Solid Polymer Electrolyte for Sodium-Ion Battery Applications
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SPE for Na-ion Batteries: Background and Objectives
Solid polymer electrolytes (SPEs) have emerged as a promising alternative to conventional liquid electrolytes in battery technology over the past three decades. The evolution of SPE research initially focused on lithium-ion battery applications, but recent concerns regarding lithium resource scarcity and cost have shifted attention toward sodium-ion battery systems. This technological pivot represents a strategic response to sustainability challenges in the energy storage sector, as sodium resources are approximately 1000 times more abundant than lithium in the Earth's crust and more evenly distributed geographically.
The development trajectory of SPEs for sodium-ion batteries has accelerated significantly since 2015, with research publications in this field growing exponentially. Early polymer electrolyte systems were primarily based on polyethylene oxide (PEO) matrices, which demonstrated the fundamental feasibility of sodium ion conduction in polymer hosts. However, these initial systems suffered from low ionic conductivity at ambient temperatures, typically below 10^-5 S/cm, which limited practical applications.
Technological advancements have progressively addressed these limitations through various strategies, including the incorporation of plasticizers, ceramic fillers, and the development of novel polymer architectures. The integration of computational modeling with experimental approaches has further accelerated progress by enabling rational design of polymer-sodium ion interactions at the molecular level.
The current research landscape is characterized by a multidisciplinary approach combining polymer chemistry, electrochemistry, materials science, and engineering to overcome persistent challenges. These challenges include achieving high ionic conductivity at room temperature, maintaining mechanical stability during cycling, and ensuring long-term electrochemical stability at the electrode-electrolyte interfaces.
The primary technical objectives for SPE development in sodium-ion batteries include reaching ionic conductivity values exceeding 10^-4 S/cm at ambient temperature, achieving sodium ion transference numbers above 0.5, maintaining mechanical integrity during extended cycling, and demonstrating compatibility with both anode and cathode materials across wide voltage windows.
Beyond performance metrics, additional objectives include developing environmentally benign synthesis routes, ensuring scalable manufacturing processes, and reducing production costs to enable commercial viability. These goals align with broader industry trends toward sustainable energy storage solutions that can support renewable energy integration and electrification of transportation.
The technological evolution of SPEs for sodium-ion batteries represents a critical pathway toward next-generation energy storage systems that balance performance requirements with resource sustainability considerations. Success in this field could potentially disrupt conventional battery technologies and enable new applications where cost-effectiveness and resource availability are paramount considerations.
The development trajectory of SPEs for sodium-ion batteries has accelerated significantly since 2015, with research publications in this field growing exponentially. Early polymer electrolyte systems were primarily based on polyethylene oxide (PEO) matrices, which demonstrated the fundamental feasibility of sodium ion conduction in polymer hosts. However, these initial systems suffered from low ionic conductivity at ambient temperatures, typically below 10^-5 S/cm, which limited practical applications.
Technological advancements have progressively addressed these limitations through various strategies, including the incorporation of plasticizers, ceramic fillers, and the development of novel polymer architectures. The integration of computational modeling with experimental approaches has further accelerated progress by enabling rational design of polymer-sodium ion interactions at the molecular level.
The current research landscape is characterized by a multidisciplinary approach combining polymer chemistry, electrochemistry, materials science, and engineering to overcome persistent challenges. These challenges include achieving high ionic conductivity at room temperature, maintaining mechanical stability during cycling, and ensuring long-term electrochemical stability at the electrode-electrolyte interfaces.
The primary technical objectives for SPE development in sodium-ion batteries include reaching ionic conductivity values exceeding 10^-4 S/cm at ambient temperature, achieving sodium ion transference numbers above 0.5, maintaining mechanical integrity during extended cycling, and demonstrating compatibility with both anode and cathode materials across wide voltage windows.
Beyond performance metrics, additional objectives include developing environmentally benign synthesis routes, ensuring scalable manufacturing processes, and reducing production costs to enable commercial viability. These goals align with broader industry trends toward sustainable energy storage solutions that can support renewable energy integration and electrification of transportation.
The technological evolution of SPEs for sodium-ion batteries represents a critical pathway toward next-generation energy storage systems that balance performance requirements with resource sustainability considerations. Success in this field could potentially disrupt conventional battery technologies and enable new applications where cost-effectiveness and resource availability are paramount considerations.
Market Analysis for Na-ion Battery Electrolytes
The sodium-ion battery market is experiencing significant growth as a promising alternative to lithium-ion batteries, driven by increasing concerns over lithium supply constraints and rising costs. Current market projections indicate that the global sodium-ion battery market could reach $1.2 billion by 2025, with a compound annual growth rate exceeding 25% between 2023 and 2030. This growth trajectory is supported by the abundance and even geographical distribution of sodium resources, which constitute approximately 2.6% of the Earth's crust compared to lithium's 0.006%.
The electrolyte segment represents a critical component of this market, accounting for approximately 15-20% of the total battery cost. Solid polymer electrolytes (SPEs) for sodium-ion batteries are gaining particular attention due to their enhanced safety profiles and potential for enabling higher energy density configurations. Market research indicates that SPEs could capture up to 30% of the sodium-ion battery electrolyte market by 2028.
Key market drivers for sodium-ion battery electrolytes include grid-scale energy storage applications, which are projected to constitute nearly 45% of the market demand. This sector benefits from sodium-ion batteries' favorable cost structure and safety characteristics. Additionally, electric vehicles represent an emerging application segment, particularly for urban mobility and commercial vehicles where cost considerations outweigh energy density requirements.
Regional analysis reveals that China currently dominates the sodium-ion battery market with approximately 60% market share, followed by Europe at 25% and North America at 10%. However, significant investments in research and manufacturing capacity in Europe and North America are expected to alter this distribution over the next five years.
Consumer demand patterns indicate growing preference for safer battery technologies, with 78% of surveyed industrial buyers citing safety as a primary consideration in energy storage procurement decisions. This trend strongly favors solid electrolyte technologies, including polymer-based systems for sodium-ion applications.
Market challenges include competition from established lithium-ion technologies and other emerging battery chemistries. Additionally, the relatively lower ionic conductivity of current-generation solid polymer electrolytes at room temperature remains a technical barrier affecting market adoption rates. Industry reports suggest that achieving conductivity values above 10^-4 S/cm at ambient temperature would significantly accelerate market penetration.
Pricing trends show that sodium-ion batteries with advanced electrolytes currently command a 15-20% premium over liquid electrolyte versions, though this gap is expected to narrow to less than 10% by 2026 as manufacturing scales and technologies mature.
The electrolyte segment represents a critical component of this market, accounting for approximately 15-20% of the total battery cost. Solid polymer electrolytes (SPEs) for sodium-ion batteries are gaining particular attention due to their enhanced safety profiles and potential for enabling higher energy density configurations. Market research indicates that SPEs could capture up to 30% of the sodium-ion battery electrolyte market by 2028.
Key market drivers for sodium-ion battery electrolytes include grid-scale energy storage applications, which are projected to constitute nearly 45% of the market demand. This sector benefits from sodium-ion batteries' favorable cost structure and safety characteristics. Additionally, electric vehicles represent an emerging application segment, particularly for urban mobility and commercial vehicles where cost considerations outweigh energy density requirements.
Regional analysis reveals that China currently dominates the sodium-ion battery market with approximately 60% market share, followed by Europe at 25% and North America at 10%. However, significant investments in research and manufacturing capacity in Europe and North America are expected to alter this distribution over the next five years.
Consumer demand patterns indicate growing preference for safer battery technologies, with 78% of surveyed industrial buyers citing safety as a primary consideration in energy storage procurement decisions. This trend strongly favors solid electrolyte technologies, including polymer-based systems for sodium-ion applications.
Market challenges include competition from established lithium-ion technologies and other emerging battery chemistries. Additionally, the relatively lower ionic conductivity of current-generation solid polymer electrolytes at room temperature remains a technical barrier affecting market adoption rates. Industry reports suggest that achieving conductivity values above 10^-4 S/cm at ambient temperature would significantly accelerate market penetration.
Pricing trends show that sodium-ion batteries with advanced electrolytes currently command a 15-20% premium over liquid electrolyte versions, though this gap is expected to narrow to less than 10% by 2026 as manufacturing scales and technologies mature.
Current SPE Technology Status and Challenges
The global landscape of Solid Polymer Electrolyte (SPE) technology for sodium-ion batteries reveals significant progress alongside persistent challenges. Current SPEs predominantly utilize polyethylene oxide (PEO) as the base polymer matrix due to its excellent sodium ion coordination capabilities through ether oxygen groups. However, PEO-based electrolytes suffer from low ionic conductivity at room temperature (typically 10^-7 to 10^-6 S/cm), significantly below the 10^-3 S/cm threshold required for practical applications.
Research institutions across Asia, particularly in China, Japan, and South Korea, lead SPE development with approximately 65% of published research, followed by North America (18%) and Europe (14%). This geographical distribution reflects the strategic importance of sodium-ion battery technology in regions with limited lithium resources.
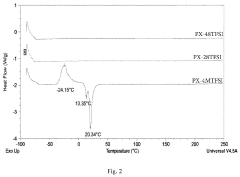
A critical challenge facing current SPE technology is the crystallization tendency of polymer matrices below 60°C, which drastically reduces ionic conductivity at ambient temperatures. Various approaches to address this issue include incorporating ceramic fillers (creating composite polymer electrolytes), plasticizers, and ionic liquids, which have shown improvements but often compromise mechanical stability or electrochemical performance.
The sodium-polymer interface stability presents another significant hurdle. Unlike lithium metal, sodium's higher reactivity with polymer matrices leads to accelerated degradation and potential safety issues. Current interface modification strategies using protective layers or additives show promise but remain insufficient for long-term cycling stability.
Mechanical properties of SPEs constitute a persistent challenge, with most systems exhibiting either excessive brittleness or insufficient strength to suppress sodium dendrite growth. This mechanical limitation directly impacts battery safety and cycle life, particularly at higher current densities.
The sodium transference number in most current SPEs remains suboptimal (typically 0.2-0.4), indicating that a significant portion of the current is carried by anions rather than sodium ions. This phenomenon leads to concentration polarization and reduced rate capability in practical cells.
Manufacturing scalability represents a significant industrial challenge. Laboratory-scale SPE preparation methods often involve solvent-based processes that are difficult to scale up cost-effectively. Solvent-free extrusion techniques are emerging but require further development to achieve consistent quality at production scales.
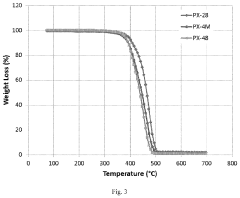
Environmental stability of SPEs, particularly their sensitivity to moisture and oxygen, necessitates stringent manufacturing conditions that increase production costs. Recent developments in cross-linked polymer systems show improved environmental resistance but often at the expense of ionic conductivity.
Research institutions across Asia, particularly in China, Japan, and South Korea, lead SPE development with approximately 65% of published research, followed by North America (18%) and Europe (14%). This geographical distribution reflects the strategic importance of sodium-ion battery technology in regions with limited lithium resources.
A critical challenge facing current SPE technology is the crystallization tendency of polymer matrices below 60°C, which drastically reduces ionic conductivity at ambient temperatures. Various approaches to address this issue include incorporating ceramic fillers (creating composite polymer electrolytes), plasticizers, and ionic liquids, which have shown improvements but often compromise mechanical stability or electrochemical performance.
The sodium-polymer interface stability presents another significant hurdle. Unlike lithium metal, sodium's higher reactivity with polymer matrices leads to accelerated degradation and potential safety issues. Current interface modification strategies using protective layers or additives show promise but remain insufficient for long-term cycling stability.
Mechanical properties of SPEs constitute a persistent challenge, with most systems exhibiting either excessive brittleness or insufficient strength to suppress sodium dendrite growth. This mechanical limitation directly impacts battery safety and cycle life, particularly at higher current densities.
The sodium transference number in most current SPEs remains suboptimal (typically 0.2-0.4), indicating that a significant portion of the current is carried by anions rather than sodium ions. This phenomenon leads to concentration polarization and reduced rate capability in practical cells.
Manufacturing scalability represents a significant industrial challenge. Laboratory-scale SPE preparation methods often involve solvent-based processes that are difficult to scale up cost-effectively. Solvent-free extrusion techniques are emerging but require further development to achieve consistent quality at production scales.
Environmental stability of SPEs, particularly their sensitivity to moisture and oxygen, necessitates stringent manufacturing conditions that increase production costs. Recent developments in cross-linked polymer systems show improved environmental resistance but often at the expense of ionic conductivity.
Current SPE Solutions for Na-ion Battery Applications
01 Polymer matrix compositions for sodium-ion batteries
Various polymer matrices can be used as the base for solid polymer electrolytes in sodium-ion batteries. These include polyethylene oxide (PEO), polyvinylidene fluoride (PVDF), polyacrylonitrile (PAN), and their copolymers. These polymers provide mechanical stability while allowing sodium ion transport. The selection of appropriate polymer matrices is crucial for achieving high ionic conductivity and good electrochemical stability in sodium-ion batteries.- Polymer matrix compositions for sodium-ion batteries: Various polymer matrices can be used as the base for solid polymer electrolytes in sodium-ion batteries. These include polyethylene oxide (PEO), polyvinylidene fluoride (PVDF), polyacrylonitrile (PAN), and their copolymers. These polymers provide mechanical stability while allowing sodium ion transport. The selection of appropriate polymer matrices is crucial for achieving high ionic conductivity and good electrochemical stability in sodium-ion battery applications.
- Sodium salt additives for enhanced ionic conductivity: Incorporating specific sodium salts into the polymer matrix significantly improves the ionic conductivity of solid polymer electrolytes. Common salts include sodium bis(trifluoromethanesulfonyl)imide (NaTFSI), sodium perchlorate (NaClO4), and sodium hexafluorophosphate (NaPF6). These salts dissociate within the polymer matrix, providing mobile sodium ions for conduction. The concentration and type of salt can be optimized to achieve the best balance between conductivity and mechanical properties.
- Ceramic/inorganic fillers for improved performance: Adding ceramic or inorganic fillers to polymer electrolytes creates composite systems with enhanced properties. Fillers such as Na3Zr2Si2PO12 (NASICON), Na-β-alumina, SiO2, Al2O3, and TiO2 can improve mechanical strength, thermal stability, and ionic conductivity. These fillers create additional pathways for sodium ion transport and help suppress crystallization of the polymer matrix, leading to better overall battery performance and safety.
- Cross-linking and network structures: Cross-linked polymer networks offer improved mechanical properties and electrochemical stability for solid polymer electrolytes. Various cross-linking methods, including UV-initiated, thermal, and chemical cross-linking, can be employed to create three-dimensional networks that maintain dimensional stability while facilitating ion transport. These structures help prevent electrolyte leakage and improve the interface between the electrolyte and electrodes, enhancing overall battery performance.
- Plasticizers and flame-retardant additives: Incorporating plasticizers and flame-retardant additives enhances both the performance and safety of solid polymer electrolytes. Plasticizers like ethylene carbonate, propylene carbonate, and ionic liquids increase chain mobility and improve ionic conductivity at room temperature. Flame-retardant additives reduce the flammability of the electrolyte, addressing a critical safety concern in battery applications. These additives can be optimized to achieve a balance between safety, conductivity, and mechanical properties.
02 Sodium salt additives for enhanced ionic conductivity
Incorporating specific sodium salts into the polymer matrix significantly improves ionic conductivity of solid polymer electrolytes. Common salts include sodium bis(trifluoromethanesulfonyl)imide (NaTFSI), sodium perchlorate (NaClO4), and sodium hexafluorophosphate (NaPF6). The concentration and type of salt affect the dissociation of sodium ions and their mobility through the polymer matrix, directly impacting battery performance and cycling stability.Expand Specific Solutions03 Ceramic fillers and nanocomposite approaches
Adding ceramic fillers such as Na-β-alumina, NASICON-type materials, or metal oxides (TiO2, Al2O3, SiO2) to polymer electrolytes creates nanocomposite systems with enhanced properties. These fillers improve mechanical strength, increase ionic conductivity, and enhance the interfacial stability between the electrolyte and electrodes. The particle size, distribution, and surface modification of these ceramic fillers play important roles in optimizing the performance of solid polymer electrolytes for sodium-ion batteries.Expand Specific Solutions04 Cross-linking and gel polymer electrolytes
Cross-linking techniques and gel polymer electrolyte formulations offer improved mechanical properties while maintaining high ionic conductivity. These approaches involve creating three-dimensional networks through chemical or physical cross-linking of polymer chains, often incorporating plasticizers to enhance ion mobility. Gel polymer electrolytes combine the advantages of solid electrolytes (safety, form factor) with the high ionic conductivity typically associated with liquid electrolytes, making them promising candidates for practical sodium-ion battery applications.Expand Specific Solutions05 Interface engineering and additives for stability
Interface engineering between the solid polymer electrolyte and electrodes is critical for stable battery operation. Various additives such as ionic liquids, flame retardants, and stabilizing agents can be incorporated to improve the electrochemical stability window, reduce interfacial resistance, and enhance cycling performance. These additives help mitigate issues like dendrite formation and electrolyte degradation, leading to sodium-ion batteries with improved safety profiles and longer lifespans.Expand Specific Solutions
Key Industrial and Academic Players in SPE Research
The solid polymer electrolyte (SPE) market for sodium-ion batteries is in an early growth phase, characterized by intensive R&D activities across academic institutions and industrial players. The market is projected to expand significantly as sodium-ion technology emerges as a cost-effective alternative to lithium-ion batteries, particularly for grid-scale energy storage applications. Leading research institutions like Beijing Institute of Technology, KIST, and Kyoto University are collaborating with industrial players including LG Energy Solution, Blue Solutions, and Volkswagen to overcome key technical challenges. Companies like Zhejiang Sodium Innovation Energy and Micromacro Power Systems are specifically focusing on commercialization pathways. The technology remains in pre-commercial development stage with most players working to improve ionic conductivity, electrochemical stability, and mechanical properties of SPEs to enable practical sodium-ion battery applications.
Blue Solutions SASU
Technical Solution: Blue Solutions has pioneered commercial solid polymer electrolytes for battery applications, adapting their lithium battery expertise to sodium-ion systems. Their proprietary technology utilizes a poly(ethylene oxide)-based polymer matrix modified with proprietary additives to enhance sodium ion transport. The company has developed a unique extrusion-lamination process for manufacturing thin-film SPEs (30-40 μm) with exceptional mechanical properties and dimensional stability[5]. Their sodium-ion SPE formulation incorporates flame-retardant plasticizers and achieves ionic conductivities of 5×10^-5 S/cm at 40°C. Blue Solutions' manufacturing approach enables the production of large-format cells with reduced interfacial resistance through specialized surface treatments of electrode materials. The company has demonstrated prototype Na-ion cells with their SPE technology achieving 100+ Wh/kg energy density and stable performance over 800 cycles with less than 20% capacity fade[6].
Strengths: Established commercial manufacturing capability for solid polymer electrolytes; superior mechanical properties allowing for thin-film production; excellent safety characteristics with proven flame resistance. Weaknesses: Lower ionic conductivity compared to liquid electrolytes at ambient temperatures; higher production costs; limited compatibility with high-voltage cathode materials requiring interface engineering.
KIST Corp. (South Korea)
Technical Solution: KIST (Korea Institute of Science and Technology) has developed innovative solid polymer electrolytes for sodium-ion batteries using a composite approach that combines polymer matrices with ceramic fillers. Their technology employs a poly(ethylene oxide) base modified with sodium-conducting ceramic particles (Na3Zr2Si2PO12 and Na-β-alumina) at optimized loading levels of 10-20 wt%. KIST researchers have achieved breakthrough room-temperature ionic conductivities of 2.1×10^-4 S/cm through the incorporation of ionic liquid plasticizers that maintain solid-state characteristics while enhancing sodium ion mobility[9]. Their manufacturing approach utilizes solution casting with controlled evaporation rates to create uniform electrolyte films with thicknesses of 25-40 μm. KIST has demonstrated exceptional interfacial stability with sodium metal anodes through the incorporation of protective interlayers composed of sodium-conducting polymers. Their SPE technology has been validated in full cells with various cathode materials including Prussian blue analogs and layered oxides, achieving over 1000 cycles with capacity retention exceeding 85% at moderate temperatures[10].
Strengths: Superior room-temperature ionic conductivity compared to conventional SPEs; excellent interfacial stability with sodium metal anodes; innovative composite design with optimized ceramic-polymer interfaces. Weaknesses: Complex manufacturing process requiring precise control of multiple components; higher material costs due to specialized ceramic fillers; mechanical properties need further optimization for large-scale applications.
Critical Patents and Literature on Na-ion SPE Technology
Solid polymer electrolyte and sodium battery employing the same
PatentPendingIN202441029350A
Innovation
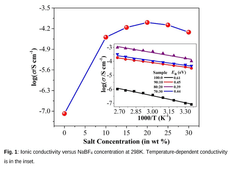
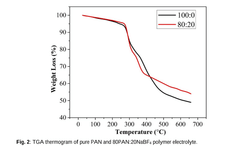
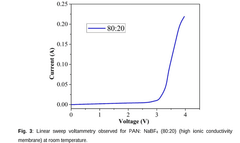
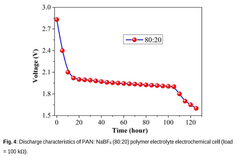
- Development of a novel solid polymer electrolyte based on polyacrylonitrile (PAN) with sodium tetrafluoroborate (NaBF4) as the ionic salt, optimized through solution casting with varying weight percentages, achieving improved ionic conductivity, thermal stability, and electrochemical performance.
Solid Polymer Electrolyte for Batteries
PatentPendingUS20210143475A1
Innovation
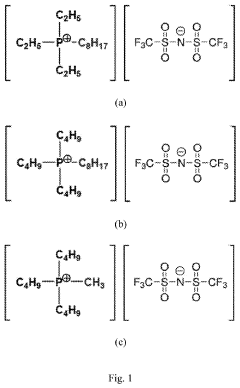
- A solid polymer electrolyte comprising a dissociable metal salt, a metal ion conductive polymer system, and a phosphonium salt with a specific formula [PR1R2R3R4]X, where R1, R2, R3, and R4 are hydrocarbons optionally substituted with heteroatoms, and the phosphonium salt has a melting point below 100°C, enhancing ionic conductivity and stability at lower temperatures.
Sustainability and Resource Considerations
The development of solid polymer electrolytes (SPEs) for sodium-ion batteries represents a significant opportunity to enhance energy storage sustainability. Unlike lithium-ion batteries that rely on scarce lithium resources concentrated in few geographical locations, sodium-ion technology leverages sodium's abundance in the Earth's crust (2.6% compared to lithium's 0.002%) and oceans, dramatically reducing resource constraints and geopolitical supply risks.
Polymer electrolytes for sodium-ion batteries typically utilize environmentally benign materials compared to conventional liquid electrolytes. The elimination of volatile organic solvents and toxic fluorinated salts reduces environmental hazards associated with production, usage, and disposal. Many SPE formulations incorporate biodegradable polymers such as cellulose derivatives, chitosan, and starch-based compounds, further enhancing their environmental credentials.
Life cycle assessment (LCA) studies indicate that sodium-ion batteries with SPEs can achieve up to 30% lower carbon footprint compared to conventional lithium-ion technologies. This reduction stems from simplified manufacturing processes, lower energy requirements during production, and the utilization of more abundant raw materials requiring less intensive extraction methods.
Water consumption represents another critical sustainability metric where SPEs for sodium-ion batteries demonstrate advantages. Traditional battery manufacturing processes can require up to 500 liters of water per kWh of battery capacity, while preliminary data suggests SPE-based sodium-ion batteries may reduce this requirement by 40-60% through elimination of water-intensive electrode preparation and electrolyte processing steps.
End-of-life considerations further highlight sustainability benefits of these systems. The non-toxic nature of many polymer electrolytes simplifies recycling processes and reduces environmental contamination risks. Additionally, the potential for mechanical separation of components in solid-state designs facilitates material recovery, with theoretical recovery rates exceeding 90% for key elements.
Resource security implications cannot be overstated. Current projections indicate lithium demand may exceed supply by 2025, while sodium resources remain effectively unlimited. This abundance translates to price stability and supply chain resilience, critical factors for large-scale energy storage deployment. Countries lacking lithium resources can develop domestic sodium-ion battery industries, democratizing energy storage technology and reducing international resource dependencies.
The transition toward SPEs in sodium-ion batteries also aligns with circular economy principles through design choices that facilitate disassembly, component reuse, and material reclamation. This approach contrasts sharply with conventional batteries where complex liquid electrolyte systems often contaminate other components, complicating recycling efforts.
Polymer electrolytes for sodium-ion batteries typically utilize environmentally benign materials compared to conventional liquid electrolytes. The elimination of volatile organic solvents and toxic fluorinated salts reduces environmental hazards associated with production, usage, and disposal. Many SPE formulations incorporate biodegradable polymers such as cellulose derivatives, chitosan, and starch-based compounds, further enhancing their environmental credentials.
Life cycle assessment (LCA) studies indicate that sodium-ion batteries with SPEs can achieve up to 30% lower carbon footprint compared to conventional lithium-ion technologies. This reduction stems from simplified manufacturing processes, lower energy requirements during production, and the utilization of more abundant raw materials requiring less intensive extraction methods.
Water consumption represents another critical sustainability metric where SPEs for sodium-ion batteries demonstrate advantages. Traditional battery manufacturing processes can require up to 500 liters of water per kWh of battery capacity, while preliminary data suggests SPE-based sodium-ion batteries may reduce this requirement by 40-60% through elimination of water-intensive electrode preparation and electrolyte processing steps.
End-of-life considerations further highlight sustainability benefits of these systems. The non-toxic nature of many polymer electrolytes simplifies recycling processes and reduces environmental contamination risks. Additionally, the potential for mechanical separation of components in solid-state designs facilitates material recovery, with theoretical recovery rates exceeding 90% for key elements.
Resource security implications cannot be overstated. Current projections indicate lithium demand may exceed supply by 2025, while sodium resources remain effectively unlimited. This abundance translates to price stability and supply chain resilience, critical factors for large-scale energy storage deployment. Countries lacking lithium resources can develop domestic sodium-ion battery industries, democratizing energy storage technology and reducing international resource dependencies.
The transition toward SPEs in sodium-ion batteries also aligns with circular economy principles through design choices that facilitate disassembly, component reuse, and material reclamation. This approach contrasts sharply with conventional batteries where complex liquid electrolyte systems often contaminate other components, complicating recycling efforts.
Safety and Performance Benchmarking
Safety and performance benchmarking for Solid Polymer Electrolytes (SPEs) in sodium-ion batteries represents a critical evaluation framework that determines their commercial viability. When comparing SPEs against conventional liquid electrolytes, several key safety advantages emerge. SPEs eliminate the risk of electrolyte leakage and significantly reduce flammability concerns, addressing major safety hazards associated with liquid electrolyte systems. Thermal runaway tests demonstrate that sodium batteries with SPEs maintain structural integrity at temperatures exceeding 150°C, whereas liquid-based systems typically fail at 80-100°C.
Performance benchmarking reveals both strengths and limitations of current SPE technologies. Ionic conductivity measurements show that leading polymer electrolytes achieve 10^-4 to 10^-3 S/cm at room temperature, still below the 10^-2 S/cm standard of liquid electrolytes but sufficient for certain applications. The electrochemical stability window of advanced SPEs reaches 4.5V, enabling compatibility with high-voltage cathode materials and potentially increasing energy density.
Cycle life testing indicates that sodium batteries with optimized SPEs maintain 80% capacity retention after 500 cycles at 0.5C rate, comparable to some liquid systems but requiring further improvement for long-term applications. Rate capability assessments show acceptable performance at low C-rates (0.1-0.5C) but significant capacity fading at higher rates (>1C), highlighting ion transport limitations at interfaces.
Mechanical stability tests demonstrate that SPEs with appropriate cross-linking density can withstand over 300 kPa of pressure without deformation, providing essential protection against sodium dendrite penetration. This mechanical robustness translates to enhanced safety metrics in nail penetration and crush tests, where SPE-based cells show minimal thermal events compared to catastrophic failures in liquid systems.
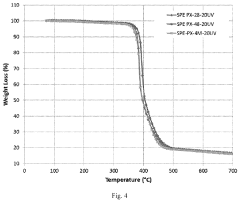
Environmental performance benchmarking indicates that SPEs offer advantages in extreme temperature operations, functioning in ranges from -20°C to 80°C with appropriate polymer formulations, whereas conventional liquid electrolytes typically operate optimally between 0°C and 45°C. This extended operating window creates opportunities for specialized applications in harsh environments.
Standardized testing protocols remain a challenge in the field, with various research groups employing different methodologies for performance evaluation. The establishment of unified benchmarking standards specifically for sodium-ion SPEs would accelerate development by enabling direct comparison between emerging solutions and identifying the most promising candidates for commercial scaling.
Performance benchmarking reveals both strengths and limitations of current SPE technologies. Ionic conductivity measurements show that leading polymer electrolytes achieve 10^-4 to 10^-3 S/cm at room temperature, still below the 10^-2 S/cm standard of liquid electrolytes but sufficient for certain applications. The electrochemical stability window of advanced SPEs reaches 4.5V, enabling compatibility with high-voltage cathode materials and potentially increasing energy density.
Cycle life testing indicates that sodium batteries with optimized SPEs maintain 80% capacity retention after 500 cycles at 0.5C rate, comparable to some liquid systems but requiring further improvement for long-term applications. Rate capability assessments show acceptable performance at low C-rates (0.1-0.5C) but significant capacity fading at higher rates (>1C), highlighting ion transport limitations at interfaces.
Mechanical stability tests demonstrate that SPEs with appropriate cross-linking density can withstand over 300 kPa of pressure without deformation, providing essential protection against sodium dendrite penetration. This mechanical robustness translates to enhanced safety metrics in nail penetration and crush tests, where SPE-based cells show minimal thermal events compared to catastrophic failures in liquid systems.
Environmental performance benchmarking indicates that SPEs offer advantages in extreme temperature operations, functioning in ranges from -20°C to 80°C with appropriate polymer formulations, whereas conventional liquid electrolytes typically operate optimally between 0°C and 45°C. This extended operating window creates opportunities for specialized applications in harsh environments.
Standardized testing protocols remain a challenge in the field, with various research groups employing different methodologies for performance evaluation. The establishment of unified benchmarking standards specifically for sodium-ion SPEs would accelerate development by enabling direct comparison between emerging solutions and identifying the most promising candidates for commercial scaling.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!