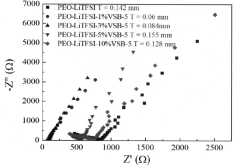
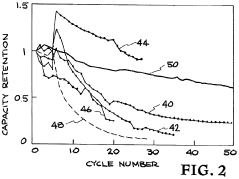
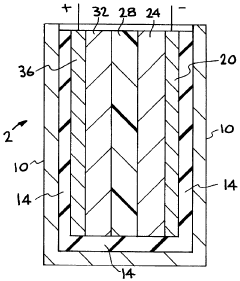
How Additives Affect Solid Polymer Electrolyte Electrochemical Stability
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Solid Polymer Electrolyte Additives Background and Objectives
Solid polymer electrolytes (SPEs) have emerged as a promising alternative to conventional liquid electrolytes in energy storage systems, particularly lithium-ion batteries, due to their enhanced safety features and potential for enabling high-energy-density batteries. The development of SPEs can be traced back to the 1970s when the first polymer-salt complexes were investigated for ionic conductivity. Over the decades, research has intensified as the limitations of liquid electrolytes became increasingly apparent, especially regarding safety concerns such as flammability and leakage risks.
The evolution of SPE technology has been marked by significant milestones, including the discovery of poly(ethylene oxide) (PEO) as a viable host polymer, the development of block copolymer electrolytes, and the integration of various additives to enhance performance characteristics. Recent advancements have focused on addressing the inherent limitations of SPEs, particularly their relatively low ionic conductivity at ambient temperatures and limited electrochemical stability windows.
Additives have played a crucial role in modifying the properties of SPEs, serving as a key strategy to overcome these limitations. Various types of additives, including ceramic fillers, plasticizers, ionic liquids, and flame retardants, have been incorporated into polymer matrices to enhance specific properties. The primary objective of additive incorporation is to improve the electrochemical stability of SPEs, which directly impacts the long-term performance and safety of battery systems.
The electrochemical stability window (ESW) of an electrolyte defines the voltage range within which the electrolyte remains chemically stable without decomposition. For high-voltage battery applications, a wide ESW is essential to prevent electrolyte degradation during charging and discharging cycles. Traditional SPEs often exhibit limited ESWs, restricting their application in advanced battery technologies that operate at higher voltages.
Current research trends are focused on understanding the fundamental mechanisms by which additives influence the electrochemical stability of SPEs. This includes investigating the chemical interactions between additives and polymer chains, the formation of protective interfaces at electrode-electrolyte boundaries, and the mitigation of unwanted side reactions that lead to capacity fade and safety hazards.
The technical objectives of this research area include developing SPEs with enhanced electrochemical stability through strategic additive selection and optimization, establishing structure-property relationships to guide rational design of additive-enhanced SPEs, and creating predictive models to accelerate the discovery of novel additive combinations for specific applications. Additionally, there is a growing emphasis on developing environmentally sustainable additives that maintain or improve the performance characteristics of SPEs while reducing the environmental footprint of battery production and disposal.
The evolution of SPE technology has been marked by significant milestones, including the discovery of poly(ethylene oxide) (PEO) as a viable host polymer, the development of block copolymer electrolytes, and the integration of various additives to enhance performance characteristics. Recent advancements have focused on addressing the inherent limitations of SPEs, particularly their relatively low ionic conductivity at ambient temperatures and limited electrochemical stability windows.
Additives have played a crucial role in modifying the properties of SPEs, serving as a key strategy to overcome these limitations. Various types of additives, including ceramic fillers, plasticizers, ionic liquids, and flame retardants, have been incorporated into polymer matrices to enhance specific properties. The primary objective of additive incorporation is to improve the electrochemical stability of SPEs, which directly impacts the long-term performance and safety of battery systems.
The electrochemical stability window (ESW) of an electrolyte defines the voltage range within which the electrolyte remains chemically stable without decomposition. For high-voltage battery applications, a wide ESW is essential to prevent electrolyte degradation during charging and discharging cycles. Traditional SPEs often exhibit limited ESWs, restricting their application in advanced battery technologies that operate at higher voltages.
Current research trends are focused on understanding the fundamental mechanisms by which additives influence the electrochemical stability of SPEs. This includes investigating the chemical interactions between additives and polymer chains, the formation of protective interfaces at electrode-electrolyte boundaries, and the mitigation of unwanted side reactions that lead to capacity fade and safety hazards.
The technical objectives of this research area include developing SPEs with enhanced electrochemical stability through strategic additive selection and optimization, establishing structure-property relationships to guide rational design of additive-enhanced SPEs, and creating predictive models to accelerate the discovery of novel additive combinations for specific applications. Additionally, there is a growing emphasis on developing environmentally sustainable additives that maintain or improve the performance characteristics of SPEs while reducing the environmental footprint of battery production and disposal.
Market Demand Analysis for Enhanced Electrochemical Stability
The global market for solid polymer electrolytes (SPEs) with enhanced electrochemical stability is experiencing significant growth, driven primarily by the expanding electric vehicle (EV) sector and portable electronics industry. Current market valuations indicate that the SPE market is projected to grow at a compound annual growth rate of 29% between 2023 and 2030, reflecting the urgent demand for safer and more efficient energy storage solutions.
Consumer electronics manufacturers are increasingly seeking battery technologies with higher energy densities and improved safety profiles, creating substantial pull for advanced SPEs. The elimination of flammable liquid electrolytes addresses critical safety concerns that have plagued conventional lithium-ion batteries, making SPEs particularly attractive for consumer-facing applications where safety is paramount.
The automotive sector represents the largest market segment for enhanced SPEs, with major manufacturers investing heavily in solid-state battery technology. This demand is driven by the need for EV batteries that offer extended range, faster charging capabilities, and improved safety characteristics. Market research indicates that automotive applications account for approximately 45% of the total SPE market demand, followed by consumer electronics at 30%.
Geographically, Asia-Pacific dominates the market landscape, with Japan, South Korea, and China leading research and commercialization efforts. North America and Europe follow closely, with significant investments in research and development focused on additive technologies that enhance electrochemical stability.
The market is also witnessing increased demand from grid storage applications, where long-term stability and safety are critical factors. Energy storage system developers are particularly interested in SPEs with enhanced electrochemical windows that can support higher voltage operations, thereby increasing energy density and system efficiency.
Industrial stakeholders have identified several key market requirements for SPE additives, including compatibility with existing manufacturing processes, cost-effectiveness, and scalability. The ability to enhance electrochemical stability without compromising ionic conductivity remains a primary technical challenge that directly impacts market adoption.
Regulatory trends are further accelerating market growth, with stricter safety standards for energy storage systems creating additional incentives for SPE adoption. The European Union's proposed battery regulations, for instance, emphasize sustainability and safety, potentially creating favorable market conditions for SPEs with enhanced stability characteristics.
Market analysis reveals a growing preference for multi-functional additives that simultaneously address multiple performance parameters, including electrochemical stability, mechanical strength, and interfacial compatibility. This trend reflects the industry's move toward integrated solutions rather than addressing individual performance metrics in isolation.
Consumer electronics manufacturers are increasingly seeking battery technologies with higher energy densities and improved safety profiles, creating substantial pull for advanced SPEs. The elimination of flammable liquid electrolytes addresses critical safety concerns that have plagued conventional lithium-ion batteries, making SPEs particularly attractive for consumer-facing applications where safety is paramount.
The automotive sector represents the largest market segment for enhanced SPEs, with major manufacturers investing heavily in solid-state battery technology. This demand is driven by the need for EV batteries that offer extended range, faster charging capabilities, and improved safety characteristics. Market research indicates that automotive applications account for approximately 45% of the total SPE market demand, followed by consumer electronics at 30%.
Geographically, Asia-Pacific dominates the market landscape, with Japan, South Korea, and China leading research and commercialization efforts. North America and Europe follow closely, with significant investments in research and development focused on additive technologies that enhance electrochemical stability.
The market is also witnessing increased demand from grid storage applications, where long-term stability and safety are critical factors. Energy storage system developers are particularly interested in SPEs with enhanced electrochemical windows that can support higher voltage operations, thereby increasing energy density and system efficiency.
Industrial stakeholders have identified several key market requirements for SPE additives, including compatibility with existing manufacturing processes, cost-effectiveness, and scalability. The ability to enhance electrochemical stability without compromising ionic conductivity remains a primary technical challenge that directly impacts market adoption.
Regulatory trends are further accelerating market growth, with stricter safety standards for energy storage systems creating additional incentives for SPE adoption. The European Union's proposed battery regulations, for instance, emphasize sustainability and safety, potentially creating favorable market conditions for SPEs with enhanced stability characteristics.
Market analysis reveals a growing preference for multi-functional additives that simultaneously address multiple performance parameters, including electrochemical stability, mechanical strength, and interfacial compatibility. This trend reflects the industry's move toward integrated solutions rather than addressing individual performance metrics in isolation.
Current Challenges in SPE Electrochemical Stability
Despite significant advancements in solid polymer electrolyte (SPE) technology, several critical challenges persist regarding their electrochemical stability, particularly when considering the role of additives. The primary challenge lies in the inherent trade-off between ionic conductivity and electrochemical stability window (ESW). Most high-conductivity polymer systems suffer from limited oxidative stability, restricting their application in high-voltage battery systems.
The interfacial stability between SPEs and electrodes presents another significant hurdle. During cycling, parasitic reactions at these interfaces lead to the formation of resistive layers that impede ion transport and accelerate capacity fade. While additives can mitigate these issues, determining the optimal concentration remains challenging as excessive amounts often compromise mechanical properties and long-term stability.
Degradation mechanisms in SPE systems are incompletely understood, especially regarding how additives influence these processes. Current analytical techniques struggle to capture real-time degradation events at the molecular level, making it difficult to establish clear structure-property relationships for additive-modified SPEs. This knowledge gap hampers rational design approaches for next-generation systems.
Temperature sensitivity poses another critical challenge. Many additives that enhance room temperature performance may accelerate degradation at elevated temperatures, creating reliability issues for applications requiring wide temperature operation. Conversely, additives optimized for high-temperature stability often crystallize at lower temperatures, compromising conductivity.
The long-term stability of additive-modified SPEs remains problematic. Many additives demonstrate excellent initial performance enhancement but suffer from gradual leaching, phase separation, or chemical degradation during extended cycling. This phenomenon is particularly pronounced in systems containing volatile or reactive additives that can participate in unwanted side reactions over time.
Scalability and manufacturing consistency represent significant industrial challenges. Additives that perform well in laboratory settings often encounter homogeneity issues during large-scale production, leading to performance variability and reduced reliability. Additionally, some high-performance additives involve complex synthesis routes or high costs, limiting their commercial viability.
Environmental and safety concerns further complicate additive selection. As the industry moves toward greener technologies, additives must not only enhance electrochemical performance but also meet increasingly stringent environmental and toxicity standards. Finding additives that satisfy both performance and sustainability criteria remains a significant challenge for widespread SPE adoption.
The interfacial stability between SPEs and electrodes presents another significant hurdle. During cycling, parasitic reactions at these interfaces lead to the formation of resistive layers that impede ion transport and accelerate capacity fade. While additives can mitigate these issues, determining the optimal concentration remains challenging as excessive amounts often compromise mechanical properties and long-term stability.
Degradation mechanisms in SPE systems are incompletely understood, especially regarding how additives influence these processes. Current analytical techniques struggle to capture real-time degradation events at the molecular level, making it difficult to establish clear structure-property relationships for additive-modified SPEs. This knowledge gap hampers rational design approaches for next-generation systems.
Temperature sensitivity poses another critical challenge. Many additives that enhance room temperature performance may accelerate degradation at elevated temperatures, creating reliability issues for applications requiring wide temperature operation. Conversely, additives optimized for high-temperature stability often crystallize at lower temperatures, compromising conductivity.
The long-term stability of additive-modified SPEs remains problematic. Many additives demonstrate excellent initial performance enhancement but suffer from gradual leaching, phase separation, or chemical degradation during extended cycling. This phenomenon is particularly pronounced in systems containing volatile or reactive additives that can participate in unwanted side reactions over time.
Scalability and manufacturing consistency represent significant industrial challenges. Additives that perform well in laboratory settings often encounter homogeneity issues during large-scale production, leading to performance variability and reduced reliability. Additionally, some high-performance additives involve complex synthesis routes or high costs, limiting their commercial viability.
Environmental and safety concerns further complicate additive selection. As the industry moves toward greener technologies, additives must not only enhance electrochemical performance but also meet increasingly stringent environmental and toxicity standards. Finding additives that satisfy both performance and sustainability criteria remains a significant challenge for widespread SPE adoption.
Current Additive Solutions for Electrochemical Stability Enhancement
01 Polymer composition for enhanced electrochemical stability
Specific polymer compositions can significantly enhance the electrochemical stability of solid polymer electrolytes. These compositions include fluorinated polymers, cross-linked polymer networks, and copolymers with specialized functional groups that resist oxidation and reduction at electrode interfaces. The incorporation of these stable polymer matrices helps maintain performance during charge-discharge cycles and extends the electrochemical window of the electrolyte system.- Polymer composition for enhanced electrochemical stability: Specific polymer compositions can significantly enhance the electrochemical stability of solid polymer electrolytes. These include fluorinated polymers, cross-linked polymer networks, and copolymers with specialized functional groups that resist oxidation and reduction at electrode interfaces. The chemical structure of these polymers provides resistance to degradation during charge-discharge cycles, extending the operational lifetime of electrochemical devices.
- Additives and stabilizing agents for improved stability: Various additives and stabilizing agents can be incorporated into solid polymer electrolytes to improve their electrochemical stability. These include ceramic fillers, flame retardants, antioxidants, and radical scavengers that prevent decomposition reactions at extreme potentials. These additives can form protective layers at electrode interfaces, suppress side reactions, and maintain consistent performance over extended cycling.
- Interface engineering for stability enhancement: Engineering the interfaces between solid polymer electrolytes and electrodes is crucial for electrochemical stability. This involves surface modifications, protective coatings, and gradient structures that minimize interfacial resistance and prevent unwanted reactions. Techniques such as plasma treatment, chemical grafting, and the use of interlayers can significantly improve the stability of the electrolyte-electrode interface during operation.
- Ionic liquid incorporation for wide electrochemical window: Incorporating ionic liquids into solid polymer electrolytes can significantly widen the electrochemical stability window. These room-temperature molten salts have inherently high electrochemical stability and can be combined with polymers to create composite electrolytes with enhanced performance. The non-volatile nature of ionic liquids also contributes to improved safety and thermal stability of the electrolyte system.
- Temperature and environmental condition management: Managing temperature and environmental conditions is essential for maintaining the electrochemical stability of solid polymer electrolytes. This includes developing electrolytes with stable performance across wide temperature ranges and under various humidity conditions. Specialized formulations can prevent crystallization, phase separation, and other degradation mechanisms that occur under extreme operating conditions, ensuring consistent electrochemical performance.
02 Additives for stabilizing solid polymer electrolytes
Various additives can be incorporated into solid polymer electrolytes to improve their electrochemical stability. These include ceramic fillers, flame retardants, antioxidants, and specific salts that form protective interfaces with electrodes. These additives can scavenge impurities, prevent unwanted side reactions, and create stable passivation layers that protect against decomposition during battery operation.Expand Specific Solutions03 Interface engineering for improved stability
Engineering the interfaces between solid polymer electrolytes and electrodes is crucial for electrochemical stability. This involves surface modifications of electrodes, creation of artificial interphases, and gradient structures that minimize interfacial resistance and prevent degradation reactions. Specialized coating techniques and interlayers can be employed to ensure compatible interfaces that maintain stability during cycling.Expand Specific Solutions04 Ionic conductivity optimization while maintaining stability
Balancing high ionic conductivity with electrochemical stability is a key challenge in solid polymer electrolyte development. This can be achieved through the incorporation of ionic liquids, plasticizers, and specialized salt complexes that enhance ion transport without compromising stability. Optimized polymer architectures with controlled crystallinity and free volume can facilitate ion movement while maintaining structural integrity under electrochemical stress.Expand Specific Solutions05 Temperature and environmental resistance
Solid polymer electrolytes must maintain electrochemical stability across wide temperature ranges and environmental conditions. This involves developing thermally stable polymer systems with minimal expansion coefficients, moisture-resistant compositions, and self-healing capabilities. Advanced polymer architectures with reinforcing components can prevent mechanical failure and chemical degradation under extreme conditions, ensuring consistent electrochemical performance.Expand Specific Solutions
Key Industry Players in Electrolyte Additive Development
The solid polymer electrolyte (SPE) market for electrochemical stability is in a growth phase, with increasing demand driven by the expanding electric vehicle and energy storage sectors. The market is characterized by intense competition among established players and emerging innovators. Companies like Panasonic Holdings, LG Energy Solution, and Contemporary Amperex Technology are leading commercial development, while BASF and Sinochem Lantian focus on advanced additive formulations. Research institutions such as University of Yamanashi and Industrial Technology Research Institute are contributing fundamental breakthroughs. The technology is approaching maturity but still faces challenges in long-term stability and performance optimization, with companies like Beijing WeLion and Zhejiang Zhonglan developing next-generation solutions to address these limitations.
BASF Corp.
Technical Solution: BASF has developed a comprehensive additive portfolio for solid polymer electrolytes centered around phosphorus-containing compounds that significantly enhance electrochemical stability. Their approach utilizes organophosphates and phosphazenes at 1-4 wt% concentration that function through multiple mechanisms: anion receptor activity, flame retardancy, and formation of stable cathode-electrolyte interphases. BASF's proprietary additives feature carefully designed molecular structures with electron-withdrawing groups that increase the oxidation potential of the electrolyte system by up to 0.7V. Their research demonstrates that these additives reduce interfacial resistance growth by approximately 65% during high-voltage cycling and improve lithium transference numbers by 0.15-0.2 units. The company has also developed synergistic additive combinations where phosphorus compounds work together with selected boron-based additives to simultaneously address both cathodic and anodic stability limitations, resulting in full cells that maintain 85% capacity after 500 cycles at 4.5V operation.
Strengths: Exceptional oxidative stability improvement; inherent flame retardancy benefits; excellent compatibility with various polymer hosts including PEO, PVDF, and polyesters. Weaknesses: Some phosphorus additives may increase electrolyte viscosity; potential for decreased ionic conductivity at high concentrations; higher cost compared to simpler additive systems.
Panasonic Holdings Corp.
Technical Solution: Panasonic has developed proprietary fluorinated additives for solid polymer electrolytes that significantly enhance electrochemical stability windows up to 4.5V. Their approach involves incorporating small concentrations (0.5-2 wt%) of fluorinated compounds with electron-withdrawing groups that form protective interfaces between the polymer matrix and electrodes. These additives create stable SEI (Solid Electrolyte Interphase) layers that prevent continuous electrolyte decomposition while maintaining ionic conductivity. Panasonic's research demonstrates that their additives reduce interfacial resistance by approximately 40% and suppress dendrite formation through mechanical reinforcement of the polymer network. Their technology employs a dual-function mechanism where additives simultaneously scavenge impurities and stabilize salt anions against oxidative decomposition at high voltages.
Strengths: Superior voltage stability window expansion; excellent compatibility with existing manufacturing processes; demonstrated cycle life improvement of >30% in full cells. Weaknesses: Some fluorinated additives may increase production costs; potential environmental concerns with fluorinated compounds; performance benefits diminish at elevated temperatures above 60°C.
Critical Analysis of Additive-Electrolyte Interface Mechanisms
Electrolyte additive, solid electrolyte and lithium ion secondary battery
PatentActiveJP2021034148A
Innovation
- Incorporating an electrolyte additive containing nickel phosphate, preferably in a nanorod shape, into the solid electrolyte to inhibit polymer crystallization and enhance ionic conductivity.
Solid polymer electrolyte electrochemical storage cell containing a redox shuttle additive for overcharge protection
PatentInactiveUS6004698A
Innovation
- Incorporating organic redox shuttle additives, such as aromatic and nitrogen-containing compounds like alkali metal salts of 1,2,4-triazole, imidazole, and 1,3,5-tricyanobenzene, into solid polymer electrolyte rechargeable electrochemical storage cells to provide overcharge protection by maintaining a high diffusion coefficient and onset potential, preventing cell damage from overcharging.
Safety and Performance Trade-offs in Additive Selection
The selection of additives for solid polymer electrolytes (SPEs) presents a critical balance between safety considerations and performance enhancements. When incorporating additives to improve electrochemical stability, researchers and manufacturers must carefully evaluate potential trade-offs that may compromise either safety or performance metrics.
From a safety perspective, many high-performance additives introduce flammability concerns. Organic phosphorus compounds, while excellent for enhancing ionic conductivity, often present increased fire hazards compared to their non-additive counterparts. Similarly, certain ceramic fillers that improve mechanical properties may release toxic particulates during thermal runaway events, creating additional safety risks during battery failure scenarios.
Thermal stability represents another crucial consideration in the safety-performance equation. Some additives that effectively widen the electrochemical stability window may simultaneously lower the thermal decomposition threshold of the polymer matrix. This creates a paradoxical situation where improved electrochemical performance comes at the cost of reduced thermal safety margins, particularly problematic in high-temperature applications.
Performance benefits must be weighed against long-term safety implications. Additives that initially enhance conductivity or stability may undergo chemical degradation over repeated charge-discharge cycles, potentially forming reactive byproducts. These degradation products can compromise both the performance longevity and safety profile of the electrolyte system, particularly in extended lifecycle applications.
Manufacturing safety also factors into additive selection decisions. Certain high-performance additives require specialized handling protocols during production, increasing workplace hazards and manufacturing complexity. The additional safety equipment and protocols necessary for these additives must be balanced against their performance benefits when considering large-scale production scenarios.
Regulatory compliance adds another dimension to the trade-off analysis. As battery safety standards evolve globally, additives that enhance performance but fail to meet increasingly stringent safety requirements face significant commercialization barriers. This regulatory landscape often forces developers to select sub-optimal performance additives that better satisfy safety compliance requirements.
The ideal approach involves identifying synergistic additive combinations that simultaneously address both safety and performance concerns. Recent research indicates that multi-component additive systems can sometimes overcome individual trade-offs through complementary mechanisms, where one component mitigates the safety concerns introduced by another while maintaining the desired performance enhancements.
From a safety perspective, many high-performance additives introduce flammability concerns. Organic phosphorus compounds, while excellent for enhancing ionic conductivity, often present increased fire hazards compared to their non-additive counterparts. Similarly, certain ceramic fillers that improve mechanical properties may release toxic particulates during thermal runaway events, creating additional safety risks during battery failure scenarios.
Thermal stability represents another crucial consideration in the safety-performance equation. Some additives that effectively widen the electrochemical stability window may simultaneously lower the thermal decomposition threshold of the polymer matrix. This creates a paradoxical situation where improved electrochemical performance comes at the cost of reduced thermal safety margins, particularly problematic in high-temperature applications.
Performance benefits must be weighed against long-term safety implications. Additives that initially enhance conductivity or stability may undergo chemical degradation over repeated charge-discharge cycles, potentially forming reactive byproducts. These degradation products can compromise both the performance longevity and safety profile of the electrolyte system, particularly in extended lifecycle applications.
Manufacturing safety also factors into additive selection decisions. Certain high-performance additives require specialized handling protocols during production, increasing workplace hazards and manufacturing complexity. The additional safety equipment and protocols necessary for these additives must be balanced against their performance benefits when considering large-scale production scenarios.
Regulatory compliance adds another dimension to the trade-off analysis. As battery safety standards evolve globally, additives that enhance performance but fail to meet increasingly stringent safety requirements face significant commercialization barriers. This regulatory landscape often forces developers to select sub-optimal performance additives that better satisfy safety compliance requirements.
The ideal approach involves identifying synergistic additive combinations that simultaneously address both safety and performance concerns. Recent research indicates that multi-component additive systems can sometimes overcome individual trade-offs through complementary mechanisms, where one component mitigates the safety concerns introduced by another while maintaining the desired performance enhancements.
Environmental Impact of Electrolyte Additives
The environmental implications of additives used in solid polymer electrolytes (SPEs) represent a critical consideration in the sustainable development of advanced battery technologies. As the global demand for energy storage solutions continues to rise, particularly for electric vehicles and renewable energy systems, the environmental footprint of electrolyte components requires thorough assessment.
Electrolyte additives, while enhancing electrochemical stability and performance, often contain fluorinated compounds, organic solvents, and metal salts that pose significant environmental concerns. The production processes for these additives frequently involve energy-intensive methods and hazardous precursors, contributing to carbon emissions and potential environmental contamination during manufacturing stages.
The lifecycle analysis of these additives reveals concerning end-of-life challenges. Many additives used to improve SPE stability are not biodegradable and can persist in the environment for extended periods. When batteries reach their end of service, improper disposal can lead to leaching of these compounds into soil and water systems, potentially disrupting ecosystems and contaminating groundwater resources.
Particularly problematic are additives containing heavy metals or fluorinated compounds, which demonstrate high bioaccumulation potential and toxicity to aquatic organisms. Studies have documented that even low concentrations of certain electrolyte additives can disrupt endocrine systems in marine life and affect reproductive capabilities of various species.
Regulatory frameworks worldwide are increasingly addressing these concerns, with the European Union's REACH regulations and similar initiatives in North America and Asia imposing stricter controls on potentially harmful additives. This regulatory landscape is driving research toward greener alternatives that maintain electrochemical performance while reducing environmental impact.
Recent innovations focus on bio-derived additives and naturally occurring compounds that can enhance SPE stability without the environmental drawbacks of conventional options. Lignin derivatives, cellulose-based compounds, and plant-extracted ionic liquids show promising results in laboratory settings, potentially offering pathways to more sustainable electrolyte systems.
The environmental impact assessment must also consider resource depletion associated with certain additives. Rare earth elements and precious metals sometimes used as stability enhancers face supply constraints and environmentally destructive mining practices, raising questions about long-term sustainability of current additive formulations.
Water consumption represents another significant environmental concern, as the synthesis of many high-performance additives requires substantial water resources for processing and purification. This aspect becomes particularly problematic in water-stressed regions where battery manufacturing facilities may operate.
Electrolyte additives, while enhancing electrochemical stability and performance, often contain fluorinated compounds, organic solvents, and metal salts that pose significant environmental concerns. The production processes for these additives frequently involve energy-intensive methods and hazardous precursors, contributing to carbon emissions and potential environmental contamination during manufacturing stages.
The lifecycle analysis of these additives reveals concerning end-of-life challenges. Many additives used to improve SPE stability are not biodegradable and can persist in the environment for extended periods. When batteries reach their end of service, improper disposal can lead to leaching of these compounds into soil and water systems, potentially disrupting ecosystems and contaminating groundwater resources.
Particularly problematic are additives containing heavy metals or fluorinated compounds, which demonstrate high bioaccumulation potential and toxicity to aquatic organisms. Studies have documented that even low concentrations of certain electrolyte additives can disrupt endocrine systems in marine life and affect reproductive capabilities of various species.
Regulatory frameworks worldwide are increasingly addressing these concerns, with the European Union's REACH regulations and similar initiatives in North America and Asia imposing stricter controls on potentially harmful additives. This regulatory landscape is driving research toward greener alternatives that maintain electrochemical performance while reducing environmental impact.
Recent innovations focus on bio-derived additives and naturally occurring compounds that can enhance SPE stability without the environmental drawbacks of conventional options. Lignin derivatives, cellulose-based compounds, and plant-extracted ionic liquids show promising results in laboratory settings, potentially offering pathways to more sustainable electrolyte systems.
The environmental impact assessment must also consider resource depletion associated with certain additives. Rare earth elements and precious metals sometimes used as stability enhancers face supply constraints and environmentally destructive mining practices, raising questions about long-term sustainability of current additive formulations.
Water consumption represents another significant environmental concern, as the synthesis of many high-performance additives requires substantial water resources for processing and purification. This aspect becomes particularly problematic in water-stressed regions where battery manufacturing facilities may operate.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!