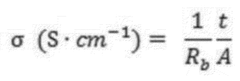How Solid Polymer Electrolyte Affects Electrode Kinetics in Lithium-Ion Cells
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SPE Technology Background and Objectives
Solid Polymer Electrolytes (SPEs) have emerged as a promising alternative to conventional liquid electrolytes in lithium-ion battery technology since their initial development in the 1970s. The evolution of SPE technology has been driven by the increasing demand for safer, more efficient, and higher energy density energy storage solutions. Traditional lithium-ion batteries utilizing liquid electrolytes face inherent safety risks including electrolyte leakage, flammability, and potential thermal runaway events that have limited their application in certain high-demand scenarios.
The fundamental concept behind SPEs involves replacing volatile liquid components with solid polymer matrices that can conduct lithium ions while maintaining mechanical stability. This technological approach addresses multiple challenges simultaneously: enhancing safety through elimination of flammable components, improving electrochemical stability at electrode interfaces, and enabling novel battery architectures including flexible and thin-film designs.
Historical development of SPEs has progressed through several distinct phases, beginning with polyethylene oxide (PEO)-based systems discovered by Wright and Armand, advancing through composite and block copolymer approaches in the 1990s and 2000s, and more recently exploring cross-linked networks and polymer blends with ceramic fillers. Each evolutionary step has aimed to overcome the persistent challenges of ionic conductivity at ambient temperatures while maintaining mechanical integrity.
The current technological landscape shows significant progress in understanding how polymer chemistry, morphology, and additives influence ion transport mechanisms at the electrode-electrolyte interface. Recent breakthroughs in polymer science have enabled the development of SPEs with conductivities approaching 10^-3 S/cm at room temperature, a critical threshold for practical applications.
The primary objectives of current SPE research focus on elucidating the fundamental mechanisms by which these materials affect electrode kinetics in lithium-ion cells. Specifically, researchers aim to understand interfacial phenomena including ion desolvation processes, charge transfer resistance, and the formation of solid-electrolyte interphase (SEI) layers unique to polymer systems. Additional goals include optimizing polymer architectures to facilitate faster ion transport while maintaining dimensional stability during cycling.
Looking forward, SPE technology aims to enable next-generation battery designs with improved energy density, cycle life, and safety profiles. The field is moving toward multifunctional electrolyte systems that not only conduct ions but actively participate in stabilizing electrode interfaces during extended cycling. Understanding these complex interactions represents a critical frontier in advancing lithium-ion technology toward applications in electric vehicles, grid storage, and portable electronics.
The fundamental concept behind SPEs involves replacing volatile liquid components with solid polymer matrices that can conduct lithium ions while maintaining mechanical stability. This technological approach addresses multiple challenges simultaneously: enhancing safety through elimination of flammable components, improving electrochemical stability at electrode interfaces, and enabling novel battery architectures including flexible and thin-film designs.
Historical development of SPEs has progressed through several distinct phases, beginning with polyethylene oxide (PEO)-based systems discovered by Wright and Armand, advancing through composite and block copolymer approaches in the 1990s and 2000s, and more recently exploring cross-linked networks and polymer blends with ceramic fillers. Each evolutionary step has aimed to overcome the persistent challenges of ionic conductivity at ambient temperatures while maintaining mechanical integrity.
The current technological landscape shows significant progress in understanding how polymer chemistry, morphology, and additives influence ion transport mechanisms at the electrode-electrolyte interface. Recent breakthroughs in polymer science have enabled the development of SPEs with conductivities approaching 10^-3 S/cm at room temperature, a critical threshold for practical applications.
The primary objectives of current SPE research focus on elucidating the fundamental mechanisms by which these materials affect electrode kinetics in lithium-ion cells. Specifically, researchers aim to understand interfacial phenomena including ion desolvation processes, charge transfer resistance, and the formation of solid-electrolyte interphase (SEI) layers unique to polymer systems. Additional goals include optimizing polymer architectures to facilitate faster ion transport while maintaining dimensional stability during cycling.
Looking forward, SPE technology aims to enable next-generation battery designs with improved energy density, cycle life, and safety profiles. The field is moving toward multifunctional electrolyte systems that not only conduct ions but actively participate in stabilizing electrode interfaces during extended cycling. Understanding these complex interactions represents a critical frontier in advancing lithium-ion technology toward applications in electric vehicles, grid storage, and portable electronics.
Market Demand Analysis for SPE in Li-ion Batteries
The global market for solid polymer electrolytes (SPEs) in lithium-ion batteries is experiencing significant growth, driven by increasing demand for safer and higher energy density battery solutions. The market value for SPEs is projected to reach $2.5 billion by 2027, growing at a compound annual growth rate of approximately 18% from 2022 to 2027.
Consumer electronics continues to be the largest application segment for SPE-based lithium-ion batteries, accounting for nearly 40% of the market share. This is primarily due to the growing consumer preference for devices with longer battery life and enhanced safety features. The reduced risk of thermal runaway and leakage associated with SPEs makes them particularly attractive for wearable technology and high-end portable electronics.
Electric vehicles represent the fastest-growing segment for SPE technology, with major automotive manufacturers increasingly investing in solid-state battery research. The automotive industry's shift toward electrification has created substantial demand for batteries that offer higher energy density, faster charging capabilities, and improved safety profiles. SPEs address these requirements by enabling higher voltage operation and potentially reducing charging times by up to 30% compared to conventional liquid electrolyte systems.
The energy storage sector is also showing increased interest in SPE technology, particularly for grid-scale applications where safety and longevity are paramount concerns. Utility companies are exploring SPE-based solutions for renewable energy integration, with pilot projects demonstrating up to 25% improvement in cycle life compared to traditional lithium-ion batteries.
Regional market analysis indicates that Asia Pacific dominates the SPE market with approximately 45% share, led by Japan, South Korea, and China. These countries have established robust supply chains and significant R&D investments in advanced battery technologies. North America and Europe follow with 30% and 20% market shares respectively, with both regions showing accelerated growth due to supportive government policies promoting clean energy technologies.
Customer surveys indicate that battery safety remains the primary concern for 68% of end-users across all segments, followed by energy density (57%) and cycle life (52%). This aligns perfectly with the value proposition of SPE technology, which offers improvements in all three areas. The willingness to pay premium prices for enhanced safety features has increased by 15% since 2020, creating favorable market conditions for SPE adoption.
Industry forecasts suggest that as manufacturing processes mature and economies of scale are achieved, the cost premium for SPE-based batteries could decrease from the current 30-40% to approximately 15% by 2025, further accelerating market penetration across all application segments.
Consumer electronics continues to be the largest application segment for SPE-based lithium-ion batteries, accounting for nearly 40% of the market share. This is primarily due to the growing consumer preference for devices with longer battery life and enhanced safety features. The reduced risk of thermal runaway and leakage associated with SPEs makes them particularly attractive for wearable technology and high-end portable electronics.
Electric vehicles represent the fastest-growing segment for SPE technology, with major automotive manufacturers increasingly investing in solid-state battery research. The automotive industry's shift toward electrification has created substantial demand for batteries that offer higher energy density, faster charging capabilities, and improved safety profiles. SPEs address these requirements by enabling higher voltage operation and potentially reducing charging times by up to 30% compared to conventional liquid electrolyte systems.
The energy storage sector is also showing increased interest in SPE technology, particularly for grid-scale applications where safety and longevity are paramount concerns. Utility companies are exploring SPE-based solutions for renewable energy integration, with pilot projects demonstrating up to 25% improvement in cycle life compared to traditional lithium-ion batteries.
Regional market analysis indicates that Asia Pacific dominates the SPE market with approximately 45% share, led by Japan, South Korea, and China. These countries have established robust supply chains and significant R&D investments in advanced battery technologies. North America and Europe follow with 30% and 20% market shares respectively, with both regions showing accelerated growth due to supportive government policies promoting clean energy technologies.
Customer surveys indicate that battery safety remains the primary concern for 68% of end-users across all segments, followed by energy density (57%) and cycle life (52%). This aligns perfectly with the value proposition of SPE technology, which offers improvements in all three areas. The willingness to pay premium prices for enhanced safety features has increased by 15% since 2020, creating favorable market conditions for SPE adoption.
Industry forecasts suggest that as manufacturing processes mature and economies of scale are achieved, the cost premium for SPE-based batteries could decrease from the current 30-40% to approximately 15% by 2025, further accelerating market penetration across all application segments.
Current Challenges in SPE-Electrode Interface Kinetics
Despite significant advancements in solid polymer electrolyte (SPE) technology, the electrode-electrolyte interface remains a critical bottleneck limiting the performance of solid-state lithium-ion batteries. The primary challenge stems from the inherent incompatibility between the rigid polymer matrix and solid electrode materials, creating substantial interfacial resistance that impedes ion transport and electrode kinetics.
Contact issues represent one of the most persistent challenges. Unlike liquid electrolytes that can easily wet electrode surfaces, SPEs struggle to maintain intimate contact with electrodes during cycling. This problem is exacerbated by volume changes in electrode materials during lithiation/delithiation processes, leading to delamination and increased interfacial resistance over time.
The formation of interphases at the SPE-electrode boundary introduces additional complexity. These solid electrolyte interphase (SEI) layers, while necessary for battery stability, often exhibit poor ionic conductivity in solid-state systems. The chemical composition and morphology of these interphases significantly affect charge transfer kinetics and overall cell performance, yet controlling their formation remains challenging in polymer-based systems.
Space charge layers at interfaces further complicate electrode kinetics. The accumulation of charged species at the SPE-electrode boundary creates localized electric fields that can impede ion migration. This phenomenon is particularly problematic with high-voltage cathode materials, where space charge effects can dramatically increase activation barriers for lithium-ion transport.
Mechanical stress during cycling presents another significant hurdle. The mismatch in mechanical properties between rigid electrodes and relatively soft polymer electrolytes leads to stress concentration at interfaces. This mechanical instability not only affects contact quality but can also accelerate degradation mechanisms that further compromise electrode kinetics.
Temperature sensitivity compounds these challenges, as most polymer electrolytes exhibit strong temperature-dependent conductivity. At lower temperatures, reduced chain mobility severely limits ion transport at interfaces, creating a significant activation barrier for electrode reactions. This temperature dependence restricts the practical operating window of SPE-based batteries.
Recent research has identified lithium dendrite growth at the anode-electrolyte interface as another critical concern. Despite the mechanical resistance offered by solid electrolytes, non-uniform lithium deposition can still occur at high current densities, penetrating through polymer matrices and potentially causing short circuits while disrupting interfacial kinetics.
Contact issues represent one of the most persistent challenges. Unlike liquid electrolytes that can easily wet electrode surfaces, SPEs struggle to maintain intimate contact with electrodes during cycling. This problem is exacerbated by volume changes in electrode materials during lithiation/delithiation processes, leading to delamination and increased interfacial resistance over time.
The formation of interphases at the SPE-electrode boundary introduces additional complexity. These solid electrolyte interphase (SEI) layers, while necessary for battery stability, often exhibit poor ionic conductivity in solid-state systems. The chemical composition and morphology of these interphases significantly affect charge transfer kinetics and overall cell performance, yet controlling their formation remains challenging in polymer-based systems.
Space charge layers at interfaces further complicate electrode kinetics. The accumulation of charged species at the SPE-electrode boundary creates localized electric fields that can impede ion migration. This phenomenon is particularly problematic with high-voltage cathode materials, where space charge effects can dramatically increase activation barriers for lithium-ion transport.
Mechanical stress during cycling presents another significant hurdle. The mismatch in mechanical properties between rigid electrodes and relatively soft polymer electrolytes leads to stress concentration at interfaces. This mechanical instability not only affects contact quality but can also accelerate degradation mechanisms that further compromise electrode kinetics.
Temperature sensitivity compounds these challenges, as most polymer electrolytes exhibit strong temperature-dependent conductivity. At lower temperatures, reduced chain mobility severely limits ion transport at interfaces, creating a significant activation barrier for electrode reactions. This temperature dependence restricts the practical operating window of SPE-based batteries.
Recent research has identified lithium dendrite growth at the anode-electrolyte interface as another critical concern. Despite the mechanical resistance offered by solid electrolytes, non-uniform lithium deposition can still occur at high current densities, penetrating through polymer matrices and potentially causing short circuits while disrupting interfacial kinetics.
Current SPE-Electrode Interface Solutions
01 Polymer electrolyte composition for improved electrode kinetics
Specific polymer electrolyte compositions can significantly enhance electrode kinetics in battery systems. These compositions typically include polymers with high ionic conductivity, such as polyethylene oxide (PEO) derivatives, combined with lithium salts. The addition of plasticizers and ceramic fillers can further improve the ionic conductivity and interfacial properties, leading to better electrode reaction rates and overall battery performance.- Polymer electrolyte composition for improved electrode kinetics: Specific polymer electrolyte compositions can significantly enhance electrode kinetics in battery systems. These compositions typically include polymers with high ionic conductivity, such as polyethylene oxide (PEO) derivatives, combined with lithium salts. The addition of plasticizers and ceramic fillers can further improve the ionic conductivity and interfacial properties, leading to better electrode reaction rates and overall battery performance.
- Interface engineering for solid polymer electrolytes: Engineering the interface between solid polymer electrolytes and electrodes is crucial for optimizing electrode kinetics. Techniques include surface modification of electrodes, incorporation of interfacial layers, and development of composite interfaces. These approaches reduce interfacial resistance, enhance ion transport across the interface, and improve the electrochemical stability of the system, resulting in better rate capability and cycling performance.
- Temperature effects on solid polymer electrolyte performance: Temperature significantly influences the electrode kinetics in solid polymer electrolyte systems. At elevated temperatures, polymer chain mobility increases, leading to enhanced ionic conductivity and faster electrode reactions. Various approaches to optimize temperature-dependent performance include the development of thermally stable polymer matrices, incorporation of temperature-responsive additives, and design of electrolytes with lower glass transition temperatures.
- Composite and gel polymer electrolytes for enhanced kinetics: Composite and gel polymer electrolytes offer improved electrode kinetics compared to conventional solid polymer electrolytes. These systems combine polymers with inorganic fillers, liquid electrolytes, or ionic liquids to create hybrid structures with enhanced ionic conductivity and mechanical properties. The resulting materials provide better electrode-electrolyte contact, reduced interfacial resistance, and improved ion transport pathways, leading to superior electrochemical performance.
- Novel polymer architectures for fast electrode kinetics: Advanced polymer architectures are being developed to address the limitations of traditional solid polymer electrolytes in terms of electrode kinetics. These include block copolymers, cross-linked networks, single-ion conducting polymers, and polymers with specialized functional groups. These novel structures provide optimized ion transport channels, enhanced mechanical stability, and improved electrode compatibility, resulting in faster electrode reactions and better overall battery performance.
02 Interface engineering for solid polymer electrolytes
Engineering the interface between solid polymer electrolytes and electrodes is crucial for optimizing electrode kinetics. Various approaches include surface modification of electrodes, incorporation of interlayers, and development of gradient electrolyte structures. These techniques aim to reduce interfacial resistance, enhance ion transport across interfaces, and improve the electrochemical stability of the electrode-electrolyte interface, resulting in faster electrode reactions.Expand Specific Solutions03 Temperature effects on polymer electrolyte electrode kinetics
Temperature significantly influences the electrode kinetics in solid polymer electrolyte systems. At elevated temperatures, polymer chain mobility increases, leading to enhanced ionic conductivity and faster electrode reactions. Various approaches to optimize temperature-dependent performance include the development of thermally stable polymer matrices, incorporation of temperature-responsive additives, and design of composite structures that maintain good electrode kinetics across a wide temperature range.Expand Specific Solutions04 Composite and gel polymer electrolytes for enhanced kinetics
Composite and gel polymer electrolytes offer improved electrode kinetics compared to conventional solid polymer electrolytes. These systems typically combine polymer matrices with liquid electrolytes, ceramic fillers, or conductive nanoparticles. The resulting hybrid structures provide enhanced ionic pathways, reduced interfacial resistance, and improved mechanical stability, leading to faster electrode reactions and better overall electrochemical performance in battery applications.Expand Specific Solutions05 Novel polymer architectures for accelerated electrode processes
Advanced polymer architectures, including block copolymers, crosslinked networks, and functionalized polymers, can significantly enhance electrode kinetics. These novel structures create optimized ion transport channels, improve polymer-electrode interactions, and maintain mechanical integrity during cycling. Strategic design of polymer chain configurations and functional groups can facilitate faster ion movement at the electrode interface, resulting in improved rate capability and power density in solid-state battery systems.Expand Specific Solutions
Key Industry Players in SPE Development
The solid polymer electrolyte (SPE) market in lithium-ion cells is currently in a growth phase, with increasing adoption driven by demands for safer and higher-energy-density batteries. The global market size is expanding rapidly, projected to reach significant value as electric vehicle adoption accelerates. Technologically, SPEs are advancing from early-stage development toward commercial viability, with varying degrees of maturity. Leading players like LG Energy Solution, LG Chem, and Panasonic are investing heavily in R&D to overcome key challenges in ionic conductivity and electrode interface stability. Academic institutions including University of California and Kyoto University collaborate with industrial partners such as Sumitomo Chemical and Wildcat Discovery Technologies to bridge fundamental research with commercial applications. The competitive landscape features automotive manufacturers (Nissan, Volkswagen, Honda) integrating vertically to secure battery technology advantages.
LG Chem Ltd.
Technical Solution: LG Chem has developed a composite solid polymer electrolyte (SPE) system that combines polyethylene oxide (PEO) with ceramic fillers such as Al2O3 and Li7La3Zr2O12 (LLZO). Their approach focuses on enhancing ionic conductivity while maintaining mechanical stability. The company has implemented a cross-linking technique that creates a three-dimensional network structure within the polymer matrix, which significantly improves the mechanical properties without compromising ion transport. Their latest SPE formulations achieve ionic conductivities of 10^-4 S/cm at room temperature[1], addressing one of the key limitations of traditional polymer electrolytes. LG Chem has also developed surface modification techniques for the ceramic fillers to improve their compatibility with the polymer matrix, resulting in more homogeneous distribution and enhanced interfacial properties between the electrolyte and electrodes[3].
Strengths: Superior mechanical stability compared to liquid electrolytes, enhanced safety profile with reduced flammability, and excellent electrochemical stability window (>4.5V). Weaknesses: Still faces challenges with lower ionic conductivity at room temperature compared to liquid electrolytes, and manufacturing scalability issues for mass production of uniform polymer-ceramic composite films.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has pioneered a dual-phase solid polymer electrolyte system that combines a high molecular weight PEO backbone with low molecular weight PEG plasticizers and lithium salts (LiTFSI). Their proprietary technology incorporates nano-sized ceramic fillers (SiO2 and TiO2) that create favorable Li+ transport pathways at the polymer-ceramic interfaces. The company has developed a gradient concentration approach where the polymer electrolyte composition varies across its thickness to optimize both electrode interfaces simultaneously. Their latest generation SPEs demonstrate ionic conductivities reaching 5×10^-4 S/cm at 30°C[2] and maintain stable cycling performance over 1000 cycles with minimal capacity degradation. LG Energy Solution has also implemented a surface coating technology for cathode materials that improves compatibility with their polymer electrolytes, reducing interfacial resistance and enhancing rate capability[4].
Strengths: Excellent cycling stability with minimal capacity fade, good mechanical properties that help prevent lithium dendrite growth, and compatibility with high-voltage cathode materials. Weaknesses: Manufacturing complexity of gradient structures increases production costs, and the system still requires elevated temperatures (>40°C) for optimal performance in high-power applications.
Critical SPE-Electrode Kinetics Research Breakthroughs
Solid polymer electrolyte, method for manufacturing same, and lithium polymer secondary battery manufactured using same
PatentWO2012099331A2
Innovation
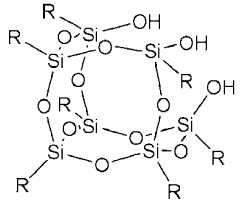
- A solid polymer electrolyte is developed by incorporating polyhedral oligomer silsesquioxane with a hydroxyl group into polyethylene oxide or polypropylene oxide, along with lithium salts and a carbonate compound, to enhance mechanical strength and reduce crystallinity, thereby improving ionic conductivity.
Solid polymer electrolyte composition, and solid polymer electrolyte containing same
PatentWO2020060292A1
Innovation
- A solid polymer electrolyte composition comprising a polymer with alkylene oxide and a reactive double bond, a multifunctional crosslinkable polymer, and an ionic liquid with an amide-based solvent and lithium salt, which is photocured to enhance ionic conductivity, mechanical strength, and flame retardancy.
Safety and Performance Trade-offs in SPE Implementation
The implementation of Solid Polymer Electrolytes (SPEs) in lithium-ion cells presents a complex landscape of safety advantages and performance challenges that must be carefully balanced. SPEs offer significant safety improvements over traditional liquid electrolytes, primarily due to their non-flammable nature and mechanical stability. This characteristic substantially reduces the risk of thermal runaway events and electrolyte leakage, addressing critical safety concerns that have plagued conventional lithium-ion battery technologies.
However, these safety benefits come with notable performance trade-offs. The ionic conductivity of most polymer electrolytes remains significantly lower than liquid counterparts at room temperature, typically 10^-5 to 10^-4 S/cm versus 10^-3 S/cm for liquid electrolytes. This conductivity limitation directly impacts power density and rate capability, restricting the application of SPE-based cells in high-power scenarios.
The mechanical properties of SPEs present another dimension to this trade-off equation. While increased mechanical strength enhances safety and prevents dendrite formation, it simultaneously creates challenges for electrode-electrolyte interfacial contact. Poor interfacial contact increases charge transfer resistance, negatively affecting electrode kinetics and overall cell performance.
Temperature dependence represents a critical factor in this safety-performance balance. Many SPEs exhibit adequate conductivity only at elevated temperatures (>60°C), which necessitates thermal management systems that add complexity, weight, and cost to battery designs. This requirement partially offsets the safety advantages gained from the non-flammable nature of SPEs.
Cycling stability presents another nuanced trade-off. SPEs typically demonstrate excellent electrochemical stability windows, supporting long-term cycling. However, the formation of resistive interfacial layers during cycling can progressively degrade performance, particularly at the cathode interface where oxidative decomposition may occur.
Manufacturing considerations further complicate implementation decisions. SPE-based cells often allow for simplified production processes and potentially enable novel cell designs like bipolar stacking. These advantages must be weighed against the challenges of achieving consistent electrolyte thickness and uniform contact with electrode surfaces at scale.
Recent research has focused on composite and hybrid electrolyte systems that aim to optimize this safety-performance balance. Incorporating ceramic fillers or creating polymer-ceramic composite electrolytes has shown promise in enhancing both ionic conductivity and mechanical properties simultaneously, potentially offering pathways to mitigate these trade-offs in next-generation battery designs.
However, these safety benefits come with notable performance trade-offs. The ionic conductivity of most polymer electrolytes remains significantly lower than liquid counterparts at room temperature, typically 10^-5 to 10^-4 S/cm versus 10^-3 S/cm for liquid electrolytes. This conductivity limitation directly impacts power density and rate capability, restricting the application of SPE-based cells in high-power scenarios.
The mechanical properties of SPEs present another dimension to this trade-off equation. While increased mechanical strength enhances safety and prevents dendrite formation, it simultaneously creates challenges for electrode-electrolyte interfacial contact. Poor interfacial contact increases charge transfer resistance, negatively affecting electrode kinetics and overall cell performance.
Temperature dependence represents a critical factor in this safety-performance balance. Many SPEs exhibit adequate conductivity only at elevated temperatures (>60°C), which necessitates thermal management systems that add complexity, weight, and cost to battery designs. This requirement partially offsets the safety advantages gained from the non-flammable nature of SPEs.
Cycling stability presents another nuanced trade-off. SPEs typically demonstrate excellent electrochemical stability windows, supporting long-term cycling. However, the formation of resistive interfacial layers during cycling can progressively degrade performance, particularly at the cathode interface where oxidative decomposition may occur.
Manufacturing considerations further complicate implementation decisions. SPE-based cells often allow for simplified production processes and potentially enable novel cell designs like bipolar stacking. These advantages must be weighed against the challenges of achieving consistent electrolyte thickness and uniform contact with electrode surfaces at scale.
Recent research has focused on composite and hybrid electrolyte systems that aim to optimize this safety-performance balance. Incorporating ceramic fillers or creating polymer-ceramic composite electrolytes has shown promise in enhancing both ionic conductivity and mechanical properties simultaneously, potentially offering pathways to mitigate these trade-offs in next-generation battery designs.
Manufacturing Scalability of SPE Technologies
The manufacturing scalability of Solid Polymer Electrolyte (SPE) technologies represents a critical factor in their commercial viability for lithium-ion cell applications. Current manufacturing processes for SPEs face significant challenges when transitioning from laboratory-scale production to industrial-scale manufacturing. Traditional solution casting methods, while effective for research purposes, demonstrate limited throughput and consistency when scaled up.
Industrial implementation of SPE technologies requires adaptation of existing battery production lines, which have been optimized for liquid electrolyte systems. The integration of solid polymer electrolytes necessitates modifications to electrode coating processes, assembly techniques, and quality control protocols. These adaptations often require substantial capital investment, creating barriers to widespread adoption.
Material supply chains for high-performance polymer electrolytes remain underdeveloped compared to conventional liquid electrolyte components. The specialized polymers, lithium salts, and additives required for advanced SPEs are frequently produced in limited quantities at premium prices. Establishing robust supply networks capable of delivering consistent, high-quality materials at industrial scale represents a significant hurdle.
Process optimization for SPE manufacturing presents unique challenges related to thickness control, interfacial contact, and defect minimization. Current extrusion and roll-to-roll processing techniques struggle to produce uniform polymer electrolyte films with the necessary mechanical properties while maintaining electrochemical performance. The trade-off between manufacturing speed and product quality remains a central concern.
Quality assurance methodologies for SPE production require further development. Unlike liquid electrolytes, which can be tested through established analytical techniques, solid polymer electrolytes demand specialized testing protocols to verify ionic conductivity, mechanical integrity, and electrochemical stability at production scale. In-line monitoring systems capable of detecting defects in polymer films are still evolving.
Cost considerations significantly impact the scalability of SPE technologies. Current manufacturing approaches for high-performance polymer electrolytes typically result in production costs 3-5 times higher than conventional liquid electrolyte systems. Achieving cost parity will require innovations in materials, processing techniques, and equipment design specifically tailored to SPE production requirements.
Recent advancements in additive manufacturing and continuous processing technologies offer promising pathways to improve SPE manufacturing scalability. These approaches potentially enable more precise control over electrolyte morphology and interfacial properties while reducing material waste and processing steps. However, these technologies remain in early development stages for battery applications.
Industrial implementation of SPE technologies requires adaptation of existing battery production lines, which have been optimized for liquid electrolyte systems. The integration of solid polymer electrolytes necessitates modifications to electrode coating processes, assembly techniques, and quality control protocols. These adaptations often require substantial capital investment, creating barriers to widespread adoption.
Material supply chains for high-performance polymer electrolytes remain underdeveloped compared to conventional liquid electrolyte components. The specialized polymers, lithium salts, and additives required for advanced SPEs are frequently produced in limited quantities at premium prices. Establishing robust supply networks capable of delivering consistent, high-quality materials at industrial scale represents a significant hurdle.
Process optimization for SPE manufacturing presents unique challenges related to thickness control, interfacial contact, and defect minimization. Current extrusion and roll-to-roll processing techniques struggle to produce uniform polymer electrolyte films with the necessary mechanical properties while maintaining electrochemical performance. The trade-off between manufacturing speed and product quality remains a central concern.
Quality assurance methodologies for SPE production require further development. Unlike liquid electrolytes, which can be tested through established analytical techniques, solid polymer electrolytes demand specialized testing protocols to verify ionic conductivity, mechanical integrity, and electrochemical stability at production scale. In-line monitoring systems capable of detecting defects in polymer films are still evolving.
Cost considerations significantly impact the scalability of SPE technologies. Current manufacturing approaches for high-performance polymer electrolytes typically result in production costs 3-5 times higher than conventional liquid electrolyte systems. Achieving cost parity will require innovations in materials, processing techniques, and equipment design specifically tailored to SPE production requirements.
Recent advancements in additive manufacturing and continuous processing technologies offer promising pathways to improve SPE manufacturing scalability. These approaches potentially enable more precise control over electrolyte morphology and interfacial properties while reducing material waste and processing steps. However, these technologies remain in early development stages for battery applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!