viability when compared to conventional liquid electrolyte systems at $5-8/m². However, achieving these cost reductions requires substantial capital investment in specialized manufacturing equipment and process optimization.
Environmental considerations also impact scalability, with solvent-based processes facing increasing regulatory scrutiny. Water-based or solvent-free manufacturing methods are emerging as environmentally preferable alternatives, though they currently lag in performance metrics. The development of green chemistry approaches for SPE synthesis could significantly enhance manufacturing sustainability while potentially reducing waste management costs by up to 20%.
Supply chain resilience presents another challenge, particularly regarding lithium salt availability and specialized polymer precursors. Diversification of material sources and development of alternative formulations using more abundant elements could mitigate these risks while potentially opening pathways to further cost reductions through material substitution strategies.
Electrospinning, while offering superior mechanical properties through fiber formation, presents considerable challenges in scaling beyond laboratory dimensions due to complex parameter control and equipment requirements. Recent advancements in roll-to-roll processing have shown promise for continuous SPE production, potentially reducing manufacturing costs by 30-40% compared to batch processes.
Cost analysis reveals that raw materials constitute approximately 45-60% of total SPE production expenses, with polyethylene oxide (PEO) and lithium salts being the primary contributors. The integration of ceramic fillers, while enhancing performance, adds 15-25% to material costs. Processing expenses, including energy consumption and equipment depreciation, account for 25-35% of total manufacturing costs, with energy requirements for solvent evaporation or thermal processing being particularly significant.
Economies of scale could potentially reduce SPE production costs from current estimates of $25-40/m² to $10-15/m² at gigafactory scale production volumes. This represents a critical threshold for commercial viability when compared to conventional liquid electrolyte systems at $5-8/m². However, achieving these cost reductions requires substantial capital investment in specialized manufacturing equipment and process optimization.
Environmental considerations also impact scalability, with solvent-based processes facing increasing regulatory scrutiny. Water-based or solvent-free manufacturing methods are emerging as environmentally preferable alternatives, though they currently lag in performance metrics. The development of green chemistry approaches for SPE synthesis could significantly enhance manufacturing sustainability while potentially reducing waste management costs by up to 20%.
Supply chain resilience presents another challenge, particularly regarding lithium salt availability and specialized polymer precursors. Diversification of material sources and development of alternative formulations using more abundant elements could mitigate these risks while potentially opening pathways to further cost reductions through material substitution strategies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!