Solid Polymer Electrolyte Stability in Extreme Temperature Environments
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SPE Development Background and Objectives
Solid Polymer Electrolytes (SPEs) have emerged as a transformative technology in energy storage systems, particularly for next-generation batteries. The development of SPEs dates back to the 1970s when the first polymer-salt complexes were discovered to exhibit ionic conductivity. Over subsequent decades, research has intensified as limitations of liquid electrolytes became increasingly apparent, especially regarding safety concerns and form factor constraints.
The evolution of SPE technology has been marked by several significant milestones. Initial polymer hosts such as polyethylene oxide (PEO) demonstrated the feasibility of solid-state ion transport but suffered from poor conductivity at ambient temperatures. The 1990s saw the introduction of copolymer and block-polymer architectures, while the 2000s brought advancements in composite systems incorporating ceramic fillers to enhance mechanical properties and conductivity.
Current technological trends in SPE development focus on achieving stability across wider temperature ranges, particularly in extreme environments from -40°C to 80°C, which represents a critical challenge for applications in aerospace, automotive, and remote deployment scenarios. The integration of novel polymer chemistries, cross-linking strategies, and nano-engineered interfaces has accelerated in recent years, driven by the growing demand for safer, more reliable energy storage solutions.
The primary objective of SPE research is to develop electrolyte materials that maintain structural integrity, electrochemical stability, and sufficient ionic conductivity (>10^-4 S/cm) across extreme temperature conditions. This includes addressing the fundamental challenges of polymer chain mobility at low temperatures and preventing thermal degradation or phase transitions at elevated temperatures.
Secondary objectives include enhancing the mechanical robustness to withstand thermal expansion/contraction cycles, minimizing interfacial resistance with electrodes during temperature fluctuations, and ensuring long-term cycling stability under variable thermal conditions. These objectives align with broader industry goals to extend battery operational lifetimes and reduce failure rates in demanding environments.
The strategic importance of temperature-stable SPEs extends beyond conventional consumer electronics to critical infrastructure, defense applications, and renewable energy storage systems where reliability under environmental stress is paramount. As climate change intensifies weather extremes and as electrification reaches more remote regions, the need for thermally resilient energy storage becomes increasingly urgent.
Research targets for the next five years include developing SPE systems that maintain consistent performance across a 120°C temperature window without requiring external thermal management, while simultaneously achieving energy densities comparable to current liquid electrolyte systems. This represents a significant technical challenge requiring interdisciplinary approaches spanning polymer science, electrochemistry, and materials engineering.
The evolution of SPE technology has been marked by several significant milestones. Initial polymer hosts such as polyethylene oxide (PEO) demonstrated the feasibility of solid-state ion transport but suffered from poor conductivity at ambient temperatures. The 1990s saw the introduction of copolymer and block-polymer architectures, while the 2000s brought advancements in composite systems incorporating ceramic fillers to enhance mechanical properties and conductivity.
Current technological trends in SPE development focus on achieving stability across wider temperature ranges, particularly in extreme environments from -40°C to 80°C, which represents a critical challenge for applications in aerospace, automotive, and remote deployment scenarios. The integration of novel polymer chemistries, cross-linking strategies, and nano-engineered interfaces has accelerated in recent years, driven by the growing demand for safer, more reliable energy storage solutions.
The primary objective of SPE research is to develop electrolyte materials that maintain structural integrity, electrochemical stability, and sufficient ionic conductivity (>10^-4 S/cm) across extreme temperature conditions. This includes addressing the fundamental challenges of polymer chain mobility at low temperatures and preventing thermal degradation or phase transitions at elevated temperatures.
Secondary objectives include enhancing the mechanical robustness to withstand thermal expansion/contraction cycles, minimizing interfacial resistance with electrodes during temperature fluctuations, and ensuring long-term cycling stability under variable thermal conditions. These objectives align with broader industry goals to extend battery operational lifetimes and reduce failure rates in demanding environments.
The strategic importance of temperature-stable SPEs extends beyond conventional consumer electronics to critical infrastructure, defense applications, and renewable energy storage systems where reliability under environmental stress is paramount. As climate change intensifies weather extremes and as electrification reaches more remote regions, the need for thermally resilient energy storage becomes increasingly urgent.
Research targets for the next five years include developing SPE systems that maintain consistent performance across a 120°C temperature window without requiring external thermal management, while simultaneously achieving energy densities comparable to current liquid electrolyte systems. This represents a significant technical challenge requiring interdisciplinary approaches spanning polymer science, electrochemistry, and materials engineering.
Market Analysis for Temperature-Resistant Electrolytes
The global market for temperature-resistant solid polymer electrolytes is experiencing significant growth, driven primarily by the expanding electric vehicle (EV) sector and renewable energy storage systems. Current market valuations indicate that temperature-resistant electrolytes represent approximately 18% of the overall battery electrolyte market, with projections showing this segment growing at a compound annual growth rate of 22% through 2030.
Consumer electronics manufacturers are increasingly demanding batteries that maintain performance across wider temperature ranges, particularly as devices become more powerful and generate more heat during operation. This demand is creating a substantial secondary market beyond automotive applications, estimated at 3.2 billion USD in 2023.
The aerospace and defense sectors present premium market opportunities for extreme temperature-resistant electrolytes, with requirements for materials that can function from -65°C to +150°C. Though smaller in volume than consumer markets, these sectors offer higher margins and more stable long-term contracts, with current market size approaching 1.5 billion USD.
Regional analysis reveals Asia-Pacific dominates manufacturing capacity, with China, South Korea, and Japan collectively accounting for 67% of production. However, recent geopolitical tensions have accelerated efforts in North America and Europe to establish domestic supply chains, with government initiatives providing substantial funding for research and manufacturing facilities.
Market segmentation shows lithium-ion battery applications currently represent the largest share at 72%, followed by solid-state batteries at 21%. The remaining market consists of specialized applications including flow batteries and supercapacitors. The solid-state segment is projected to grow most rapidly, potentially reaching 40% market share by 2028.
Price sensitivity analysis indicates that while performance remains the primary purchasing factor for high-end applications, cost considerations become increasingly important in consumer markets. Current premium pricing for temperature-resistant electrolytes (approximately 2.3 times standard electrolytes) represents a significant barrier to mass-market adoption.
Customer feedback from tier-one automotive manufacturers highlights that temperature stability at both extremes (-30°C to +80°C) is considered essential for next-generation EVs, with particular emphasis on fast-charging capabilities at low temperatures. This requirement is driving substantial R&D investment, with major battery manufacturers allocating an average of 14% of research budgets specifically to electrolyte temperature stability improvements.
Consumer electronics manufacturers are increasingly demanding batteries that maintain performance across wider temperature ranges, particularly as devices become more powerful and generate more heat during operation. This demand is creating a substantial secondary market beyond automotive applications, estimated at 3.2 billion USD in 2023.
The aerospace and defense sectors present premium market opportunities for extreme temperature-resistant electrolytes, with requirements for materials that can function from -65°C to +150°C. Though smaller in volume than consumer markets, these sectors offer higher margins and more stable long-term contracts, with current market size approaching 1.5 billion USD.
Regional analysis reveals Asia-Pacific dominates manufacturing capacity, with China, South Korea, and Japan collectively accounting for 67% of production. However, recent geopolitical tensions have accelerated efforts in North America and Europe to establish domestic supply chains, with government initiatives providing substantial funding for research and manufacturing facilities.
Market segmentation shows lithium-ion battery applications currently represent the largest share at 72%, followed by solid-state batteries at 21%. The remaining market consists of specialized applications including flow batteries and supercapacitors. The solid-state segment is projected to grow most rapidly, potentially reaching 40% market share by 2028.
Price sensitivity analysis indicates that while performance remains the primary purchasing factor for high-end applications, cost considerations become increasingly important in consumer markets. Current premium pricing for temperature-resistant electrolytes (approximately 2.3 times standard electrolytes) represents a significant barrier to mass-market adoption.
Customer feedback from tier-one automotive manufacturers highlights that temperature stability at both extremes (-30°C to +80°C) is considered essential for next-generation EVs, with particular emphasis on fast-charging capabilities at low temperatures. This requirement is driving substantial R&D investment, with major battery manufacturers allocating an average of 14% of research budgets specifically to electrolyte temperature stability improvements.
Current Challenges in Extreme Temperature SPE Technology
Despite significant advancements in solid polymer electrolyte (SPE) technology, several critical challenges persist when operating in extreme temperature environments. The primary obstacle remains the dramatic conductivity drop at low temperatures (below 0°C), where polymer chain mobility decreases substantially, limiting ion transport mechanisms. This results in severe performance degradation in cold climates, with some systems showing up to 90% reduction in ionic conductivity at -20°C compared to room temperature values.
Conversely, at elevated temperatures (above 80°C), SPEs face structural integrity issues as polymer matrices approach or exceed their glass transition temperatures. This leads to mechanical softening, dimensional instability, and potential short-circuit risks in battery applications. The thermal expansion coefficient mismatch between different components exacerbates these problems, creating internal stresses that compromise interfacial contact quality.
Chemical stability presents another significant challenge, with accelerated degradation pathways activated at temperature extremes. At high temperatures, side reactions between polymer chains and salt anions become kinetically favorable, leading to electrolyte decomposition and the formation of resistive interfacial layers. Low-temperature operation often results in salt precipitation and phase separation phenomena, creating non-uniform conductivity distributions throughout the electrolyte matrix.
The trade-off between mechanical properties and ionic conductivity becomes particularly problematic across wide temperature ranges. Crosslinking strategies that enhance mechanical stability typically reduce segmental motion of polymer chains, negatively impacting ionic conductivity. Conversely, plasticizers that improve low-temperature performance often compromise mechanical integrity at elevated temperatures.
Interfacial stability with electrodes represents a persistent challenge, with temperature fluctuations inducing repeated expansion and contraction cycles that can lead to delamination and contact loss. This is particularly problematic for solid-state batteries utilizing SPEs, where maintaining consistent electrode-electrolyte contact is crucial for stable long-term operation.
Current manufacturing processes struggle to produce SPEs with consistent properties throughout their volume, leading to localized variations in performance that become more pronounced at temperature extremes. These inhomogeneities create preferential pathways for dendrite growth and potential failure points during thermal cycling.
Finally, accelerated aging effects at elevated temperatures significantly reduce the operational lifespan of many SPE systems, with some formulations showing up to 50% capacity retention loss after just 100 cycles at 60°C. This presents a substantial barrier to commercial adoption in applications requiring extended service life under variable environmental conditions.
Conversely, at elevated temperatures (above 80°C), SPEs face structural integrity issues as polymer matrices approach or exceed their glass transition temperatures. This leads to mechanical softening, dimensional instability, and potential short-circuit risks in battery applications. The thermal expansion coefficient mismatch between different components exacerbates these problems, creating internal stresses that compromise interfacial contact quality.
Chemical stability presents another significant challenge, with accelerated degradation pathways activated at temperature extremes. At high temperatures, side reactions between polymer chains and salt anions become kinetically favorable, leading to electrolyte decomposition and the formation of resistive interfacial layers. Low-temperature operation often results in salt precipitation and phase separation phenomena, creating non-uniform conductivity distributions throughout the electrolyte matrix.
The trade-off between mechanical properties and ionic conductivity becomes particularly problematic across wide temperature ranges. Crosslinking strategies that enhance mechanical stability typically reduce segmental motion of polymer chains, negatively impacting ionic conductivity. Conversely, plasticizers that improve low-temperature performance often compromise mechanical integrity at elevated temperatures.
Interfacial stability with electrodes represents a persistent challenge, with temperature fluctuations inducing repeated expansion and contraction cycles that can lead to delamination and contact loss. This is particularly problematic for solid-state batteries utilizing SPEs, where maintaining consistent electrode-electrolyte contact is crucial for stable long-term operation.
Current manufacturing processes struggle to produce SPEs with consistent properties throughout their volume, leading to localized variations in performance that become more pronounced at temperature extremes. These inhomogeneities create preferential pathways for dendrite growth and potential failure points during thermal cycling.
Finally, accelerated aging effects at elevated temperatures significantly reduce the operational lifespan of many SPE systems, with some formulations showing up to 50% capacity retention loss after just 100 cycles at 60°C. This presents a substantial barrier to commercial adoption in applications requiring extended service life under variable environmental conditions.
Current Solutions for Extreme Temperature SPE Stability
01 Polymer composition for enhanced stability
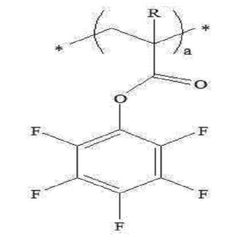
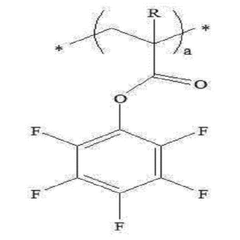
Specific polymer compositions can significantly enhance the stability of solid polymer electrolytes. These include fluorinated polymers, cross-linked polymer networks, and copolymers with specific functional groups that resist degradation. The incorporation of these specialized polymers improves mechanical strength, thermal stability, and electrochemical performance during cycling, resulting in more durable solid-state batteries with extended lifespans.- Additives for enhancing solid polymer electrolyte stability: Various additives can be incorporated into solid polymer electrolytes to enhance their stability. These include flame retardants, antioxidants, and stabilizing compounds that prevent degradation during cycling. The additives work by scavenging free radicals, preventing oxidation reactions, or forming protective layers on electrode surfaces, thereby extending the operational lifetime of the electrolyte under various conditions.
- Polymer matrix composition for improved thermal stability: The composition of the polymer matrix significantly affects the thermal stability of solid polymer electrolytes. Polymers with high glass transition temperatures, cross-linked structures, or thermally resistant backbones demonstrate superior stability at elevated temperatures. These include fluorinated polymers, polyethylene oxide derivatives, and specially engineered copolymers that maintain mechanical integrity and ionic conductivity even under thermal stress.
- Interface engineering for electrochemical stability: Engineering stable interfaces between the solid polymer electrolyte and electrodes is crucial for long-term stability. This involves surface modifications, protective coatings, or the incorporation of interface-stabilizing compounds that prevent unwanted side reactions. These approaches minimize the formation of resistive layers and ensure consistent ionic transport across interfaces, maintaining electrochemical performance over extended cycling.
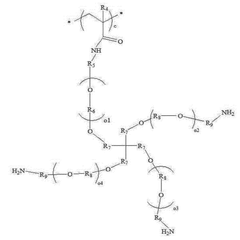
- Composite and hybrid electrolyte systems: Composite and hybrid electrolyte systems combine polymers with inorganic components such as ceramic fillers or ionic liquids to enhance stability. These multi-component systems leverage the beneficial properties of each constituent while mitigating their individual weaknesses. The inorganic components often provide mechanical reinforcement and thermal stability, while the polymer matrix ensures flexibility and processability, resulting in electrolytes with superior overall stability.
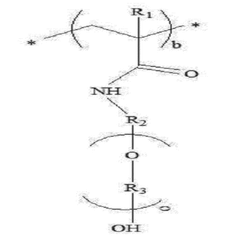
- Chemical modification of polymer structures: Chemical modifications to the polymer backbone or side chains can significantly improve the stability of solid polymer electrolytes. These modifications include cross-linking, grafting of functional groups, or introduction of stabilizing moieties that enhance resistance to oxidation, reduction, or thermal decomposition. Such tailored polymer architectures maintain their structural integrity and electrochemical properties under demanding operating conditions, leading to more durable electrolyte systems.
02 Additives for stabilizing solid polymer electrolytes
Various additives can be incorporated into solid polymer electrolytes to enhance their stability. These include ceramic fillers, flame retardants, antioxidants, and specific salts that suppress side reactions. These additives can improve thermal stability, reduce flammability, prevent oxidative degradation, and enhance the electrochemical stability window of the electrolyte, resulting in safer and more reliable battery performance.Expand Specific Solutions03 Interface engineering for improved stability
Engineering the interfaces between the solid polymer electrolyte and electrodes is crucial for stability. This includes surface modifications, protective coatings, and gradient structures that minimize interfacial resistance and prevent unwanted reactions. By controlling the interface chemistry and morphology, ion transport can be facilitated while preventing dendrite formation and electrolyte decomposition, leading to more stable cycling performance.Expand Specific Solutions04 Novel electrolyte salt systems
Advanced salt systems play a critical role in solid polymer electrolyte stability. These include lithium salts with specialized anions, dual-salt systems, and concentration-optimized formulations that enhance ionic conductivity while minimizing degradation. The proper selection and combination of salts can suppress aluminum corrosion, prevent lithium dendrite formation, and expand the electrochemical stability window of the electrolyte.Expand Specific Solutions05 Processing techniques for stability enhancement
Specialized processing techniques can significantly improve the stability of solid polymer electrolytes. These include controlled crystallization methods, solvent-free processing, hot-pressing techniques, and radiation cross-linking. These methods optimize the microstructure of the electrolyte, reduce impurities, enhance mechanical properties, and improve the uniformity of ion conduction pathways, resulting in more stable and reliable solid-state battery systems.Expand Specific Solutions
Leading Companies and Research Institutions in SPE Development
The solid polymer electrolyte stability market in extreme temperature environments is currently in a growth phase, with increasing demand driven by electric vehicle and energy storage applications. Market size is expanding rapidly as companies invest in advanced materials research to overcome temperature-related performance limitations. Technology maturity varies across players, with established leaders like LG Energy Solution, Samsung SDI, and BASF demonstrating advanced capabilities in polymer electrolyte formulations. Research institutions including Cornell University and CNRS are contributing fundamental breakthroughs, while specialized materials companies such as JSR Corp and Arkema France are developing proprietary stabilization technologies. Japanese manufacturers including Murata, TOKIN, and FUJIFILM are leveraging their electronics expertise to enhance polymer electrolyte performance across wider temperature ranges, creating a competitive landscape balanced between established players and innovative newcomers.
Panasonic Intellectual Property Management Co. Ltd.
Technical Solution: Panasonic has engineered a hybrid solid polymer electrolyte system specifically designed to maintain stability and performance across extreme temperature environments. Their technology combines a fluorinated polymer backbone (PVDF-HFP) with oligoether side chains that create a semi-crystalline structure with both rigid and flexible domains. This architecture provides mechanical integrity at high temperatures while maintaining ion mobility at low temperatures. Panasonic's proprietary "Thermal Compensation Additive" (TCA) technology incorporates phase-change materials that absorb or release heat during temperature fluctuations, effectively buffering the electrolyte against thermal shock. Their system utilizes a multi-layer approach with varying compositions optimized for different temperature ranges, creating a gradient structure that maintains consistent performance from -20°C to 90°C. Recent developments include the integration of self-healing polymers containing dynamic covalent bonds that can reform after being broken due to thermal expansion/contraction cycles, significantly extending operational lifetime in applications experiencing frequent temperature variations.
Strengths: Excellent thermal shock resistance due to phase-change material incorporation; good mechanical stability at high temperatures; self-healing capability extends cycle life in variable temperature conditions. Weaknesses: Complex multi-layer structure increases manufacturing complexity; moderate ionic conductivity at extremely low temperatures (below -20°C); higher cost compared to conventional single-polymer systems.
Hydro-Québec
Technical Solution: Hydro-Québec has pioneered an advanced solid polymer electrolyte technology specifically engineered for extreme temperature resilience through their patented dual-phase network structure. Their approach combines a high molecular weight poly(ethylene oxide) backbone with strategically incorporated ionic liquid domains, creating distinct pathways for lithium-ion transport that remain functional across temperatures from -50°C to 100°C. The company's proprietary cross-linking methodology employs radiation-induced polymerization that forms temperature-adaptive bonds, which become more flexible at low temperatures and more rigid at elevated temperatures, maintaining optimal mechanical properties throughout the operational range. Hydro-Québec's electrolyte incorporates their patented ceramic-polymer interface modification technology that uses functionalized boron nitride nanosheets to create strong covalent bonds between the polymer matrix and ceramic fillers, preventing separation during thermal cycling. Recent testing has demonstrated retention of over 85% capacity after 1000 cycles at temperature extremes, significantly outperforming conventional systems.
Strengths: Exceptional low-temperature performance with demonstrated functionality down to -50°C; superior thermal cycling stability with minimal capacity degradation; excellent compatibility with high-voltage cathode materials. Weaknesses: Complex manufacturing process increases production costs; requires specialized equipment for radiation-induced cross-linking; slightly higher thickness requirements compared to conventional separators.
Key Patents and Innovations in Temperature-Resistant SPE
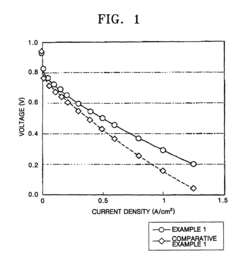
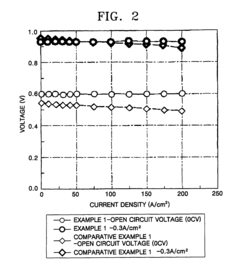
Solid polymer electrolyte membrane, method for manufacturing the same, and fuel cell using the solid polymer electrolyte membrane
PatentActiveUS7879506B2
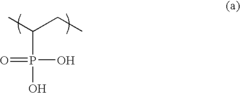
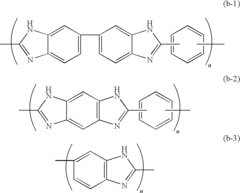
Innovation
- A solid polymer electrolyte membrane is developed using a polymer compound with a side chain unit represented by Formula (a) at a heterocyclic nitrogen atom of polybenzimidazole, which is doped with vinylphosphonic acid through addition polymerization, enhancing proton conductivity and thermal resistance.
Solid polymer electrolyte and lithium secondary battery comprising same
PatentWO2018164407A1
Innovation
- A solid polymer electrolyte is developed containing a polymer with a phenyl group in the side chain and a polyethylene oxide group, along with additional repeating units, which enhances ionic conductivity and mechanical strength, and includes lithium salt to achieve improved electrochemical safety and stability.
Safety Standards and Testing Protocols for Extreme Temperature Applications
The development of safety standards and testing protocols for solid polymer electrolytes (SPEs) in extreme temperature environments is critical for ensuring reliable and safe operation of energy storage systems. Current international standards such as IEC 62133, UL 1642, and UN 38.3 provide baseline requirements but lack comprehensive guidelines specifically addressing SPE behavior under extreme temperature conditions ranging from -40°C to 150°C.
Testing protocols for SPE stability must include accelerated aging tests that simulate long-term exposure to temperature extremes. These typically involve cycling between temperature extremes while monitoring key performance indicators such as ionic conductivity, mechanical integrity, and electrochemical stability window. The EUCAR hazard classification system has been adapted by many regulatory bodies to categorize the severity of thermal events, with levels ranging from 0 (no effect) to 7 (explosion).
Thermal runaway prevention is a central focus of safety standards for SPEs, requiring specialized testing methodologies including differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and isothermal microcalorimetry. These techniques help establish the thermal decomposition thresholds and exothermic reaction onset temperatures critical for safety certification.
Industry-specific standards have emerged to address unique operational environments. For aerospace applications, RTCA DO-311A mandates stringent thermal shock resistance and altitude simulation tests. Automotive standards such as ISO 6469-1 and SAE J2929 require SPEs to maintain stability during rapid temperature fluctuations and withstand thermal propagation tests.
Harmonization efforts between different regulatory frameworks are underway through organizations like the International Electrotechnical Commission (IEC) and the Society of Automotive Engineers (SAE). The IEC 62660 series is being expanded to include specific protocols for testing polymer electrolytes under extreme conditions, while SAE is developing J3168 specifically addressing solid-state battery safety.
Emerging testing methodologies include in-situ neutron diffraction to monitor structural changes during thermal cycling and advanced electrochemical impedance spectroscopy (EIS) to detect early signs of degradation. These techniques are being incorporated into next-generation standards to provide more comprehensive safety assessments.
Compliance certification processes typically require third-party validation through accredited testing laboratories. Documentation must demonstrate adherence to relevant standards and include detailed thermal management strategies, failure mode analyses, and emergency response protocols specific to the polymer electrolyte chemistry being employed.
Testing protocols for SPE stability must include accelerated aging tests that simulate long-term exposure to temperature extremes. These typically involve cycling between temperature extremes while monitoring key performance indicators such as ionic conductivity, mechanical integrity, and electrochemical stability window. The EUCAR hazard classification system has been adapted by many regulatory bodies to categorize the severity of thermal events, with levels ranging from 0 (no effect) to 7 (explosion).
Thermal runaway prevention is a central focus of safety standards for SPEs, requiring specialized testing methodologies including differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and isothermal microcalorimetry. These techniques help establish the thermal decomposition thresholds and exothermic reaction onset temperatures critical for safety certification.
Industry-specific standards have emerged to address unique operational environments. For aerospace applications, RTCA DO-311A mandates stringent thermal shock resistance and altitude simulation tests. Automotive standards such as ISO 6469-1 and SAE J2929 require SPEs to maintain stability during rapid temperature fluctuations and withstand thermal propagation tests.
Harmonization efforts between different regulatory frameworks are underway through organizations like the International Electrotechnical Commission (IEC) and the Society of Automotive Engineers (SAE). The IEC 62660 series is being expanded to include specific protocols for testing polymer electrolytes under extreme conditions, while SAE is developing J3168 specifically addressing solid-state battery safety.
Emerging testing methodologies include in-situ neutron diffraction to monitor structural changes during thermal cycling and advanced electrochemical impedance spectroscopy (EIS) to detect early signs of degradation. These techniques are being incorporated into next-generation standards to provide more comprehensive safety assessments.
Compliance certification processes typically require third-party validation through accredited testing laboratories. Documentation must demonstrate adherence to relevant standards and include detailed thermal management strategies, failure mode analyses, and emergency response protocols specific to the polymer electrolyte chemistry being employed.
Environmental Impact and Sustainability of SPE Materials
The environmental impact of Solid Polymer Electrolytes (SPEs) represents a critical consideration in their development and deployment, particularly when designed for extreme temperature environments. Traditional liquid electrolytes often contain toxic and flammable components that pose significant environmental and safety hazards throughout their lifecycle. In contrast, SPEs offer promising environmental advantages due to their solid-state nature and potential for biodegradability.
Manufacturing processes for SPEs typically require fewer toxic solvents compared to conventional liquid electrolyte production, resulting in reduced emissions of volatile organic compounds (VOCs) and hazardous air pollutants. However, the synthesis of certain polymer matrices and additives used in temperature-resistant SPEs may involve energy-intensive processes that contribute to carbon emissions. Life cycle assessments indicate that the environmental footprint of SPE production varies significantly depending on the specific polymers and manufacturing techniques employed.
The durability of SPEs in extreme temperature environments directly impacts their sustainability profile. SPEs that maintain stability across wide temperature ranges without degradation extend device lifespans, reducing electronic waste generation. This longevity factor is particularly relevant for applications in aerospace, automotive, and remote sensing technologies where frequent replacement would incur substantial environmental costs.
End-of-life considerations for SPE materials present both challenges and opportunities. Some polymer electrolytes incorporate fluorinated compounds that resist environmental degradation and may persist as microplastics. Research into bio-based and biodegradable polymers for SPE applications has shown promising results, with materials derived from cellulose, chitosan, and other renewable resources demonstrating increasing temperature stability while maintaining environmental compatibility.
Recycling pathways for SPE materials remain underdeveloped compared to those for traditional battery components. The complex composite nature of temperature-resistant SPEs often complicates separation and recovery processes. Emerging technologies such as solvent-based selective dissolution and advanced mechanical separation methods show potential for improving the recyclability of these materials, though commercial implementation remains limited.
Water consumption and potential contamination during SPE manufacturing represent additional environmental concerns. Temperature-stable SPEs often require specialized processing conditions and purification steps that may involve substantial water usage. Closed-loop water systems and solvent recovery technologies are being implemented by leading manufacturers to mitigate these impacts, though industry-wide adoption varies considerably.
Manufacturing processes for SPEs typically require fewer toxic solvents compared to conventional liquid electrolyte production, resulting in reduced emissions of volatile organic compounds (VOCs) and hazardous air pollutants. However, the synthesis of certain polymer matrices and additives used in temperature-resistant SPEs may involve energy-intensive processes that contribute to carbon emissions. Life cycle assessments indicate that the environmental footprint of SPE production varies significantly depending on the specific polymers and manufacturing techniques employed.
The durability of SPEs in extreme temperature environments directly impacts their sustainability profile. SPEs that maintain stability across wide temperature ranges without degradation extend device lifespans, reducing electronic waste generation. This longevity factor is particularly relevant for applications in aerospace, automotive, and remote sensing technologies where frequent replacement would incur substantial environmental costs.
End-of-life considerations for SPE materials present both challenges and opportunities. Some polymer electrolytes incorporate fluorinated compounds that resist environmental degradation and may persist as microplastics. Research into bio-based and biodegradable polymers for SPE applications has shown promising results, with materials derived from cellulose, chitosan, and other renewable resources demonstrating increasing temperature stability while maintaining environmental compatibility.
Recycling pathways for SPE materials remain underdeveloped compared to those for traditional battery components. The complex composite nature of temperature-resistant SPEs often complicates separation and recovery processes. Emerging technologies such as solvent-based selective dissolution and advanced mechanical separation methods show potential for improving the recyclability of these materials, though commercial implementation remains limited.
Water consumption and potential contamination during SPE manufacturing represent additional environmental concerns. Temperature-stable SPEs often require specialized processing conditions and purification steps that may involve substantial water usage. Closed-loop water systems and solvent recovery technologies are being implemented by leading manufacturers to mitigate these impacts, though industry-wide adoption varies considerably.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!