Research on Nanostructured Solid Polymer Electrolytes for Enhanced Performance
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nanostructured SPE Background and Objectives
Solid polymer electrolytes (SPEs) have emerged as a promising alternative to liquid electrolytes in energy storage devices, particularly lithium-ion batteries, due to their enhanced safety features and potential for higher energy density. The evolution of SPE technology began in the 1970s with the discovery of ionic conductivity in polymer-salt complexes, primarily polyethylene oxide (PEO). Over subsequent decades, research has focused on improving the inherently low ionic conductivity of these materials at ambient temperatures, which has been the primary limitation for widespread commercial adoption.
The technological trajectory has seen significant advancements through various approaches, including the development of copolymers, polymer blends, and the incorporation of plasticizers. However, the most promising recent development has been the integration of nanostructured materials into polymer matrices, creating composite systems with enhanced properties. These nanostructured SPEs represent a convergence of polymer science, nanotechnology, and electrochemistry, offering multifunctional benefits beyond simple ionic conductivity enhancement.
Current technological trends indicate a growing focus on tailoring the interface between nanofillers and polymer matrices, understanding ion transport mechanisms at the nanoscale, and developing sustainable manufacturing processes for these advanced materials. The field is moving toward multifunctional electrolytes that simultaneously address multiple challenges including conductivity, mechanical stability, and electrochemical performance.
The primary objectives of research in nanostructured SPEs include achieving room-temperature ionic conductivity exceeding 10^-3 S/cm while maintaining mechanical stability and electrochemical compatibility with electrode materials. Additionally, researchers aim to develop scalable and cost-effective manufacturing processes that can transition these materials from laboratory curiosities to commercial products.
Another critical goal is understanding the fundamental mechanisms by which nanostructures enhance ionic conductivity, including their effects on polymer crystallinity, segmental motion, and the creation of preferential ion transport pathways. This mechanistic understanding is essential for rational design of next-generation materials rather than empirical optimization.
The long-term vision for nanostructured SPEs extends beyond lithium-ion batteries to emerging technologies such as solid-state sodium batteries, lithium-sulfur systems, and flexible/wearable energy storage devices. Success in this field could revolutionize energy storage technology, enabling safer, higher-capacity batteries with longer cycle life and faster charging capabilities, ultimately supporting the global transition to renewable energy and electrified transportation.
The technological trajectory has seen significant advancements through various approaches, including the development of copolymers, polymer blends, and the incorporation of plasticizers. However, the most promising recent development has been the integration of nanostructured materials into polymer matrices, creating composite systems with enhanced properties. These nanostructured SPEs represent a convergence of polymer science, nanotechnology, and electrochemistry, offering multifunctional benefits beyond simple ionic conductivity enhancement.
Current technological trends indicate a growing focus on tailoring the interface between nanofillers and polymer matrices, understanding ion transport mechanisms at the nanoscale, and developing sustainable manufacturing processes for these advanced materials. The field is moving toward multifunctional electrolytes that simultaneously address multiple challenges including conductivity, mechanical stability, and electrochemical performance.
The primary objectives of research in nanostructured SPEs include achieving room-temperature ionic conductivity exceeding 10^-3 S/cm while maintaining mechanical stability and electrochemical compatibility with electrode materials. Additionally, researchers aim to develop scalable and cost-effective manufacturing processes that can transition these materials from laboratory curiosities to commercial products.
Another critical goal is understanding the fundamental mechanisms by which nanostructures enhance ionic conductivity, including their effects on polymer crystallinity, segmental motion, and the creation of preferential ion transport pathways. This mechanistic understanding is essential for rational design of next-generation materials rather than empirical optimization.
The long-term vision for nanostructured SPEs extends beyond lithium-ion batteries to emerging technologies such as solid-state sodium batteries, lithium-sulfur systems, and flexible/wearable energy storage devices. Success in this field could revolutionize energy storage technology, enabling safer, higher-capacity batteries with longer cycle life and faster charging capabilities, ultimately supporting the global transition to renewable energy and electrified transportation.
Market Analysis for Advanced Battery Electrolytes
The global market for advanced battery electrolytes is experiencing unprecedented growth, primarily driven by the expanding electric vehicle (EV) sector and increasing demand for high-performance energy storage solutions. The market value for advanced electrolytes reached approximately $4.1 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 15.7% through 2030, potentially reaching $12.5 billion by the end of the forecast period.
Solid polymer electrolytes (SPEs), particularly nanostructured variants, represent one of the fastest-growing segments within this market. Their appeal stems from enhanced safety profiles compared to traditional liquid electrolytes, eliminating leakage risks and reducing flammability concerns that have plagued conventional lithium-ion batteries. This safety advantage is becoming increasingly critical as battery energy densities continue to rise.
Regional analysis reveals Asia-Pacific as the dominant market for advanced electrolytes, accounting for approximately 45% of global demand. This dominance is attributed to the concentration of battery manufacturing facilities in China, South Korea, and Japan. North America and Europe follow with market shares of 28% and 22% respectively, with both regions showing accelerated growth rates as they establish domestic battery supply chains to reduce dependence on Asian manufacturers.
Consumer electronics currently represents the largest application segment for advanced electrolytes at 38% market share, though automotive applications are growing at the fastest rate and are expected to become the dominant segment by 2026. Grid storage applications constitute a smaller but rapidly expanding market segment, projected to grow at a CAGR of 21% through 2030.
Key market drivers include stringent government regulations promoting electric mobility, continuous improvements in battery performance requirements, and declining costs of advanced materials. The push for higher energy density batteries with faster charging capabilities has particularly accelerated interest in nanostructured polymer electrolytes, which can facilitate improved ion transport while maintaining structural integrity.
Market challenges include high production costs compared to conventional liquid electrolytes, scaling manufacturing processes for consistent quality, and competition from other emerging electrolyte technologies such as ceramic and glass-based solid electrolytes. The cost premium for nanostructured SPEs currently ranges from 30-40% above conventional electrolytes, though this gap is narrowing as production scales increase.
Customer demand patterns indicate growing preference for batteries with longer cycle life and improved safety characteristics, even at premium price points. This trend is particularly pronounced in high-end consumer electronics and luxury electric vehicles, creating market entry opportunities for advanced nanostructured electrolyte technologies despite their current cost disadvantages.
Solid polymer electrolytes (SPEs), particularly nanostructured variants, represent one of the fastest-growing segments within this market. Their appeal stems from enhanced safety profiles compared to traditional liquid electrolytes, eliminating leakage risks and reducing flammability concerns that have plagued conventional lithium-ion batteries. This safety advantage is becoming increasingly critical as battery energy densities continue to rise.
Regional analysis reveals Asia-Pacific as the dominant market for advanced electrolytes, accounting for approximately 45% of global demand. This dominance is attributed to the concentration of battery manufacturing facilities in China, South Korea, and Japan. North America and Europe follow with market shares of 28% and 22% respectively, with both regions showing accelerated growth rates as they establish domestic battery supply chains to reduce dependence on Asian manufacturers.
Consumer electronics currently represents the largest application segment for advanced electrolytes at 38% market share, though automotive applications are growing at the fastest rate and are expected to become the dominant segment by 2026. Grid storage applications constitute a smaller but rapidly expanding market segment, projected to grow at a CAGR of 21% through 2030.
Key market drivers include stringent government regulations promoting electric mobility, continuous improvements in battery performance requirements, and declining costs of advanced materials. The push for higher energy density batteries with faster charging capabilities has particularly accelerated interest in nanostructured polymer electrolytes, which can facilitate improved ion transport while maintaining structural integrity.
Market challenges include high production costs compared to conventional liquid electrolytes, scaling manufacturing processes for consistent quality, and competition from other emerging electrolyte technologies such as ceramic and glass-based solid electrolytes. The cost premium for nanostructured SPEs currently ranges from 30-40% above conventional electrolytes, though this gap is narrowing as production scales increase.
Customer demand patterns indicate growing preference for batteries with longer cycle life and improved safety characteristics, even at premium price points. This trend is particularly pronounced in high-end consumer electronics and luxury electric vehicles, creating market entry opportunities for advanced nanostructured electrolyte technologies despite their current cost disadvantages.
Current Challenges in Solid Polymer Electrolyte Technology
Despite significant advancements in solid polymer electrolyte (SPE) technology, several critical challenges continue to impede their widespread commercial adoption in energy storage applications. The primary limitation remains insufficient ionic conductivity at ambient temperatures, with most conventional SPEs exhibiting conductivities below 10^-4 S/cm at room temperature, significantly lower than the 10^-3 S/cm threshold required for practical applications. This conductivity limitation stems from the semi-crystalline nature of polymer matrices, where crystalline regions restrict ion mobility.
Another persistent challenge is the poor mechanical stability of high-conductivity SPEs. Systems that achieve enhanced ionic conductivity often sacrifice mechanical integrity, creating a fundamental conductivity-mechanical strength trade-off. This compromise becomes particularly problematic in lithium metal battery applications, where electrolyte mechanical strength is crucial for preventing dendrite formation and ensuring long-term cycling stability.
The electrode-electrolyte interfacial resistance presents another significant hurdle. Solid polymer electrolytes typically form high-resistance interfaces with electrode materials, particularly cathodes, leading to increased cell impedance and reduced power density. This interfacial challenge is exacerbated by the limited electrochemical stability window of many polymer systems, which restricts their compatibility with high-voltage cathode materials.
Long-term cycling stability remains problematic, with many nanostructured SPEs showing performance degradation over extended cycling. This degradation often results from polymer chain reorganization, nanoparticle agglomeration, or chemical degradation at interfaces. Additionally, the thermal stability of polymer-based electrolytes under extreme temperature conditions continues to be a concern for safety-critical applications.
Manufacturing scalability presents significant technical barriers, particularly for nanostructured SPEs. Current laboratory-scale synthesis methods for advanced nanocomposite electrolytes often involve complex, multi-step processes that are challenging to scale to industrial production levels. The precise control of nanostructure morphology and uniform dispersion of functional additives becomes increasingly difficult at larger scales.
From a commercial perspective, the cost-performance ratio of advanced SPEs remains unfavorable compared to conventional liquid electrolytes. The specialized polymers, nanomaterials, and complex processing techniques required for high-performance SPEs significantly increase production costs, creating market entry barriers despite their potential safety advantages.
Addressing these interconnected challenges requires interdisciplinary approaches that simultaneously target ionic conductivity enhancement, mechanical reinforcement, interfacial engineering, and manufacturing scalability to develop commercially viable nanostructured solid polymer electrolytes.
Another persistent challenge is the poor mechanical stability of high-conductivity SPEs. Systems that achieve enhanced ionic conductivity often sacrifice mechanical integrity, creating a fundamental conductivity-mechanical strength trade-off. This compromise becomes particularly problematic in lithium metal battery applications, where electrolyte mechanical strength is crucial for preventing dendrite formation and ensuring long-term cycling stability.
The electrode-electrolyte interfacial resistance presents another significant hurdle. Solid polymer electrolytes typically form high-resistance interfaces with electrode materials, particularly cathodes, leading to increased cell impedance and reduced power density. This interfacial challenge is exacerbated by the limited electrochemical stability window of many polymer systems, which restricts their compatibility with high-voltage cathode materials.
Long-term cycling stability remains problematic, with many nanostructured SPEs showing performance degradation over extended cycling. This degradation often results from polymer chain reorganization, nanoparticle agglomeration, or chemical degradation at interfaces. Additionally, the thermal stability of polymer-based electrolytes under extreme temperature conditions continues to be a concern for safety-critical applications.
Manufacturing scalability presents significant technical barriers, particularly for nanostructured SPEs. Current laboratory-scale synthesis methods for advanced nanocomposite electrolytes often involve complex, multi-step processes that are challenging to scale to industrial production levels. The precise control of nanostructure morphology and uniform dispersion of functional additives becomes increasingly difficult at larger scales.
From a commercial perspective, the cost-performance ratio of advanced SPEs remains unfavorable compared to conventional liquid electrolytes. The specialized polymers, nanomaterials, and complex processing techniques required for high-performance SPEs significantly increase production costs, creating market entry barriers despite their potential safety advantages.
Addressing these interconnected challenges requires interdisciplinary approaches that simultaneously target ionic conductivity enhancement, mechanical reinforcement, interfacial engineering, and manufacturing scalability to develop commercially viable nanostructured solid polymer electrolytes.
State-of-the-Art Nanostructured SPE Solutions
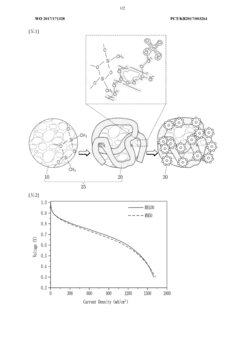
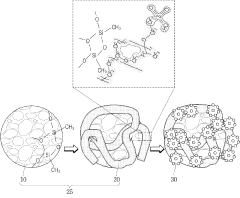
01 Nanostructured polymer electrolytes with enhanced ionic conductivity
Nanostructured solid polymer electrolytes can be designed to enhance ionic conductivity through the incorporation of nanoscale structures that create efficient ion transport pathways. These electrolytes typically feature polymer matrices with nanoscale domains that facilitate ion movement while maintaining mechanical stability. The nanostructuring can involve block copolymers that self-assemble into ordered morphologies, creating dedicated ion-conducting channels that significantly improve performance in battery applications.- Nanostructured polymer electrolytes with enhanced ionic conductivity: Nanostructured solid polymer electrolytes can be designed to enhance ionic conductivity through the incorporation of nanoscale materials or structures. These electrolytes typically feature optimized ion transport pathways, reduced crystallinity, and improved interfacial properties. The nanostructuring approach allows for higher lithium-ion mobility while maintaining mechanical stability, resulting in superior electrochemical performance for battery applications.
- Composite polymer electrolytes with inorganic nanofillers: Incorporating inorganic nanofillers such as ceramic nanoparticles, metal oxides, or silica into polymer matrices creates composite electrolytes with enhanced mechanical and electrochemical properties. These nanofillers can improve the amorphous phase content of the polymer, create additional ion transport pathways, and enhance the interfacial stability between the electrolyte and electrodes. The resulting nanocomposite electrolytes demonstrate higher ionic conductivity, better thermal stability, and improved electrochemical performance.
- Block copolymer-based nanostructured electrolytes: Block copolymers can self-assemble into nanoscale domains, creating well-defined ion-conducting channels that enhance ionic transport. These nanostructured electrolytes combine mechanically robust blocks with ion-conducting blocks to achieve both high mechanical strength and excellent ionic conductivity. The morphology and performance of these electrolytes can be tuned by adjusting the molecular weight, composition, and processing conditions of the block copolymers.
- Crosslinked polymer networks with nanoscale architecture: Crosslinked polymer networks with controlled nanoscale architecture offer improved mechanical properties while maintaining high ionic conductivity. These systems typically involve chemical or physical crosslinking strategies that create a three-dimensional network with optimized free volume for ion transport. The crosslinking density and distribution can be engineered to balance mechanical strength and ion mobility, resulting in solid polymer electrolytes with enhanced electrochemical stability and cycling performance.
- Surface-modified nanostructured polymer electrolytes: Surface modification of nanostructured polymer electrolytes can significantly improve their interfacial properties and overall performance. Techniques such as plasma treatment, chemical functionalization, or coating with specialized materials can enhance the compatibility between the electrolyte and electrodes, reduce interfacial resistance, and improve cycling stability. These modifications often target the reduction of unwanted side reactions and the formation of stable solid-electrolyte interfaces, leading to better long-term battery performance.
02 Ceramic/polymer composite electrolytes for improved stability
Incorporating ceramic nanoparticles into polymer electrolyte matrices creates composite systems with enhanced thermal and electrochemical stability. These nanostructured composites combine the flexibility of polymers with the rigidity and stability of ceramic materials. The ceramic nanofillers can improve the mechanical properties of the electrolyte while also enhancing ionic conductivity through interactions at the polymer-ceramic interfaces. This approach addresses the mechanical weakness often associated with pure polymer electrolytes while maintaining good electrochemical performance.Expand Specific Solutions03 Cross-linked polymer networks for enhanced mechanical properties
Cross-linked nanostructured polymer electrolytes offer improved mechanical strength and dimensional stability while maintaining adequate ionic conductivity. The cross-linking creates a three-dimensional network that restricts polymer chain mobility, reducing crystallinity and enhancing amorphous regions where ion transport occurs. These systems can be designed with controlled cross-link density to balance mechanical properties with electrochemical performance, making them suitable for applications requiring robust electrolytes that can withstand physical stresses.Expand Specific Solutions04 Polymer blends and copolymers for optimized performance
Blending different polymers or using copolymer systems creates nanostructured electrolytes with tailored properties. These systems can combine polymers with complementary characteristics to achieve an optimal balance of ionic conductivity, mechanical strength, and electrochemical stability. Block copolymers can self-assemble into nanoscale domains that create dedicated pathways for ion transport while maintaining structural integrity. The composition and architecture of these polymer systems can be precisely engineered to meet specific performance requirements.Expand Specific Solutions05 Functionalized nanofillers for interface engineering
Surface-modified or functionalized nanofillers can be incorporated into polymer electrolytes to engineer the interfaces between components. These functionalized particles create favorable interactions with both the polymer matrix and mobile ions, reducing interfacial resistance and enhancing overall performance. The surface chemistry of nanofillers can be tailored to improve compatibility with the polymer host, prevent aggregation, and create additional ion transport pathways. This approach enables precise control over the nanostructure of the electrolyte system and optimizes its electrochemical properties.Expand Specific Solutions
Leading Research Groups and Industrial Stakeholders
The nanostructured solid polymer electrolyte (SPE) market is currently in a growth phase, with increasing demand driven by the expanding electric vehicle and energy storage sectors. The global market is projected to reach significant scale as battery technologies evolve toward safer, higher-performance solutions. Leading companies like BYD, JSR Corp., and Honda Motor are advancing commercial applications, while research institutions such as KAIST, Lawrence Livermore National Security, and CSIR are developing next-generation technologies. The competitive landscape features established chemical manufacturers (ZEON Corp., Nippon Soda, LOTTE Chemical) alongside specialized players like Blue Solutions Canada and Seeo. Technical challenges remain in balancing ionic conductivity, mechanical stability, and electrochemical performance, with recent innovations focusing on composite systems and novel polymer architectures to overcome traditional limitations.
BYD Co., Ltd.
Technical Solution: BYD has developed advanced nanostructured solid polymer electrolytes (SPEs) incorporating ceramic nanoparticles into polymer matrices to create hybrid electrolytes. Their proprietary technology utilizes polyvinylidene fluoride (PVDF) and polyethylene oxide (PEO) based matrices with nano-sized ceramic fillers such as Al2O3, SiO2, and TiO2 to enhance ionic conductivity while maintaining mechanical stability. BYD's approach includes surface modification of nanoparticles to improve compatibility with polymer matrices and reduce interfacial resistance. Their SPEs demonstrate ionic conductivities exceeding 10^-4 S/cm at room temperature while maintaining excellent electrochemical stability windows up to 4.5V. BYD has integrated these electrolytes into their commercial battery systems, particularly in their Blade Battery technology, enabling safer and more energy-dense battery solutions.
Strengths: Vertical integration allowing direct implementation in commercial products; established manufacturing infrastructure for scale-up; proprietary surface modification techniques for nanoparticles. Weaknesses: Higher production costs compared to liquid electrolytes; challenges in maintaining consistent quality across large-scale production; temperature-dependent performance limitations.
Seeo, Inc.
Technical Solution: Seeo (acquired by Bosch in 2015) developed proprietary nanostructured solid polymer electrolytes based on polystyrene-polyethylene oxide-polystyrene (PS-PEO-PS) block copolymers. Their technology creates self-assembled nanostructures with distinct mechanical and ion-conducting domains, where rigid polystyrene blocks provide structural integrity while PEO domains facilitate lithium-ion transport. Seeo's approach incorporates lithium salts (LiTFSI) and proprietary additives to enhance ionic conductivity while maintaining dimensional stability. Their electrolytes feature nanoscale ceramic fillers (5-15 nm) with tailored surface chemistry to improve interfacial compatibility and reduce resistance at electrode interfaces. The company demonstrated ionic conductivities exceeding 10^-4 S/cm at room temperature with excellent electrochemical stability up to 4.5V. Seeo's technology enables the fabrication of thin-film solid electrolytes (15-30 μm) with sufficient mechanical properties to inhibit lithium dendrite growth while maintaining flexibility for roll-to-roll processing.
Strengths: Highly scalable manufacturing process compatible with existing production equipment; excellent mechanical properties preventing dendrite formation; good electrochemical stability. Weaknesses: Lower ionic conductivity compared to liquid electrolytes, particularly at lower temperatures; challenges in achieving consistent electrode-electrolyte interfaces; higher material costs compared to conventional separators.
Key Patents and Scientific Breakthroughs in SPE Design
Polymer solid electrolyte composition for lithium secondary battery, and use thereof
PatentWO2024117623A1
Innovation
- A polymer solid electrolyte composition incorporating a mesoporous tungsten oxynitride nanomaterial with specific composition and structure, combined with a polymer component, lithium salt, and organic solvent, which enhances electrical conductivity and mechanical strength by controlling the content and form of the mesoporous tungsten oxynitride nanomaterial.
Nanostructured electrode for polymer electrolyte membrane fuel cell, and manufacturing method therefor
PatentWO2017171328A1
Innovation
- A nanostructured electrode is developed, featuring a three-dimensional nanostructure composed of nanoporous airgel and an ionomer with a catalyst dispersed within, where the airgel serves as a template for the ionomer to form a nanostructure, enhancing catalyst utilization and mass transfer efficiency.
Safety and Stability Considerations for Commercial Applications
The commercialization of nanostructured solid polymer electrolytes (NSPEs) requires rigorous safety and stability assessments to ensure reliable performance in real-world applications. Thermal stability remains a primary concern, as commercial battery systems must operate across wide temperature ranges (-20°C to 60°C) while maintaining consistent ionic conductivity. Current NSPEs exhibit promising stability at moderate temperatures but often experience performance degradation at temperature extremes, particularly above 80°C where polymer chain reorganization can compromise mechanical integrity.
Chemical stability presents another critical challenge, as NSPEs must resist oxidation at the cathode interface (typically >4V vs. Li/Li+) and reduction at the anode interface (<1V vs. Li/Li+). Long-term exposure to these electrochemical environments can trigger degradation pathways that release harmful byproducts or compromise electrolyte functionality. Recent research incorporating fluorinated polymers and ceramic nanofillers has demonstrated improved electrochemical stability windows approaching 4.5V, though this remains below the 5V threshold desired for next-generation high-voltage cathode materials.
Mechanical stability during cycling represents a significant hurdle for commercial viability. NSPEs must maintain intimate electrode contact through thousands of charge-discharge cycles while accommodating volumetric changes in electrode materials. Current systems typically show acceptable performance for 500-1000 cycles, falling short of the 2000+ cycles required for automotive applications. The incorporation of self-healing polymers and dynamic crosslinking strategies shows promise for addressing this limitation.
Manufacturing scalability introduces additional safety considerations, as production processes must transition from laboratory-scale synthesis to industrial volumes while maintaining precise nanostructure control. Solvent-free processing methods are gaining attention to eliminate flammable processing chemicals and reduce environmental impact, though these approaches often require higher processing temperatures that may introduce thermal degradation risks.
Regulatory compliance frameworks for NSPE-based devices remain underdeveloped, creating uncertainty for commercial deployment. Current safety standards (IEC 62133, UL 1642) were established for liquid electrolyte systems and may not adequately address the unique failure modes of solid-state systems. Industry consortia are actively developing specialized testing protocols that evaluate dendrite resistance, interfacial stability, and mechanical robustness under abuse conditions to establish appropriate safety certification pathways for NSPE technologies.
Chemical stability presents another critical challenge, as NSPEs must resist oxidation at the cathode interface (typically >4V vs. Li/Li+) and reduction at the anode interface (<1V vs. Li/Li+). Long-term exposure to these electrochemical environments can trigger degradation pathways that release harmful byproducts or compromise electrolyte functionality. Recent research incorporating fluorinated polymers and ceramic nanofillers has demonstrated improved electrochemical stability windows approaching 4.5V, though this remains below the 5V threshold desired for next-generation high-voltage cathode materials.
Mechanical stability during cycling represents a significant hurdle for commercial viability. NSPEs must maintain intimate electrode contact through thousands of charge-discharge cycles while accommodating volumetric changes in electrode materials. Current systems typically show acceptable performance for 500-1000 cycles, falling short of the 2000+ cycles required for automotive applications. The incorporation of self-healing polymers and dynamic crosslinking strategies shows promise for addressing this limitation.
Manufacturing scalability introduces additional safety considerations, as production processes must transition from laboratory-scale synthesis to industrial volumes while maintaining precise nanostructure control. Solvent-free processing methods are gaining attention to eliminate flammable processing chemicals and reduce environmental impact, though these approaches often require higher processing temperatures that may introduce thermal degradation risks.
Regulatory compliance frameworks for NSPE-based devices remain underdeveloped, creating uncertainty for commercial deployment. Current safety standards (IEC 62133, UL 1642) were established for liquid electrolyte systems and may not adequately address the unique failure modes of solid-state systems. Industry consortia are actively developing specialized testing protocols that evaluate dendrite resistance, interfacial stability, and mechanical robustness under abuse conditions to establish appropriate safety certification pathways for NSPE technologies.
Environmental Impact and Sustainability Assessment
The development and implementation of nanostructured solid polymer electrolytes (NSPEs) present significant environmental implications that warrant thorough assessment. Traditional lithium-ion batteries containing liquid electrolytes pose substantial environmental hazards due to their flammability, toxicity, and potential for leakage. NSPEs offer a promising alternative with reduced environmental impact through elimination of volatile organic solvents and decreased risk of hazardous material release during production, use, and disposal phases.
Life cycle assessment (LCA) studies indicate that NSPEs can potentially reduce the carbon footprint of battery production by 15-30% compared to conventional liquid electrolyte systems. This reduction stems primarily from simplified manufacturing processes and decreased energy requirements during production. Additionally, the extended cycle life and enhanced thermal stability of NSPEs contribute to longer battery lifespans, thereby reducing electronic waste generation and resource consumption associated with battery replacement.
The raw materials used in NSPE production present both challenges and opportunities from a sustainability perspective. While some nanomaterials require energy-intensive processing, many polymer matrices can be derived from renewable sources or recycled materials. Recent innovations have demonstrated successful incorporation of bio-based polymers and environmentally benign nanofillers, reducing dependence on petroleum-derived compounds and critical minerals.
End-of-life management represents a crucial consideration for NSPE technology. Current research indicates that solid polymer electrolytes may facilitate more efficient recycling processes compared to liquid systems. The structural stability of NSPEs allows for more straightforward separation and recovery of valuable battery components, potentially increasing recycling yields by up to 25% and reducing hazardous waste generation during recycling operations.
Water consumption and pollution metrics also favor NSPE technology. Manufacturing processes for these materials typically require 40-60% less water than conventional electrolyte production, with significantly reduced wastewater contamination levels. This advantage becomes particularly relevant in regions facing water scarcity challenges or stringent water quality regulations.
Regulatory frameworks worldwide are increasingly emphasizing sustainable battery technologies, creating favorable conditions for NSPE adoption. The European Union's Battery Directive revisions and similar initiatives in North America and Asia are establishing sustainability criteria that inherently favor solid-state technologies with improved environmental profiles. Companies developing NSPEs with demonstrable sustainability advantages may gain competitive advantages through regulatory compliance and access to green technology incentives.
Life cycle assessment (LCA) studies indicate that NSPEs can potentially reduce the carbon footprint of battery production by 15-30% compared to conventional liquid electrolyte systems. This reduction stems primarily from simplified manufacturing processes and decreased energy requirements during production. Additionally, the extended cycle life and enhanced thermal stability of NSPEs contribute to longer battery lifespans, thereby reducing electronic waste generation and resource consumption associated with battery replacement.
The raw materials used in NSPE production present both challenges and opportunities from a sustainability perspective. While some nanomaterials require energy-intensive processing, many polymer matrices can be derived from renewable sources or recycled materials. Recent innovations have demonstrated successful incorporation of bio-based polymers and environmentally benign nanofillers, reducing dependence on petroleum-derived compounds and critical minerals.
End-of-life management represents a crucial consideration for NSPE technology. Current research indicates that solid polymer electrolytes may facilitate more efficient recycling processes compared to liquid systems. The structural stability of NSPEs allows for more straightforward separation and recovery of valuable battery components, potentially increasing recycling yields by up to 25% and reducing hazardous waste generation during recycling operations.
Water consumption and pollution metrics also favor NSPE technology. Manufacturing processes for these materials typically require 40-60% less water than conventional electrolyte production, with significantly reduced wastewater contamination levels. This advantage becomes particularly relevant in regions facing water scarcity challenges or stringent water quality regulations.
Regulatory frameworks worldwide are increasingly emphasizing sustainable battery technologies, creating favorable conditions for NSPE adoption. The European Union's Battery Directive revisions and similar initiatives in North America and Asia are establishing sustainability criteria that inherently favor solid-state technologies with improved environmental profiles. Companies developing NSPEs with demonstrable sustainability advantages may gain competitive advantages through regulatory compliance and access to green technology incentives.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!