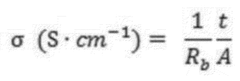Comparison of Solid Polymer Electrolytes in Lithium-Ion vs Sodium-Ion Batteries
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SPE Technology Background and Objectives
Solid Polymer Electrolytes (SPEs) have emerged as a promising alternative to conventional liquid electrolytes in battery technology, representing a significant shift in energy storage systems. The development of SPEs can be traced back to the 1970s when the first polymer-salt complexes were investigated for ionic conductivity. Over the decades, this technology has evolved from basic polyethylene oxide (PEO) systems to sophisticated composite and block copolymer architectures designed to overcome inherent limitations.
The evolution of SPE technology has been primarily driven by the need for safer, more stable, and higher-performing energy storage solutions. Traditional liquid electrolytes, while offering high ionic conductivity, present significant safety concerns including flammability and potential leakage. SPEs address these issues by providing a solid-state alternative that eliminates these risks while potentially enabling higher energy density configurations through compatibility with metallic anodes.
Recent technological advancements have accelerated SPE development, particularly in the context of both lithium-ion and sodium-ion battery systems. While lithium-ion batteries have dominated the commercial landscape, sodium-ion technology has gained attention due to sodium's greater abundance and lower cost compared to lithium. This parallel development creates a unique opportunity to compare SPE performance across these two battery chemistries.
The fundamental challenge in SPE technology lies in achieving sufficient ionic conductivity at ambient temperatures while maintaining mechanical stability. Traditional PEO-based electrolytes typically require elevated temperatures (>60°C) to achieve practical conductivity levels, limiting their application. Current research trends focus on novel polymer architectures, ceramic fillers, plasticizers, and ionic liquids to overcome these limitations.
The technical objectives for SPE development in both lithium and sodium systems include achieving room temperature ionic conductivity exceeding 10^-4 S/cm, maintaining a wide electrochemical stability window (>4.5V for Li-ion, >4.0V for Na-ion), ensuring mechanical stability sufficient to prevent dendrite formation, and demonstrating long-term cycling stability (>1000 cycles).
Comparing SPEs in lithium versus sodium systems presents unique challenges and opportunities. The larger ionic radius of Na+ (102 pm) compared to Li+ (76 pm) affects ion transport mechanisms, polymer-ion interactions, and overall electrolyte design requirements. This fundamental difference necessitates tailored approaches to SPE formulation for each system rather than direct technology transfer from lithium to sodium platforms.
This technical assessment aims to comprehensively evaluate the current state of SPE technology in both battery systems, identify key technological gaps, and outline promising research directions that could accelerate commercial implementation across multiple energy storage applications.
The evolution of SPE technology has been primarily driven by the need for safer, more stable, and higher-performing energy storage solutions. Traditional liquid electrolytes, while offering high ionic conductivity, present significant safety concerns including flammability and potential leakage. SPEs address these issues by providing a solid-state alternative that eliminates these risks while potentially enabling higher energy density configurations through compatibility with metallic anodes.
Recent technological advancements have accelerated SPE development, particularly in the context of both lithium-ion and sodium-ion battery systems. While lithium-ion batteries have dominated the commercial landscape, sodium-ion technology has gained attention due to sodium's greater abundance and lower cost compared to lithium. This parallel development creates a unique opportunity to compare SPE performance across these two battery chemistries.
The fundamental challenge in SPE technology lies in achieving sufficient ionic conductivity at ambient temperatures while maintaining mechanical stability. Traditional PEO-based electrolytes typically require elevated temperatures (>60°C) to achieve practical conductivity levels, limiting their application. Current research trends focus on novel polymer architectures, ceramic fillers, plasticizers, and ionic liquids to overcome these limitations.
The technical objectives for SPE development in both lithium and sodium systems include achieving room temperature ionic conductivity exceeding 10^-4 S/cm, maintaining a wide electrochemical stability window (>4.5V for Li-ion, >4.0V for Na-ion), ensuring mechanical stability sufficient to prevent dendrite formation, and demonstrating long-term cycling stability (>1000 cycles).
Comparing SPEs in lithium versus sodium systems presents unique challenges and opportunities. The larger ionic radius of Na+ (102 pm) compared to Li+ (76 pm) affects ion transport mechanisms, polymer-ion interactions, and overall electrolyte design requirements. This fundamental difference necessitates tailored approaches to SPE formulation for each system rather than direct technology transfer from lithium to sodium platforms.
This technical assessment aims to comprehensively evaluate the current state of SPE technology in both battery systems, identify key technological gaps, and outline promising research directions that could accelerate commercial implementation across multiple energy storage applications.
Market Analysis for SPE in Li-ion and Na-ion Batteries
The global market for solid polymer electrolytes (SPEs) in battery applications is experiencing significant growth, driven by increasing demand for safer and higher energy density energy storage solutions. The SPE market for lithium-ion batteries currently dominates, valued at approximately $1.2 billion in 2022, with projections indicating a compound annual growth rate (CAGR) of 18% through 2030. This growth is primarily fueled by electric vehicle adoption and portable electronics proliferation.
In contrast, the sodium-ion battery SPE market remains nascent but shows promising growth potential. Currently valued at around $85 million, it is expected to grow at a more aggressive CAGR of 24% through 2030, albeit from a smaller base. This accelerated growth reflects increasing interest in sodium-ion technology as a cost-effective alternative to lithium-ion systems, particularly for grid storage applications where energy density requirements are less stringent.
Regional analysis reveals Asia-Pacific dominates SPE manufacturing and implementation, with China, South Korea, and Japan collectively accounting for over 65% of global production capacity. European markets are rapidly expanding their footprint, particularly in automotive applications, while North American companies focus heavily on research and development of next-generation polymer electrolyte systems.
Consumer electronics currently represents the largest application segment for lithium-ion SPEs, accounting for 42% of market share, followed by electric vehicles at 38%. For sodium-ion SPEs, stationary energy storage dominates with 56% market share, highlighting the technology's strategic positioning for grid applications.
Key market drivers for both technologies include increasing safety concerns with liquid electrolytes, regulatory pressure for fire-resistant battery systems, and the push toward higher energy density solutions. However, sodium-ion SPE adoption faces specific market challenges, including competition from established lithium-ion technologies and the need for dedicated manufacturing infrastructure.
Cost analysis indicates that while lithium-ion SPEs currently command premium pricing, sodium-ion SPEs offer potential cost advantages of 30-40% at scale, primarily due to the abundance and lower cost of sodium raw materials. This cost differential represents a significant market opportunity, particularly in price-sensitive applications like grid storage and developing markets.
Market forecasts suggest that by 2030, SPEs could penetrate up to 25% of the total lithium-ion battery market and potentially 40% of the emerging sodium-ion battery market, representing a combined opportunity exceeding $8 billion annually. This growth trajectory is contingent upon continued improvements in ionic conductivity and mechanical properties of polymer electrolyte systems.
In contrast, the sodium-ion battery SPE market remains nascent but shows promising growth potential. Currently valued at around $85 million, it is expected to grow at a more aggressive CAGR of 24% through 2030, albeit from a smaller base. This accelerated growth reflects increasing interest in sodium-ion technology as a cost-effective alternative to lithium-ion systems, particularly for grid storage applications where energy density requirements are less stringent.
Regional analysis reveals Asia-Pacific dominates SPE manufacturing and implementation, with China, South Korea, and Japan collectively accounting for over 65% of global production capacity. European markets are rapidly expanding their footprint, particularly in automotive applications, while North American companies focus heavily on research and development of next-generation polymer electrolyte systems.
Consumer electronics currently represents the largest application segment for lithium-ion SPEs, accounting for 42% of market share, followed by electric vehicles at 38%. For sodium-ion SPEs, stationary energy storage dominates with 56% market share, highlighting the technology's strategic positioning for grid applications.
Key market drivers for both technologies include increasing safety concerns with liquid electrolytes, regulatory pressure for fire-resistant battery systems, and the push toward higher energy density solutions. However, sodium-ion SPE adoption faces specific market challenges, including competition from established lithium-ion technologies and the need for dedicated manufacturing infrastructure.
Cost analysis indicates that while lithium-ion SPEs currently command premium pricing, sodium-ion SPEs offer potential cost advantages of 30-40% at scale, primarily due to the abundance and lower cost of sodium raw materials. This cost differential represents a significant market opportunity, particularly in price-sensitive applications like grid storage and developing markets.
Market forecasts suggest that by 2030, SPEs could penetrate up to 25% of the total lithium-ion battery market and potentially 40% of the emerging sodium-ion battery market, representing a combined opportunity exceeding $8 billion annually. This growth trajectory is contingent upon continued improvements in ionic conductivity and mechanical properties of polymer electrolyte systems.
Current SPE Development Status and Challenges
Solid Polymer Electrolytes (SPEs) have emerged as promising alternatives to conventional liquid electrolytes in both lithium-ion and sodium-ion battery systems. Currently, the development of SPEs faces several distinct challenges in each battery type, though with notable overlapping concerns.
In lithium-ion battery applications, SPEs have reached a relatively mature stage with commercial implementations beginning to appear in niche markets. PEO-based systems dominate the landscape, achieving ionic conductivities of 10^-4 to 10^-3 S/cm at operating temperatures. However, these values remain significantly lower than liquid electrolyte counterparts (10^-2 S/cm), particularly at room temperature. The crystallization tendency of PEO below 60°C continues to be a major limitation for widespread adoption.
For sodium-ion batteries, SPE development remains at an earlier stage. The larger ionic radius of Na+ (1.02Å) compared to Li+ (0.76Å) creates fundamental challenges in polymer chain coordination and ion transport mechanisms. Current Na-ion SPEs typically demonstrate conductivities one order of magnitude lower than their Li-ion counterparts under similar conditions, with values rarely exceeding 10^-5 S/cm at ambient temperature.
A common challenge across both systems is the mechanical integrity of polymer electrolytes. Pure polymer systems often lack sufficient mechanical strength, while excessive ceramic filler addition to create composite electrolytes can compromise conductivity. The interfacial resistance between the electrolyte and electrodes presents another significant hurdle, particularly pronounced in sodium systems where less research has been conducted on interface engineering.
Stability issues manifest differently between the two battery types. In lithium systems, dendrite formation remains a persistent concern despite the mechanical barrier provided by solid electrolytes. For sodium systems, the chemical compatibility between Na-metal anodes and polymer hosts presents unique challenges, with accelerated degradation observed in several polymer families that show stability with lithium.
Processing and manufacturing scalability represent additional barriers to commercialization. Current laboratory-scale production methods for high-performance SPEs often involve complex solvent-based processes that are difficult to scale industrially. The thickness control and uniformity requirements for solid-state batteries demand precision that current roll-to-roll or extrusion processes struggle to achieve consistently.
Recent innovations have focused on cross-linked polymer networks and block copolymer architectures to simultaneously address conductivity and mechanical property requirements. Single-ion conducting polymers with immobilized anions have shown promise in reducing concentration polarization effects, though synthesis complexity remains high. Composite approaches incorporating nanoscale ceramic fillers continue to evolve, with surface-modified particles showing improved compatibility in both Li and Na systems.
In lithium-ion battery applications, SPEs have reached a relatively mature stage with commercial implementations beginning to appear in niche markets. PEO-based systems dominate the landscape, achieving ionic conductivities of 10^-4 to 10^-3 S/cm at operating temperatures. However, these values remain significantly lower than liquid electrolyte counterparts (10^-2 S/cm), particularly at room temperature. The crystallization tendency of PEO below 60°C continues to be a major limitation for widespread adoption.
For sodium-ion batteries, SPE development remains at an earlier stage. The larger ionic radius of Na+ (1.02Å) compared to Li+ (0.76Å) creates fundamental challenges in polymer chain coordination and ion transport mechanisms. Current Na-ion SPEs typically demonstrate conductivities one order of magnitude lower than their Li-ion counterparts under similar conditions, with values rarely exceeding 10^-5 S/cm at ambient temperature.
A common challenge across both systems is the mechanical integrity of polymer electrolytes. Pure polymer systems often lack sufficient mechanical strength, while excessive ceramic filler addition to create composite electrolytes can compromise conductivity. The interfacial resistance between the electrolyte and electrodes presents another significant hurdle, particularly pronounced in sodium systems where less research has been conducted on interface engineering.
Stability issues manifest differently between the two battery types. In lithium systems, dendrite formation remains a persistent concern despite the mechanical barrier provided by solid electrolytes. For sodium systems, the chemical compatibility between Na-metal anodes and polymer hosts presents unique challenges, with accelerated degradation observed in several polymer families that show stability with lithium.
Processing and manufacturing scalability represent additional barriers to commercialization. Current laboratory-scale production methods for high-performance SPEs often involve complex solvent-based processes that are difficult to scale industrially. The thickness control and uniformity requirements for solid-state batteries demand precision that current roll-to-roll or extrusion processes struggle to achieve consistently.
Recent innovations have focused on cross-linked polymer networks and block copolymer architectures to simultaneously address conductivity and mechanical property requirements. Single-ion conducting polymers with immobilized anions have shown promise in reducing concentration polarization effects, though synthesis complexity remains high. Composite approaches incorporating nanoscale ceramic fillers continue to evolve, with surface-modified particles showing improved compatibility in both Li and Na systems.
Current SPE Solutions for Li-ion and Na-ion Batteries
01 Ionic conductivity comparison between lithium and sodium solid polymer electrolytes
Solid polymer electrolytes (SPEs) show different ionic conductivity behaviors when used with lithium versus sodium ions. Generally, lithium-ion SPEs demonstrate higher ionic conductivity at room temperature compared to sodium-ion counterparts due to the smaller ionic radius of lithium. However, some specially designed polymer matrices can enhance sodium ion transport through larger channels and coordination sites, narrowing this performance gap. The conductivity difference impacts battery performance metrics including energy density and power capability.- Ionic conductivity comparison between lithium and sodium solid polymer electrolytes: Solid polymer electrolytes (SPEs) show different ionic conductivity behaviors when used with lithium versus sodium ions. Generally, lithium-ion SPEs demonstrate higher ionic conductivity at room temperature due to the smaller ionic radius of Li+ compared to Na+. However, sodium-based systems can achieve competitive conductivity levels at elevated temperatures or with specific polymer matrices optimized for larger cation transport. The difference in coordination chemistry between the two ions affects their mobility through polymer chains and impacts overall battery performance.
- Mechanical stability and interfacial properties of solid polymer electrolytes: Solid polymer electrolytes exhibit different mechanical and interfacial behaviors in lithium-ion versus sodium-ion battery systems. Lithium-based SPEs typically form more stable solid electrolyte interphases (SEI) with electrodes, while sodium-based systems often face challenges with dendrite formation and interfacial resistance. Various polymer compositions and additives can enhance the mechanical strength and flexibility of the electrolyte membrane, which is crucial for preventing short circuits and maintaining long-term cycling stability. Cross-linked polymer networks and composite formulations have shown improved performance in both battery types.
- Temperature dependence and thermal stability comparison: The temperature performance of solid polymer electrolytes differs significantly between lithium and sodium battery systems. Sodium-ion SPEs often show better performance at higher temperatures compared to their lithium counterparts, while lithium-ion SPEs generally perform better at ambient and lower temperatures. This temperature-dependent behavior affects the practical operating range of the respective battery technologies. Thermal stability is another critical factor, with some polymer matrices showing different degradation patterns depending on the cation species, which impacts the safety profile and lifespan of the batteries.
- Novel polymer compositions and additives for enhanced performance: Innovative polymer compositions and additives have been developed to enhance the performance of solid polymer electrolytes in both lithium and sodium battery systems. These include the incorporation of ceramic fillers, ionic liquids, plasticizers, and flame retardants. Certain polymer blends show preferential performance with either lithium or sodium ions based on their chemical structure and coordination capabilities. PEO-based electrolytes modified with specific functional groups have demonstrated improved ion transport for sodium, while polycarbonate and polyester-based systems often favor lithium ion conduction. These compositional variations allow for tailored electrolyte solutions for specific battery applications.
- Cycling performance and long-term stability comparison: The cycling performance and long-term stability of batteries utilizing solid polymer electrolytes show marked differences between lithium and sodium systems. Lithium-ion batteries with SPEs typically demonstrate superior capacity retention and longer cycle life compared to their sodium counterparts, partly due to the more stable interfaces formed with electrode materials. However, sodium systems can achieve competitive cycling performance through specialized polymer designs that accommodate the larger ion size and different coordination chemistry. Factors affecting the comparative cycling stability include electrolyte degradation mechanisms, ion trapping phenomena, and electrode compatibility, all of which vary between the two battery chemistries.
02 Mechanical stability and interfacial properties of solid polymer electrolytes
Solid polymer electrolytes exhibit different mechanical and interfacial behaviors in lithium-ion versus sodium-ion battery systems. Lithium-ion SPEs typically form more stable solid electrolyte interphases (SEI) with electrodes, while sodium-ion SPEs often face challenges with interfacial resistance and dendrite formation. However, sodium-ion SPEs may demonstrate better mechanical flexibility and lower volume expansion during cycling. Various polymer compositions and additives are employed to enhance mechanical properties while maintaining ionic conductivity for both battery types.Expand Specific Solutions03 Thermal stability and operating temperature range comparison
The thermal stability and operating temperature range differ significantly between lithium-ion and sodium-ion solid polymer electrolytes. Lithium-ion SPEs typically demonstrate better performance at lower temperatures but may face safety concerns at elevated temperatures. In contrast, sodium-ion SPEs often exhibit enhanced thermal stability at higher temperatures, making them potentially more suitable for high-temperature applications. Various polymer blends and ceramic fillers are incorporated to extend the operating temperature range for both systems, with different optimal compositions depending on the cation type.Expand Specific Solutions04 Cycling stability and lifetime performance comparison
Cycling stability and lifetime performance vary between lithium-ion and sodium-ion batteries using solid polymer electrolytes. Lithium-ion systems typically demonstrate superior long-term cycling stability with less capacity fade over extended cycles. Sodium-ion SPE batteries often show higher initial capacity degradation but can achieve comparable stability through specialized polymer formulations. The differences stem from distinct electrode-electrolyte interface evolution, ion transport mechanisms, and structural changes during repeated charge-discharge cycles. Various crosslinking strategies and composite approaches are employed to enhance cycling performance for both battery types.Expand Specific Solutions05 Cost-effectiveness and sustainability comparison
Sodium-ion batteries with solid polymer electrolytes generally offer cost advantages over their lithium-ion counterparts due to the greater natural abundance and more even global distribution of sodium resources. While lithium-ion systems deliver higher energy density, sodium-ion SPE batteries present a more economical and environmentally sustainable alternative, particularly for large-scale energy storage applications where energy density is less critical. The polymer matrices used in sodium systems can often be produced from more sustainable precursors, further enhancing their environmental profile. Manufacturing processes for sodium-ion SPE batteries typically require less energy and generate fewer harmful byproducts.Expand Specific Solutions
Key Industrial Players in SPE Development
The solid polymer electrolyte market for lithium-ion and sodium-ion batteries is in a growth phase, with lithium-ion technology being more mature while sodium-ion solutions are emerging as promising alternatives. The global market is expanding rapidly, driven by demand for safer, higher-performance energy storage solutions. Companies like LG Chem, Solvay, and Sekisui Chemical lead in lithium-ion polymer electrolyte development with established commercial products, while Zhejiang Sodium Innovation Energy and research institutions such as Huazhong University of Science & Technology are advancing sodium-ion polymer electrolyte technologies. Automotive manufacturers including Volkswagen and Ford are increasingly investing in both technologies, recognizing the strategic importance of solid electrolytes for next-generation batteries with improved safety profiles and energy density.
Shenzhen Capchem Technology Co., Ltd.
Technical Solution: Capchem has developed a comprehensive portfolio of solid polymer electrolytes for both lithium and sodium-ion batteries, with particular emphasis on their proprietary "CapSPE" technology. Their approach utilizes a blend of high molecular weight PEO (Mw > 1,000,000) with carefully selected lithium or sodium salts and ceramic fillers. For lithium-ion applications, their SPEs incorporate LiTFSI and proprietary lithium borate salts, achieving ionic conductivities of 1.2×10^-4 S/cm at 60°C. Their sodium-ion formulations utilize NaFSI and NaTFSI salts with specially designed polymer architectures that accommodate the larger sodium ion radius. Capchem's comparative analysis reveals that while their Li-ion SPEs demonstrate higher absolute conductivity (approximately 2-3 times greater than Na counterparts at equivalent temperatures), their Na-ion SPEs exhibit superior electrochemical stability windows (up to 4.3V vs. Na/Na+) and better compatibility with hard carbon anodes commonly used in sodium-ion batteries. A key innovation is their dual-phase polymer system that creates interconnected pathways for ion transport while maintaining mechanical integrity. Their manufacturing process allows for direct coating onto electrodes, enabling simplified battery assembly and improved interfacial contact.
Strengths: Excellent thermal stability up to 150°C for both Li and Na systems; superior electrochemical stability windows compared to liquid electrolytes; established manufacturing processes for commercial-scale production. Weaknesses: Lower room temperature ionic conductivity compared to liquid electrolytes; sodium-ion formulations require operating at elevated temperatures (>40°C) for practical conductivity values; higher cost compared to conventional liquid electrolyte systems.
Zhejiang Sodium Innovation Energy Co., Ltd.
Technical Solution: Zhejiang Sodium Innovation Energy has developed specialized solid polymer electrolytes optimized specifically for sodium-ion batteries, addressing the fundamental challenges of larger Na+ ion transport. Their proprietary "NaSPE" technology utilizes a poly(ethylene oxide) backbone modified with sodium-philic functional groups that create preferential coordination sites for sodium ions. This approach achieves ionic conductivities of 10^-4 S/cm at 60°C and 10^-5 S/cm at room temperature. Their comparative studies with lithium systems reveal that while lithium SPEs generally show higher absolute conductivity values, their sodium-specific polymer architecture demonstrates superior cation transference numbers (t+ > 0.5) for sodium ions compared to conventional PEO systems. The company has pioneered a dual-salt strategy combining NaTFSI and NaFSI to optimize both conductivity and interfacial stability. Their solid polymer electrolytes incorporate proprietary ceramic fillers (Na-NASICON type) at 10-15 wt% to create mechanically robust composite electrolytes with enhanced sodium ion transport channels. Recent innovations include UV-crosslinkable formulations that enable direct in-situ polymerization on electrodes, simplifying manufacturing processes.
Strengths: Specifically engineered for sodium-ion chemistry with optimized Na+ transport pathways; excellent compatibility with hard carbon anodes commonly used in Na-ion batteries; cost-effective formulation compared to lithium-based alternatives. Weaknesses: Lower absolute ionic conductivity compared to liquid electrolytes; requires elevated operating temperatures (40-60°C) for optimal performance; limited long-term cycling data compared to more established lithium SPE systems.
Critical SPE Materials and Interface Chemistry
Solid polymer electrolyte and sodium battery employing the same
PatentPendingIN202441029350A
Innovation
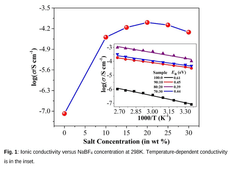
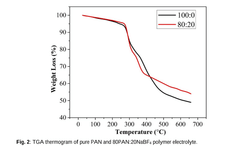
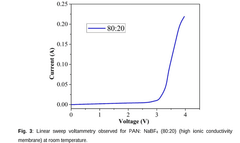
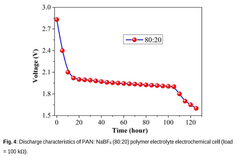
- Development of a novel solid polymer electrolyte based on polyacrylonitrile (PAN) with sodium tetrafluoroborate (NaBF4) as the ionic salt, optimized through solution casting with varying weight percentages, achieving improved ionic conductivity, thermal stability, and electrochemical performance.
Solid polymer electrolyte composition, and solid polymer electrolyte containing same
PatentWO2020060292A1
Innovation
- A solid polymer electrolyte composition comprising a polymer with alkylene oxide and a reactive double bond, a multifunctional crosslinkable polymer, and an ionic liquid with an amide-based solvent and lithium salt, which is photocured to enhance ionic conductivity, mechanical strength, and flame retardancy.
Safety and Performance Benchmarking
Safety performance benchmarking of solid polymer electrolytes (SPEs) in lithium-ion and sodium-ion batteries reveals significant differences that impact their commercial viability. Thermal stability tests demonstrate that SPEs generally exhibit superior safety characteristics compared to conventional liquid electrolytes in both battery types, with reduced flammability and minimal leakage risks. However, SPEs in sodium-ion batteries typically maintain stability at slightly lower temperature thresholds (120-150°C) compared to their lithium-ion counterparts (150-180°C), necessitating different thermal management strategies.
Mechanical integrity assessments show that PEO-based electrolytes maintain better interfacial contact in lithium-ion systems, while NASICON-type polymer composites demonstrate superior performance in sodium-ion configurations. This difference stems from the larger ionic radius of Na+ (1.02Å) compared to Li+ (0.76Å), affecting ion transport mechanisms and interfacial stability during cycling.
Cycling performance data indicates that lithium-ion batteries with optimized SPEs can achieve 80-85% capacity retention after 500 cycles, while sodium-ion systems currently reach 70-75% under similar conditions. The rate capability gap is more pronounced, with Li-ion SPE cells delivering 65-70% capacity at 2C rates, whereas Na-ion SPE cells typically manage only 50-55% at equivalent rates.
Electrochemical stability window measurements reveal another critical difference: lithium-ion SPEs generally demonstrate stability windows of 4.0-4.5V, while sodium-ion SPEs often exhibit narrower windows of 3.5-4.0V. This limitation restricts the cathode materials selection for Na-ion systems and impacts their energy density potential.
Self-discharge rates present an interesting contrast, with sodium-ion SPE batteries showing marginally better shelf-life in certain polymer compositions, particularly those incorporating sodium-conducting ceramic fillers. Accelerated aging tests at 60°C show capacity losses of 3-5% per month for optimized Na-ion SPE cells versus 4-6% for comparable Li-ion systems.
Safety response under abuse conditions demonstrates that both systems resist thermal runaway more effectively than liquid electrolyte counterparts. Nail penetration tests show temperature increases limited to 50-80°C for SPE-based cells versus 120-200°C for conventional cells. However, lithium-ion SPE cells still exhibit slightly higher temperature spikes during short-circuit events compared to sodium-ion alternatives, reflecting the inherently different reactivity profiles of the two alkali metals.
Mechanical integrity assessments show that PEO-based electrolytes maintain better interfacial contact in lithium-ion systems, while NASICON-type polymer composites demonstrate superior performance in sodium-ion configurations. This difference stems from the larger ionic radius of Na+ (1.02Å) compared to Li+ (0.76Å), affecting ion transport mechanisms and interfacial stability during cycling.
Cycling performance data indicates that lithium-ion batteries with optimized SPEs can achieve 80-85% capacity retention after 500 cycles, while sodium-ion systems currently reach 70-75% under similar conditions. The rate capability gap is more pronounced, with Li-ion SPE cells delivering 65-70% capacity at 2C rates, whereas Na-ion SPE cells typically manage only 50-55% at equivalent rates.
Electrochemical stability window measurements reveal another critical difference: lithium-ion SPEs generally demonstrate stability windows of 4.0-4.5V, while sodium-ion SPEs often exhibit narrower windows of 3.5-4.0V. This limitation restricts the cathode materials selection for Na-ion systems and impacts their energy density potential.
Self-discharge rates present an interesting contrast, with sodium-ion SPE batteries showing marginally better shelf-life in certain polymer compositions, particularly those incorporating sodium-conducting ceramic fillers. Accelerated aging tests at 60°C show capacity losses of 3-5% per month for optimized Na-ion SPE cells versus 4-6% for comparable Li-ion systems.
Safety response under abuse conditions demonstrates that both systems resist thermal runaway more effectively than liquid electrolyte counterparts. Nail penetration tests show temperature increases limited to 50-80°C for SPE-based cells versus 120-200°C for conventional cells. However, lithium-ion SPE cells still exhibit slightly higher temperature spikes during short-circuit events compared to sodium-ion alternatives, reflecting the inherently different reactivity profiles of the two alkali metals.
Sustainability and Resource Considerations
The sustainability profile of solid polymer electrolytes (SPEs) in battery technologies represents a critical consideration in the ongoing transition toward greener energy storage solutions. When comparing lithium-ion and sodium-ion battery systems, several key sustainability factors emerge that differentiate their environmental footprints and resource implications.
Lithium resources face significant geographical concentration, with over 75% of global reserves located in the "Lithium Triangle" of Argentina, Bolivia, and Chile. This concentration raises concerns about supply chain resilience and geopolitical dependencies. In contrast, sodium resources are abundantly available worldwide, with substantial reserves in seawater and rock salt deposits, offering a more democratized resource distribution that reduces extraction-related environmental impacts.
The extraction processes for lithium typically involve extensive water consumption in arid regions, with estimates suggesting up to 2 million liters of water required per ton of lithium produced. This creates substantial ecological pressure on fragile ecosystems. Sodium extraction methods generally demonstrate lower environmental impact profiles, requiring less water and energy input, which translates to reduced carbon emissions throughout the supply chain.
From a lifecycle perspective, SPEs in sodium-ion batteries present advantages in end-of-life management. The biodegradability potential of certain sodium-compatible polymer electrolytes exceeds that of their lithium counterparts, with some bio-derived polymers showing 40-60% degradation within standardized testing periods compared to 10-25% for conventional lithium-compatible polymers.
Carbon footprint analyses reveal that the production of SPEs for lithium-ion batteries generates approximately 15-20% higher greenhouse gas emissions compared to equivalent sodium-ion battery components. This difference stems primarily from the energy-intensive processing and purification requirements for lithium-compatible polymers and their associated additives.
Economic sustainability metrics also favor sodium-based systems, with raw material costs for sodium-compatible SPEs averaging 30-40% lower than lithium alternatives. This cost advantage could accelerate market adoption and enable broader deployment of stationary energy storage solutions in developing economies, supporting global energy equity goals.
The recyclability characteristics of SPEs differ significantly between the two battery chemistries. Lithium-based systems often require more complex separation processes due to the higher reactivity of lithium compounds, while sodium-based polymers typically allow for simpler recycling protocols with reduced hazardous waste generation, supporting circular economy principles.
Lithium resources face significant geographical concentration, with over 75% of global reserves located in the "Lithium Triangle" of Argentina, Bolivia, and Chile. This concentration raises concerns about supply chain resilience and geopolitical dependencies. In contrast, sodium resources are abundantly available worldwide, with substantial reserves in seawater and rock salt deposits, offering a more democratized resource distribution that reduces extraction-related environmental impacts.
The extraction processes for lithium typically involve extensive water consumption in arid regions, with estimates suggesting up to 2 million liters of water required per ton of lithium produced. This creates substantial ecological pressure on fragile ecosystems. Sodium extraction methods generally demonstrate lower environmental impact profiles, requiring less water and energy input, which translates to reduced carbon emissions throughout the supply chain.
From a lifecycle perspective, SPEs in sodium-ion batteries present advantages in end-of-life management. The biodegradability potential of certain sodium-compatible polymer electrolytes exceeds that of their lithium counterparts, with some bio-derived polymers showing 40-60% degradation within standardized testing periods compared to 10-25% for conventional lithium-compatible polymers.
Carbon footprint analyses reveal that the production of SPEs for lithium-ion batteries generates approximately 15-20% higher greenhouse gas emissions compared to equivalent sodium-ion battery components. This difference stems primarily from the energy-intensive processing and purification requirements for lithium-compatible polymers and their associated additives.
Economic sustainability metrics also favor sodium-based systems, with raw material costs for sodium-compatible SPEs averaging 30-40% lower than lithium alternatives. This cost advantage could accelerate market adoption and enable broader deployment of stationary energy storage solutions in developing economies, supporting global energy equity goals.
The recyclability characteristics of SPEs differ significantly between the two battery chemistries. Lithium-based systems often require more complex separation processes due to the higher reactivity of lithium compounds, while sodium-based polymers typically allow for simpler recycling protocols with reduced hazardous waste generation, supporting circular economy principles.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!