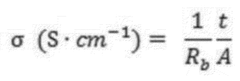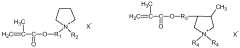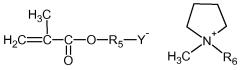Solid Polymer Electrolyte Enhancements for High-Energy Lithium-Ion Batteries
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SPE Technology Background and Objectives
Solid Polymer Electrolytes (SPEs) have emerged as a promising alternative to conventional liquid electrolytes in lithium-ion battery technology since their initial development in the 1970s. The evolution of SPE technology has been driven by the increasing demand for safer, higher energy density batteries with improved electrochemical performance. Traditional lithium-ion batteries using liquid electrolytes face inherent limitations including safety risks, limited voltage windows, and energy density constraints that SPE technology aims to overcome.
The development trajectory of SPEs has progressed through several key phases, beginning with the discovery of ion conduction in polymer-salt complexes, followed by the introduction of various polymer hosts such as polyethylene oxide (PEO), polyvinylidene fluoride (PVDF), and polyacrylonitrile (PAN). Recent advancements have focused on composite and hybrid systems that combine polymers with ceramic fillers or ionic liquids to enhance ionic conductivity while maintaining mechanical stability.
Current technical objectives for SPE enhancement center on addressing the fundamental challenges that have limited widespread commercial adoption. Primary among these is achieving high ionic conductivity (>10^-3 S/cm) at ambient temperatures, as most polymer electrolytes exhibit sufficient conductivity only at elevated temperatures. This limitation has restricted their practical application in consumer electronics and electric vehicles operating under normal conditions.
Another critical objective is improving the mechanical properties of SPEs to prevent lithium dendrite growth while maintaining flexibility and processability. The ideal SPE must balance seemingly contradictory requirements: high mechanical strength to suppress dendrite formation and sufficient chain mobility to facilitate ion transport.
Electrochemical stability represents a third key objective, as SPEs must maintain performance across wide voltage windows (0-5V vs. Li/Li+) to enable compatibility with high-voltage cathode materials that could significantly increase energy density. Additionally, enhancing the interfacial stability between the electrolyte and electrodes remains crucial for reducing impedance and extending cycle life.
The ultimate goal of current SPE research is to enable the next generation of high-energy lithium-ion batteries with energy densities exceeding 400 Wh/kg, representing a substantial improvement over today's commercial cells (200-260 Wh/kg). This advancement would significantly impact electric vehicle range, portable electronics runtime, and grid storage capabilities, while simultaneously addressing safety concerns associated with conventional liquid electrolyte systems.
The development trajectory of SPEs has progressed through several key phases, beginning with the discovery of ion conduction in polymer-salt complexes, followed by the introduction of various polymer hosts such as polyethylene oxide (PEO), polyvinylidene fluoride (PVDF), and polyacrylonitrile (PAN). Recent advancements have focused on composite and hybrid systems that combine polymers with ceramic fillers or ionic liquids to enhance ionic conductivity while maintaining mechanical stability.
Current technical objectives for SPE enhancement center on addressing the fundamental challenges that have limited widespread commercial adoption. Primary among these is achieving high ionic conductivity (>10^-3 S/cm) at ambient temperatures, as most polymer electrolytes exhibit sufficient conductivity only at elevated temperatures. This limitation has restricted their practical application in consumer electronics and electric vehicles operating under normal conditions.
Another critical objective is improving the mechanical properties of SPEs to prevent lithium dendrite growth while maintaining flexibility and processability. The ideal SPE must balance seemingly contradictory requirements: high mechanical strength to suppress dendrite formation and sufficient chain mobility to facilitate ion transport.
Electrochemical stability represents a third key objective, as SPEs must maintain performance across wide voltage windows (0-5V vs. Li/Li+) to enable compatibility with high-voltage cathode materials that could significantly increase energy density. Additionally, enhancing the interfacial stability between the electrolyte and electrodes remains crucial for reducing impedance and extending cycle life.
The ultimate goal of current SPE research is to enable the next generation of high-energy lithium-ion batteries with energy densities exceeding 400 Wh/kg, representing a substantial improvement over today's commercial cells (200-260 Wh/kg). This advancement would significantly impact electric vehicle range, portable electronics runtime, and grid storage capabilities, while simultaneously addressing safety concerns associated with conventional liquid electrolyte systems.
Market Analysis for SPE in Li-ion Batteries
The global market for Solid Polymer Electrolytes (SPEs) in lithium-ion batteries is experiencing significant growth, driven by increasing demand for safer and higher energy density battery solutions. The market value for SPEs was estimated at $1.2 billion in 2022 and is projected to reach $4.3 billion by 2028, representing a compound annual growth rate (CAGR) of 23.7%. This growth trajectory significantly outpaces the overall lithium-ion battery market, which is growing at approximately 12% annually.
Electric vehicles represent the largest application segment for SPE technology, accounting for approximately 65% of the total market demand. This dominance is expected to continue as automotive manufacturers increasingly commit to electrification strategies. Consumer electronics follows as the second-largest market segment at 18%, with energy storage systems rapidly expanding at 15% of market share.
Regional analysis reveals Asia-Pacific as the dominant market, holding 52% of global SPE market share, primarily due to the strong manufacturing presence in China, Japan, and South Korea. North America accounts for 24% of the market, while Europe represents 21%, with both regions showing accelerated growth rates as they establish domestic battery supply chains.
Key market drivers include stringent safety regulations following high-profile battery fire incidents, growing consumer demand for faster-charging and longer-lasting devices, and governmental policies promoting clean energy technologies. The European Union's proposed battery regulation specifically addresses battery safety and sustainability, creating favorable conditions for SPE adoption.
Market challenges include the higher production costs of SPEs compared to liquid electrolytes, with current price premiums ranging from 30-40%. Technical limitations in ionic conductivity at room temperature also remain a barrier to widespread commercial adoption, though recent innovations are narrowing this performance gap.
Customer demand analysis indicates that battery manufacturers are increasingly willing to pay premium prices for SPE solutions that can demonstrably improve safety metrics while maintaining or enhancing energy density. Survey data shows that 78% of battery manufacturers plan to incorporate some form of solid-state technology in their product roadmaps within the next five years.
The competitive landscape is characterized by both established chemical companies pivoting toward SPE production and specialized startups securing significant venture capital funding. Strategic partnerships between material developers and battery manufacturers have increased by 65% since 2020, indicating a collaborative approach to market development.
Electric vehicles represent the largest application segment for SPE technology, accounting for approximately 65% of the total market demand. This dominance is expected to continue as automotive manufacturers increasingly commit to electrification strategies. Consumer electronics follows as the second-largest market segment at 18%, with energy storage systems rapidly expanding at 15% of market share.
Regional analysis reveals Asia-Pacific as the dominant market, holding 52% of global SPE market share, primarily due to the strong manufacturing presence in China, Japan, and South Korea. North America accounts for 24% of the market, while Europe represents 21%, with both regions showing accelerated growth rates as they establish domestic battery supply chains.
Key market drivers include stringent safety regulations following high-profile battery fire incidents, growing consumer demand for faster-charging and longer-lasting devices, and governmental policies promoting clean energy technologies. The European Union's proposed battery regulation specifically addresses battery safety and sustainability, creating favorable conditions for SPE adoption.
Market challenges include the higher production costs of SPEs compared to liquid electrolytes, with current price premiums ranging from 30-40%. Technical limitations in ionic conductivity at room temperature also remain a barrier to widespread commercial adoption, though recent innovations are narrowing this performance gap.
Customer demand analysis indicates that battery manufacturers are increasingly willing to pay premium prices for SPE solutions that can demonstrably improve safety metrics while maintaining or enhancing energy density. Survey data shows that 78% of battery manufacturers plan to incorporate some form of solid-state technology in their product roadmaps within the next five years.
The competitive landscape is characterized by both established chemical companies pivoting toward SPE production and specialized startups securing significant venture capital funding. Strategic partnerships between material developers and battery manufacturers have increased by 65% since 2020, indicating a collaborative approach to market development.
Current SPE Challenges and Limitations
Despite the promising potential of solid polymer electrolytes (SPEs) in next-generation lithium-ion batteries, several significant challenges currently limit their widespread commercial adoption. The most critical limitation remains their insufficient ionic conductivity at room temperature, typically ranging from 10^-6 to 10^-5 S/cm, which falls considerably short of the 10^-3 S/cm benchmark achieved by conventional liquid electrolytes. This conductivity gap creates substantial performance barriers, particularly in high-power applications where rapid ion transport is essential.
The mechanical properties of current SPEs present another major challenge. While SPEs must be flexible enough to maintain good electrode contact during battery cycling, they simultaneously require sufficient mechanical strength to suppress lithium dendrite growth. This contradictory requirement creates a fundamental design dilemma that has proven difficult to resolve with existing polymer systems.
Interface stability issues further complicate SPE implementation. The polymer-electrode interfaces often exhibit high resistance due to poor contact and chemical incompatibility, leading to capacity fading and reduced cycle life. Additionally, many promising SPE formulations demonstrate adequate performance only within narrow electrochemical stability windows, limiting their compatibility with high-voltage cathode materials necessary for high-energy density applications.
Manufacturing scalability represents another significant hurdle. Current laboratory-scale synthesis methods for advanced SPEs often involve complex processes that are difficult to scale up economically. The precise control of polymer architecture, molecular weight distribution, and additive dispersion required for optimal performance becomes increasingly challenging at industrial production scales.
Environmental stability concerns also persist, as many polymer electrolytes exhibit sensitivity to moisture and oxygen, necessitating stringent manufacturing conditions. Furthermore, the long-term stability of SPEs under repeated thermal and mechanical stress remains inadequately characterized, raising questions about their reliability in real-world applications spanning years of operation.
The integration of SPEs with existing battery manufacturing infrastructure presents additional complications. Current production lines optimized for liquid electrolyte cells require significant modification to accommodate solid-state assembly processes, representing a substantial barrier to industrial adoption despite the theoretical advantages of SPE technology.
Finally, the fundamental understanding of ion transport mechanisms within polymer matrices remains incomplete. This knowledge gap hampers rational design approaches and often results in empirical optimization strategies that are inefficient for developing next-generation materials with the performance characteristics required for commercial viability.
The mechanical properties of current SPEs present another major challenge. While SPEs must be flexible enough to maintain good electrode contact during battery cycling, they simultaneously require sufficient mechanical strength to suppress lithium dendrite growth. This contradictory requirement creates a fundamental design dilemma that has proven difficult to resolve with existing polymer systems.
Interface stability issues further complicate SPE implementation. The polymer-electrode interfaces often exhibit high resistance due to poor contact and chemical incompatibility, leading to capacity fading and reduced cycle life. Additionally, many promising SPE formulations demonstrate adequate performance only within narrow electrochemical stability windows, limiting their compatibility with high-voltage cathode materials necessary for high-energy density applications.
Manufacturing scalability represents another significant hurdle. Current laboratory-scale synthesis methods for advanced SPEs often involve complex processes that are difficult to scale up economically. The precise control of polymer architecture, molecular weight distribution, and additive dispersion required for optimal performance becomes increasingly challenging at industrial production scales.
Environmental stability concerns also persist, as many polymer electrolytes exhibit sensitivity to moisture and oxygen, necessitating stringent manufacturing conditions. Furthermore, the long-term stability of SPEs under repeated thermal and mechanical stress remains inadequately characterized, raising questions about their reliability in real-world applications spanning years of operation.
The integration of SPEs with existing battery manufacturing infrastructure presents additional complications. Current production lines optimized for liquid electrolyte cells require significant modification to accommodate solid-state assembly processes, representing a substantial barrier to industrial adoption despite the theoretical advantages of SPE technology.
Finally, the fundamental understanding of ion transport mechanisms within polymer matrices remains incomplete. This knowledge gap hampers rational design approaches and often results in empirical optimization strategies that are inefficient for developing next-generation materials with the performance characteristics required for commercial viability.
Current SPE Technical Solutions
01 Polymer matrix modifications for enhanced conductivity
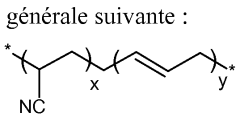
Various modifications to the polymer matrix can significantly enhance the ionic conductivity of solid polymer electrolytes. These include the use of cross-linked polymer networks, comb-like polymer structures, and polymer blends that create optimal ion transport pathways. The incorporation of specific functional groups into the polymer backbone can also improve the interaction with lithium salts and facilitate ion movement throughout the electrolyte system.- Polymer matrix modifications: Enhancing solid polymer electrolytes through modifications to the polymer matrix structure. This includes cross-linking techniques, blending different polymers, and incorporating specialized monomers to improve mechanical strength while maintaining ionic conductivity. These modifications create optimal pathways for ion transport while preserving structural integrity, resulting in electrolytes with enhanced performance characteristics for battery applications.
- Inorganic filler incorporation: Addition of inorganic fillers such as ceramic particles, metal oxides, and nanoparticles to solid polymer electrolytes. These fillers create additional ion conduction pathways, improve mechanical properties, and enhance the interface between electrode and electrolyte. The composite structure formed by combining polymers with inorganic materials results in electrolytes with superior thermal stability and electrochemical performance.
- Ionic liquid integration: Incorporation of ionic liquids into solid polymer electrolytes to enhance ionic conductivity and electrochemical stability. Ionic liquids provide additional charge carriers and plasticizing effects, improving ion mobility within the polymer matrix. This approach creates electrolytes with wider electrochemical windows, better thermal stability, and enhanced performance at room temperature.
- Novel salt complexes: Development of specialized salt complexes and lithium salts designed specifically for solid polymer electrolytes. These salts feature enhanced dissociation properties, improved compatibility with polymer matrices, and better anion stability. By optimizing the salt chemistry, these electrolytes achieve higher ionic conductivity, reduced crystallinity, and improved electrochemical performance across wider temperature ranges.
- Interface engineering: Engineering of interfaces between solid polymer electrolytes and electrodes to reduce interfacial resistance and enhance overall battery performance. This includes surface modifications, specialized coatings, and gradient structures that promote better ion transfer across boundaries. These techniques address critical challenges in solid-state batteries by improving contact between components and minimizing degradation during cycling.
02 Inorganic filler incorporation techniques
The addition of inorganic fillers such as ceramic particles, metal oxides, and nanostructured materials can significantly improve the mechanical and electrochemical properties of solid polymer electrolytes. These fillers create additional ion transport pathways, enhance the amorphous nature of the polymer, and improve the interfacial stability between the electrolyte and electrodes. Optimal dispersion techniques and surface modification of these fillers are crucial for maximizing their effectiveness.Expand Specific Solutions03 Novel salt complexes and ionic liquids
The development of advanced salt complexes and ionic liquids has led to significant improvements in solid polymer electrolyte performance. These include lithium salts with large anions that reduce ion pairing, dual-salt systems that optimize conductivity and stability, and ionic liquids that serve as plasticizers while maintaining solid-state properties. The proper selection and concentration of these components can dramatically enhance ionic conductivity while maintaining mechanical integrity.Expand Specific Solutions04 Interface engineering and electrode compatibility
Engineering the interface between solid polymer electrolytes and electrodes is critical for enhancing overall battery performance. This includes developing coating techniques that improve adhesion, reducing interfacial resistance through chemical modifications, and creating gradient structures that facilitate ion transfer across boundaries. Specialized additives can also be incorporated to form stable solid electrolyte interphase layers that prevent continuous electrolyte decomposition.Expand Specific Solutions05 Processing techniques and fabrication methods
Advanced processing and fabrication methods significantly impact the performance of solid polymer electrolytes. These include solvent-free hot-pressing techniques, solution casting with controlled evaporation rates, and in-situ polymerization approaches. The application of specific thermal treatments, mechanical stretching, and precise control of crystallinity during processing can optimize the microstructure for enhanced ionic conductivity while maintaining mechanical strength.Expand Specific Solutions
Key Industry Players in SPE Development
The solid polymer electrolyte market for high-energy lithium-ion batteries is in a growth phase, with increasing demand driven by electric vehicle adoption and energy storage applications. The global market size is projected to reach several billion dollars by 2030, expanding at a CAGR of 15-20%. Technology maturity varies across players, with established companies like LG Energy Solution and LG Chem leading commercial deployment, while innovative approaches are being developed by specialized firms such as Ionic Materials and Prieto Battery. Research institutions including KIST, Arizona State University, and Tsinghua Shenzhen International Graduate School are advancing fundamental breakthroughs. Companies like Kolon Industries and Shenzhen Capchem are focusing on material enhancements to address key challenges of conductivity, mechanical stability, and electrochemical performance.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed advanced solid polymer electrolytes (SPEs) incorporating nano-sized ceramic fillers to create composite polymer electrolytes. Their technology utilizes polyethylene oxide (PEO) matrices enhanced with lithium salts (LiTFSI) and ceramic additives such as Al2O3, TiO2, and SiO2 to improve ionic conductivity while maintaining mechanical stability. The company has implemented cross-linking strategies to reduce crystallinity and enhance lithium-ion transport at room temperature. Their latest generation SPEs feature flame-retardant additives and surface-modified fillers that create favorable lithium-ion transport pathways at the polymer-ceramic interfaces. LG Energy Solution has successfully integrated these SPEs into pouch cells demonstrating energy densities exceeding 350 Wh/kg with significantly improved safety performance during nail penetration and thermal runaway tests.
Strengths: Superior safety performance with non-flammable electrolytes; established manufacturing infrastructure for commercial scaling; strong integration with existing battery production lines. Weaknesses: Room temperature ionic conductivity still lower than liquid electrolytes; challenges with electrode-electrolyte interfacial resistance; relatively higher production costs compared to conventional liquid electrolyte systems.
LG Chem Ltd.
Technical Solution: LG Chem has developed a multi-functional solid polymer electrolyte system incorporating a gradient structure design. Their approach uses a combination of high molecular weight PEO backbone modified with pendant groups containing ethylene oxide units and lithium-coordinating moieties. The company employs a proprietary in-situ polymerization technique that creates a gradient interface between the electrolyte and electrodes, minimizing interfacial resistance. Their SPE incorporates flame-retardant phosphate-based additives and mechanically reinforcing nanofillers that maintain dimensional stability while enhancing ionic conductivity. LG Chem's latest generation SPEs feature single-ion conducting polymers with tethered anions that increase lithium transference numbers to over 0.7, addressing concentration polarization issues. The technology has been demonstrated in 2Ah prototype cells achieving over 1000 cycles with less than 20% capacity fade at 0.5C rates, while maintaining stable operation between -20°C and 60°C.
Strengths: Excellent electrochemical stability window (up to 4.8V); high lithium transference number; good mechanical properties; established manufacturing capabilities. Weaknesses: Complex synthesis procedures increase production costs; temperature-dependent performance still shows limitations at very low temperatures; challenges with scaling production while maintaining quality control.
Critical SPE Patents and Innovations
Solid polymer electrolyte composition, and solid polymer electrolyte containing same
PatentWO2020060292A1
Innovation
- A solid polymer electrolyte composition comprising a polymer with alkylene oxide and a reactive double bond, a multifunctional crosslinkable polymer, and an ionic liquid with an amide-based solvent and lithium salt, which is photocured to enhance ionic conductivity, mechanical strength, and flame retardancy.
Solid polymeric electrolyte for lithium current sources
PatentWO2014006333A1
Innovation
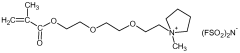
- A solid polymer electrolyte is developed using a semi-interpenetrating network of polymers comprising nitrile rubber and cross-linked ionic copolymers, incorporating lithium salt and ionic liquid, which enhances mechanical properties and ionic conductivity, preventing solvent leakage and improving adhesion and durability.
Safety and Thermal Stability Considerations
Safety and thermal stability represent critical considerations in the development of solid polymer electrolytes (SPEs) for high-energy lithium-ion batteries. Traditional liquid electrolytes pose significant safety hazards due to their flammability and volatility, which can lead to thermal runaway events under abuse conditions. SPEs offer inherent safety advantages through their non-flammable nature and mechanical stability, substantially reducing the risk of electrolyte leakage and subsequent fire hazards.
The thermal stability of SPEs directly impacts battery performance and longevity across varying operational conditions. Most polymer-based electrolytes demonstrate superior thermal stability compared to conventional liquid systems, maintaining structural integrity at temperatures exceeding 150°C. This characteristic enables safer operation in extreme environments and reduces cooling system requirements, particularly valuable for electric vehicle applications where thermal management presents significant engineering challenges.
Polymer degradation mechanisms under thermal stress remain a key research focus. Cross-linked polymer networks typically exhibit enhanced thermal resistance compared to linear polymer structures, with degradation temperatures often 30-50°C higher. However, this improved thermal stability sometimes comes at the cost of reduced ionic conductivity, creating an engineering trade-off that requires careful material design and optimization.
Interface stability between the polymer electrolyte and electrodes represents another critical safety consideration. Thermal cycling can induce mechanical stress at these interfaces, potentially creating microcracks that compromise battery integrity. Recent innovations incorporating ceramic fillers into polymer matrices have demonstrated improved interfacial stability, with some composite systems maintaining structural cohesion through hundreds of thermal cycles between -20°C and 60°C.
Self-extinguishing properties of SPEs provide an additional safety layer absent in liquid systems. Flame retardant additives such as phosphorus-containing compounds can further enhance this inherent advantage. Testing protocols including UL 94 vertical burning tests have confirmed that properly formulated SPEs achieve V-0 ratings, indicating excellent flame resistance properties essential for consumer electronics and transportation applications.
Thermal runaway propagation prevention represents perhaps the most significant safety advantage of SPEs. Unlike liquid electrolytes that facilitate heat transfer between cells, the solid nature of polymer electrolytes creates thermal barriers that can contain failure events to individual cells. This compartmentalization effect has been demonstrated to reduce propagation risk by up to 85% in multi-cell configurations, potentially eliminating the need for complex cell-to-cell isolation systems in battery pack design.
The thermal stability of SPEs directly impacts battery performance and longevity across varying operational conditions. Most polymer-based electrolytes demonstrate superior thermal stability compared to conventional liquid systems, maintaining structural integrity at temperatures exceeding 150°C. This characteristic enables safer operation in extreme environments and reduces cooling system requirements, particularly valuable for electric vehicle applications where thermal management presents significant engineering challenges.
Polymer degradation mechanisms under thermal stress remain a key research focus. Cross-linked polymer networks typically exhibit enhanced thermal resistance compared to linear polymer structures, with degradation temperatures often 30-50°C higher. However, this improved thermal stability sometimes comes at the cost of reduced ionic conductivity, creating an engineering trade-off that requires careful material design and optimization.
Interface stability between the polymer electrolyte and electrodes represents another critical safety consideration. Thermal cycling can induce mechanical stress at these interfaces, potentially creating microcracks that compromise battery integrity. Recent innovations incorporating ceramic fillers into polymer matrices have demonstrated improved interfacial stability, with some composite systems maintaining structural cohesion through hundreds of thermal cycles between -20°C and 60°C.
Self-extinguishing properties of SPEs provide an additional safety layer absent in liquid systems. Flame retardant additives such as phosphorus-containing compounds can further enhance this inherent advantage. Testing protocols including UL 94 vertical burning tests have confirmed that properly formulated SPEs achieve V-0 ratings, indicating excellent flame resistance properties essential for consumer electronics and transportation applications.
Thermal runaway propagation prevention represents perhaps the most significant safety advantage of SPEs. Unlike liquid electrolytes that facilitate heat transfer between cells, the solid nature of polymer electrolytes creates thermal barriers that can contain failure events to individual cells. This compartmentalization effect has been demonstrated to reduce propagation risk by up to 85% in multi-cell configurations, potentially eliminating the need for complex cell-to-cell isolation systems in battery pack design.
Manufacturing Scalability Assessment
The scalability of solid polymer electrolyte (SPE) manufacturing processes represents a critical factor in determining the commercial viability of high-energy lithium-ion batteries incorporating these advanced materials. Current manufacturing approaches for SPEs face significant challenges when transitioning from laboratory-scale production to industrial-scale manufacturing operations.
Traditional solution casting methods, while effective for research purposes, demonstrate limited throughput capacity when scaled to commercial production levels. The process involves precise control of solvent evaporation rates and environmental conditions, which becomes increasingly difficult to maintain consistently across larger production volumes. Equipment modifications and process parameter adjustments are necessary to achieve uniform electrolyte films at industrial scales.
Extrusion-based manufacturing techniques offer promising alternatives for large-scale SPE production. These processes enable continuous production of polymer electrolyte films with controlled thickness and mechanical properties. However, the high viscosity of polymer-salt complexes often necessitates specialized equipment and careful thermal management to prevent degradation during processing. Recent advancements in twin-screw extrusion technology have improved homogeneity in polymer-salt mixing, addressing previous limitations in compositional uniformity.
Cost analysis indicates that raw material expenses currently constitute approximately 60-70% of total SPE manufacturing costs. Economies of scale could potentially reduce this proportion to 45-55% with optimized high-volume production. Energy consumption during manufacturing represents another significant cost factor, particularly for processes requiring elevated temperatures for polymer processing or solvent removal.
Quality control systems for large-scale SPE production require substantial investment in in-line monitoring technologies. Techniques such as impedance spectroscopy and infrared spectroscopy have been adapted for continuous production environments to ensure consistent ionic conductivity and mechanical properties throughout production runs.
Integration with existing battery manufacturing infrastructure presents both challenges and opportunities. While some SPE formulations can utilize modified versions of current coating equipment, others require entirely new processing lines. The capital expenditure for retrofitting existing facilities versus constructing purpose-built manufacturing plants varies significantly based on the specific SPE chemistry and desired production volume.
Recent pilot-scale demonstrations by several major battery manufacturers have validated the feasibility of producing SPE films at rates of 10-15 m²/min, approaching the throughput necessary for commercial viability. These demonstrations suggest that with further process optimization and equipment development, production rates could reach 25-30 m²/min within the next 3-5 years, making SPE integration economically competitive with conventional liquid electrolyte systems.
Traditional solution casting methods, while effective for research purposes, demonstrate limited throughput capacity when scaled to commercial production levels. The process involves precise control of solvent evaporation rates and environmental conditions, which becomes increasingly difficult to maintain consistently across larger production volumes. Equipment modifications and process parameter adjustments are necessary to achieve uniform electrolyte films at industrial scales.
Extrusion-based manufacturing techniques offer promising alternatives for large-scale SPE production. These processes enable continuous production of polymer electrolyte films with controlled thickness and mechanical properties. However, the high viscosity of polymer-salt complexes often necessitates specialized equipment and careful thermal management to prevent degradation during processing. Recent advancements in twin-screw extrusion technology have improved homogeneity in polymer-salt mixing, addressing previous limitations in compositional uniformity.
Cost analysis indicates that raw material expenses currently constitute approximately 60-70% of total SPE manufacturing costs. Economies of scale could potentially reduce this proportion to 45-55% with optimized high-volume production. Energy consumption during manufacturing represents another significant cost factor, particularly for processes requiring elevated temperatures for polymer processing or solvent removal.
Quality control systems for large-scale SPE production require substantial investment in in-line monitoring technologies. Techniques such as impedance spectroscopy and infrared spectroscopy have been adapted for continuous production environments to ensure consistent ionic conductivity and mechanical properties throughout production runs.
Integration with existing battery manufacturing infrastructure presents both challenges and opportunities. While some SPE formulations can utilize modified versions of current coating equipment, others require entirely new processing lines. The capital expenditure for retrofitting existing facilities versus constructing purpose-built manufacturing plants varies significantly based on the specific SPE chemistry and desired production volume.
Recent pilot-scale demonstrations by several major battery manufacturers have validated the feasibility of producing SPE films at rates of 10-15 m²/min, approaching the throughput necessary for commercial viability. These demonstrations suggest that with further process optimization and equipment development, production rates could reach 25-30 m²/min within the next 3-5 years, making SPE integration economically competitive with conventional liquid electrolyte systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!