Comparison of Single-Ion vs Dual-Ion Solid Polymer Electrolytes
SEP 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Solid Polymer Electrolytes Evolution and Research Objectives
Solid polymer electrolytes (SPEs) have emerged as a promising alternative to liquid electrolytes in energy storage systems, particularly lithium-ion batteries, due to their enhanced safety features and potential for higher energy density. The evolution of SPEs can be traced back to the 1970s when P.V. Wright discovered ionic conductivity in PEO-salt complexes, marking the beginning of a new era in electrolyte research. Since then, the field has witnessed significant advancements, transitioning from simple polymer-salt mixtures to sophisticated architectures incorporating various functional groups and nanofillers.
The development trajectory of SPEs has been characterized by persistent efforts to overcome the inherent trade-off between mechanical strength and ionic conductivity. Early generations of SPEs exhibited limited conductivity at ambient temperatures, restricting their practical applications. However, breakthroughs in polymer chemistry and materials science have gradually improved their performance metrics, leading to the emergence of distinct categories including single-ion and dual-ion polymer electrolytes.
Single-ion polymer electrolytes (SIPEs) represent a significant evolutionary step, where one ion type (typically the anion) is tethered to the polymer backbone, allowing only cations to contribute to charge transport. This design addresses the concentration polarization issues prevalent in conventional dual-ion systems where both cations and anions are mobile. The concept of SIPEs was first proposed in the 1980s, but practical implementations faced numerous challenges related to synthesis complexity and insufficient conductivity.
Dual-ion polymer electrolytes (DIPEs), on the other hand, have dominated the research landscape due to their relatively simpler fabrication processes and higher initial conductivity values. These systems allow both cations and anions to migrate during electrochemical processes, which while beneficial for overall conductivity, introduces complications such as concentration gradients and decreased transference numbers.
Recent technological advancements have significantly narrowed the performance gap between these two approaches. The introduction of novel polymer architectures, such as block copolymers, comb-like structures, and interpenetrating networks, has revolutionized both SIPE and DIPE designs. Additionally, the incorporation of ceramic fillers, ionic liquids, and plasticizers has further enhanced their respective performances.
The primary research objectives in this field now focus on achieving the optimal balance between mechanical integrity, electrochemical stability, and ionic conductivity. Specifically for SIPEs, increasing the lithium-ion transference number to near unity while maintaining adequate conductivity remains a central challenge. For DIPEs, mitigating concentration polarization effects and enhancing interfacial stability with electrodes represent key research priorities.
Understanding the fundamental differences, advantages, and limitations of single-ion versus dual-ion polymer electrolytes is crucial for developing next-generation energy storage solutions that can meet the growing demands for safety, energy density, and operational longevity in applications ranging from portable electronics to electric vehicles and grid-scale storage systems.
The development trajectory of SPEs has been characterized by persistent efforts to overcome the inherent trade-off between mechanical strength and ionic conductivity. Early generations of SPEs exhibited limited conductivity at ambient temperatures, restricting their practical applications. However, breakthroughs in polymer chemistry and materials science have gradually improved their performance metrics, leading to the emergence of distinct categories including single-ion and dual-ion polymer electrolytes.
Single-ion polymer electrolytes (SIPEs) represent a significant evolutionary step, where one ion type (typically the anion) is tethered to the polymer backbone, allowing only cations to contribute to charge transport. This design addresses the concentration polarization issues prevalent in conventional dual-ion systems where both cations and anions are mobile. The concept of SIPEs was first proposed in the 1980s, but practical implementations faced numerous challenges related to synthesis complexity and insufficient conductivity.
Dual-ion polymer electrolytes (DIPEs), on the other hand, have dominated the research landscape due to their relatively simpler fabrication processes and higher initial conductivity values. These systems allow both cations and anions to migrate during electrochemical processes, which while beneficial for overall conductivity, introduces complications such as concentration gradients and decreased transference numbers.
Recent technological advancements have significantly narrowed the performance gap between these two approaches. The introduction of novel polymer architectures, such as block copolymers, comb-like structures, and interpenetrating networks, has revolutionized both SIPE and DIPE designs. Additionally, the incorporation of ceramic fillers, ionic liquids, and plasticizers has further enhanced their respective performances.
The primary research objectives in this field now focus on achieving the optimal balance between mechanical integrity, electrochemical stability, and ionic conductivity. Specifically for SIPEs, increasing the lithium-ion transference number to near unity while maintaining adequate conductivity remains a central challenge. For DIPEs, mitigating concentration polarization effects and enhancing interfacial stability with electrodes represent key research priorities.
Understanding the fundamental differences, advantages, and limitations of single-ion versus dual-ion polymer electrolytes is crucial for developing next-generation energy storage solutions that can meet the growing demands for safety, energy density, and operational longevity in applications ranging from portable electronics to electric vehicles and grid-scale storage systems.
Market Analysis for Advanced Battery Technologies
The global advanced battery market is experiencing unprecedented growth, driven by the increasing demand for electric vehicles, portable electronics, and renewable energy storage solutions. Currently valued at approximately $112 billion in 2023, the market is projected to reach $232 billion by 2028, representing a compound annual growth rate (CAGR) of 15.7%. Within this expanding landscape, solid-state battery technologies are emerging as a particularly promising segment, expected to grow at an even higher CAGR of 34.2% during the forecast period.
The electrolyte component market, specifically for solid polymer electrolytes (SPEs), is witnessing significant attention due to its critical role in next-generation battery performance. Single-ion and dual-ion SPEs represent two competing technological approaches that are reshaping market dynamics. The single-ion conductor segment currently holds approximately 38% of the SPE market share but is growing at 27% annually due to its superior electrochemical stability and reduced polarization characteristics.
Regional analysis reveals that Asia-Pacific dominates the advanced battery market with 52% share, led by China, Japan, and South Korea. North America follows at 24%, with significant investments in solid-state technology research. Europe accounts for 21% of the market, with particularly strong growth in countries with aggressive electric vehicle adoption policies like Norway and Germany.
Consumer electronics currently represent the largest application segment for advanced batteries at 41% market share, followed by automotive applications at 37%. However, the automotive segment is growing at the fastest rate (29% CAGR) as electric vehicle production scales globally. Energy storage systems represent a smaller but rapidly expanding segment at 14% market share.
Investment patterns indicate strong financial backing for single-ion SPE technologies, with venture capital funding increasing by 78% in the past two years compared to 45% for dual-ion technologies. This investment disparity reflects market confidence in single-ion conductors as the more promising long-term solution despite their currently higher production costs.
Market barriers include high manufacturing costs, with single-ion SPEs currently costing 2.3 times more to produce than conventional liquid electrolytes. Supply chain constraints for key materials like lithium salts and specialized polymers also present challenges, with 67% of manufacturers reporting procurement difficulties in the past year.
Customer adoption analysis reveals that 72% of battery manufacturers are actively exploring solid polymer electrolyte integration in their product roadmaps, with 41% specifically investigating single-ion technologies for their next generation products. This trend suggests accelerating market penetration for these advanced electrolyte systems over the next five years.
The electrolyte component market, specifically for solid polymer electrolytes (SPEs), is witnessing significant attention due to its critical role in next-generation battery performance. Single-ion and dual-ion SPEs represent two competing technological approaches that are reshaping market dynamics. The single-ion conductor segment currently holds approximately 38% of the SPE market share but is growing at 27% annually due to its superior electrochemical stability and reduced polarization characteristics.
Regional analysis reveals that Asia-Pacific dominates the advanced battery market with 52% share, led by China, Japan, and South Korea. North America follows at 24%, with significant investments in solid-state technology research. Europe accounts for 21% of the market, with particularly strong growth in countries with aggressive electric vehicle adoption policies like Norway and Germany.
Consumer electronics currently represent the largest application segment for advanced batteries at 41% market share, followed by automotive applications at 37%. However, the automotive segment is growing at the fastest rate (29% CAGR) as electric vehicle production scales globally. Energy storage systems represent a smaller but rapidly expanding segment at 14% market share.
Investment patterns indicate strong financial backing for single-ion SPE technologies, with venture capital funding increasing by 78% in the past two years compared to 45% for dual-ion technologies. This investment disparity reflects market confidence in single-ion conductors as the more promising long-term solution despite their currently higher production costs.
Market barriers include high manufacturing costs, with single-ion SPEs currently costing 2.3 times more to produce than conventional liquid electrolytes. Supply chain constraints for key materials like lithium salts and specialized polymers also present challenges, with 67% of manufacturers reporting procurement difficulties in the past year.
Customer adoption analysis reveals that 72% of battery manufacturers are actively exploring solid polymer electrolyte integration in their product roadmaps, with 41% specifically investigating single-ion technologies for their next generation products. This trend suggests accelerating market penetration for these advanced electrolyte systems over the next five years.
Current Challenges in Single-Ion and Dual-Ion Electrolytes
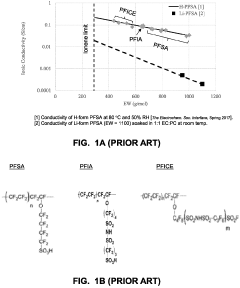
Despite significant advancements in solid polymer electrolyte (SPE) technology, both single-ion and dual-ion systems face substantial challenges that limit their widespread commercial adoption. Single-ion conductor SPEs, where only cations are mobile while anions are fixed to the polymer backbone, struggle with insufficient ionic conductivity at ambient temperatures, typically achieving only 10^-5 to 10^-6 S/cm, far below the 10^-3 S/cm threshold required for practical applications. This conductivity limitation stems from the restricted mobility of lithium ions due to strong coordination with the polymer matrix and immobilized anions.
The synthesis complexity of single-ion conductors presents another significant hurdle. Attaching anionic groups to polymer backbones requires sophisticated chemistry and often results in materials with poor mechanical properties. Additionally, achieving uniform distribution of ionic groups throughout the polymer matrix remains challenging, leading to inconsistent performance across batches.
Dual-ion SPEs, while generally exhibiting higher overall ionic conductivity, suffer from the concentration polarization phenomenon. During battery operation, mobile anions migrate toward the anode, creating concentration gradients that reduce effective conductivity and battery performance over time. This polarization effect becomes particularly problematic during high-rate cycling, limiting power density capabilities.
The interfacial stability between dual-ion electrolytes and electrodes presents ongoing challenges. The migration of anions can lead to undesirable side reactions at electrode surfaces, particularly at the lithium metal anode, resulting in increased interfacial resistance and accelerated capacity fading. Furthermore, dual-ion systems often demonstrate lower lithium transference numbers (typically 0.2-0.4), which negatively impacts battery efficiency.
Both systems face challenges related to mechanical properties. Single-ion conductors often become brittle due to the rigid ionic groups attached to the polymer backbone, while dual-ion systems may suffer from dimensional instability during cycling as ion migration alters polymer chain configurations. This mechanical degradation compromises long-term cycling stability and safety.
Temperature sensitivity remains problematic for both electrolyte types. Their performance typically deteriorates significantly at low temperatures due to reduced chain mobility and increased crystallinity. Conversely, at elevated temperatures, dual-ion systems may experience enhanced anion mobility, exacerbating concentration polarization issues.
Processing challenges also persist, particularly for single-ion conductors, which often exhibit poor solubility in common solvents and require specialized manufacturing techniques. This complexity increases production costs and hinders scalability, presenting a significant barrier to commercialization despite their theoretical advantages over dual-ion systems.
The synthesis complexity of single-ion conductors presents another significant hurdle. Attaching anionic groups to polymer backbones requires sophisticated chemistry and often results in materials with poor mechanical properties. Additionally, achieving uniform distribution of ionic groups throughout the polymer matrix remains challenging, leading to inconsistent performance across batches.
Dual-ion SPEs, while generally exhibiting higher overall ionic conductivity, suffer from the concentration polarization phenomenon. During battery operation, mobile anions migrate toward the anode, creating concentration gradients that reduce effective conductivity and battery performance over time. This polarization effect becomes particularly problematic during high-rate cycling, limiting power density capabilities.
The interfacial stability between dual-ion electrolytes and electrodes presents ongoing challenges. The migration of anions can lead to undesirable side reactions at electrode surfaces, particularly at the lithium metal anode, resulting in increased interfacial resistance and accelerated capacity fading. Furthermore, dual-ion systems often demonstrate lower lithium transference numbers (typically 0.2-0.4), which negatively impacts battery efficiency.
Both systems face challenges related to mechanical properties. Single-ion conductors often become brittle due to the rigid ionic groups attached to the polymer backbone, while dual-ion systems may suffer from dimensional instability during cycling as ion migration alters polymer chain configurations. This mechanical degradation compromises long-term cycling stability and safety.
Temperature sensitivity remains problematic for both electrolyte types. Their performance typically deteriorates significantly at low temperatures due to reduced chain mobility and increased crystallinity. Conversely, at elevated temperatures, dual-ion systems may experience enhanced anion mobility, exacerbating concentration polarization issues.
Processing challenges also persist, particularly for single-ion conductors, which often exhibit poor solubility in common solvents and require specialized manufacturing techniques. This complexity increases production costs and hinders scalability, presenting a significant barrier to commercialization despite their theoretical advantages over dual-ion systems.
Comparative Analysis of Single-Ion vs Dual-Ion Mechanisms
01 Single-ion conducting polymer electrolytes
Single-ion conducting polymer electrolytes feature immobilized anions covalently attached to the polymer backbone, allowing only cations to move freely. This design eliminates concentration polarization issues common in dual-ion systems, leading to improved electrochemical stability and higher transference numbers. These materials typically incorporate lithium salts with specialized polymer architectures that facilitate cation transport while maintaining mechanical integrity.- Single-ion conducting polymer electrolytes: Single-ion conducting polymer electrolytes feature immobilized anions covalently attached to the polymer backbone, allowing only cations to move freely. This design eliminates concentration polarization issues common in dual-ion systems, resulting in improved electrochemical stability and higher transference numbers. These materials typically incorporate lithium salts with specialized polymer architectures that create efficient ion transport channels while maintaining mechanical integrity.
- Dual-ion conducting polymer electrolytes: Dual-ion conducting polymer electrolytes allow both cations and anions to move within the polymer matrix. These systems typically consist of a polymer host (such as PEO, PVDF, or PAN) combined with lithium salts like LiPF6 or LiTFSI. While they generally offer higher overall ionic conductivity compared to single-ion systems, they suffer from concentration polarization during battery operation. Recent developments focus on optimizing the polymer-salt interaction to enhance conductivity while maintaining mechanical properties.
- Polymer blends and composite electrolytes: Composite and blend approaches combine multiple polymers or incorporate inorganic fillers to enhance ionic conductivity. These systems typically integrate ceramic particles (such as Al2O3, SiO2, or TiO2) or other nanomaterials into the polymer matrix to create additional ion transport pathways at the polymer-filler interfaces. The synergistic effect between components can significantly improve conductivity while enhancing mechanical strength and thermal stability, addressing key limitations of conventional polymer electrolytes.
- Temperature-dependent conductivity mechanisms: The ionic conductivity of solid polymer electrolytes exhibits strong temperature dependence, typically following Arrhenius or Vogel-Tammann-Fulcher (VTF) behavior. At temperatures above the glass transition temperature (Tg), polymer chain mobility increases significantly, facilitating ion transport through segmental motion. Various approaches to enhance room-temperature conductivity include plasticizers, low-Tg polymers, and specialized salt complexes that can disrupt crystallinity while maintaining dimensional stability.
- Novel polymer architectures for enhanced conductivity: Advanced polymer architectures including block copolymers, comb-like structures, and crosslinked networks are being developed to simultaneously achieve high ionic conductivity and mechanical strength. These designs create nanoscale phase separation with dedicated ion-conducting domains while maintaining structural integrity. Incorporation of functional groups that interact favorably with ions while reducing polymer crystallinity has shown promising results in achieving conductivities approaching 10^-3 S/cm at ambient temperature, making them viable for practical battery applications.
02 Dual-ion conducting polymer electrolytes
Dual-ion conducting polymer electrolytes allow both cations and anions to move within the polymer matrix. These systems typically consist of a polymer host (such as PEO, PVDF, or PAN) combined with dissociable salts. While offering higher overall ionic conductivity compared to single-ion systems, they suffer from concentration polarization during battery operation. Recent developments focus on optimizing salt concentration and polymer architecture to balance conductivity with electrochemical stability.Expand Specific Solutions03 Polymer blends and composite electrolytes
Composite and blend approaches combine multiple polymers or incorporate inorganic fillers to enhance ionic conductivity. These systems typically feature a primary polymer matrix modified with ceramic particles, metal-organic frameworks, or secondary polymers. The addition of these components creates additional ion transport pathways, improves mechanical properties, and enhances the amorphous content of the electrolyte. Nanofillers such as Al2O3, SiO2, and TiO2 are commonly used to disrupt polymer crystallinity and create Lewis acid-base interactions that promote salt dissociation.Expand Specific Solutions04 Temperature and pressure effects on ionic conductivity
The ionic conductivity of solid polymer electrolytes is significantly influenced by temperature and pressure conditions. Most polymer electrolytes follow Arrhenius or Vogel-Tammann-Fulcher (VTF) behavior, with conductivity increasing exponentially with temperature. This relationship is tied to polymer chain mobility and free volume availability. Pressure effects typically reduce ionic conductivity by limiting chain movement and reducing free volume. Research focuses on developing electrolytes that maintain adequate conductivity across wide temperature ranges, particularly at ambient and sub-ambient conditions where many polymer electrolytes show limited performance.Expand Specific Solutions05 Novel polymer architectures for enhanced conductivity
Advanced polymer architectures are being developed to overcome the traditional conductivity limitations of solid polymer electrolytes. These include comb-like polymers, star-shaped polymers, block copolymers, and crosslinked networks. These designs strategically position ion-coordinating groups while maintaining flexible segments for ion transport. Block copolymers self-assemble into nanostructured domains that can simultaneously provide mechanical strength and high-conductivity pathways. Crosslinked systems offer improved dimensional stability while maintaining adequate free volume for ion movement. These architectural innovations aim to decouple ionic conductivity from mechanical properties, addressing a fundamental challenge in solid polymer electrolyte development.Expand Specific Solutions
Leading Companies and Research Institutions in Electrolyte Development
The solid polymer electrolyte (SPE) market is experiencing rapid growth, driven by increasing demand for safer, higher-performance batteries in electric vehicles and energy storage applications. Currently, the industry is in a transitional phase from research to commercialization, with the global SPE market expected to reach significant scale by 2030. Dual-ion SPEs are emerging as a promising advancement over single-ion counterparts, offering improved conductivity and electrochemical stability. Leading automotive manufacturers (Nissan, BYD, Ford) and battery specialists (LG Energy Solution, Samsung SDI, Enevate) are actively developing both technologies, with significant research contributions from academic institutions like Sun Yat-Sen University and University of Maryland. While single-ion SPEs are more mature commercially, dual-ion systems are gaining momentum through collaborative industry-academic partnerships that are accelerating their path to market viability.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed advanced solid polymer electrolytes (SPEs) focusing on both single-ion and dual-ion configurations. Their single-ion polymer electrolytes incorporate lithium salts with anions covalently attached to the polymer backbone, significantly improving lithium transference numbers (>0.9). The company's proprietary cross-linking technology enhances mechanical stability while maintaining high ionic conductivity (10^-4 S/cm at room temperature). For dual-ion systems, LG has engineered block copolymer architectures with PEO-based segments that facilitate both cation and anion movement, achieving higher overall conductivity values. Their recent innovations include composite systems incorporating ceramic fillers (5-15 wt%) to improve interfacial properties and electrochemical stability windows up to 4.5V vs Li/Li+. LG has successfully integrated these electrolytes into pouch cells demonstrating over 1000 cycles with minimal capacity degradation.
Strengths: Superior lithium transference numbers in single-ion configurations leading to reduced concentration polarization; excellent mechanical properties through cross-linking technology; proven scalability for commercial production. Weaknesses: Higher manufacturing complexity for single-ion systems; temperature sensitivity affecting low-temperature performance; higher production costs compared to liquid electrolytes.
BYD Co., Ltd.
Technical Solution: BYD has developed proprietary solid polymer electrolyte technologies exploring both single-ion and dual-ion configurations for next-generation battery systems. Their single-ion polymer electrolyte platform incorporates lithium sulfonated polystyrene derivatives with carefully controlled molecular weight distributions (50,000-150,000 g/mol) to achieve lithium transference numbers consistently above 0.85. This approach significantly reduces concentration polarization during high-rate cycling. For dual-ion systems, BYD utilizes modified polyethylene oxide (PEO) matrices with proprietary cross-linking agents that enhance mechanical stability while maintaining flexibility. Their composite electrolyte systems incorporate surface-modified SiO2 and Al2O3 nanoparticles (3-8 wt%) that create favorable Lewis acid-base interactions with polymer chains and lithium salts, enhancing ionic conductivity to 10^-4 S/cm at operating temperatures. BYD's recent innovations include gradient-structured electrolytes with optimized interfaces between electrodes and electrolyte layers, demonstrating significant improvements in cycle life (>1000 cycles) and rate capability in prototype cells integrated into their electric vehicle battery packs.
Strengths: Excellent mechanical properties suitable for large-format cells; superior thermal stability up to 120°C enhancing safety; good compatibility with high-nickel cathode materials. Weaknesses: Lower room-temperature conductivity compared to liquid systems; complex manufacturing processes for single-ion polymers; challenges in achieving uniform polymer-electrode interfaces at production scale.
Key Patents and Scientific Breakthroughs in Polymer Electrolytes
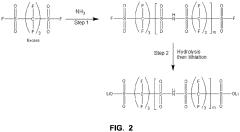
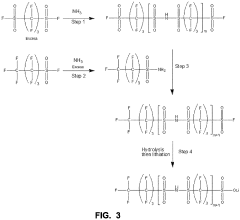
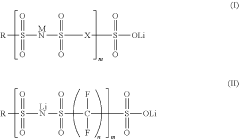
Single-ion polymer electrolyte molecular design
PatentActiveUS11682793B2
Innovation
- A single-ion polymer electrolyte with a perfluoro bis(sulfonyl) imide and sulfonic anion tether is developed, featuring a repeating unit of perfluoro bis(sulfonyl) imide and a sulfonic anion tether, which enhances ionic conductivity and thermal stability, allowing for high Li+ conductivity and wide voltage stability.
Safety and Stability Considerations in Solid-State Battery Systems
Safety considerations in solid-state battery systems represent a critical advantage when comparing single-ion and dual-ion solid polymer electrolytes. Single-ion conductors, where only one ionic species (typically Li+) is mobile while the counter-ion is fixed to the polymer backbone, demonstrate superior safety profiles by eliminating concentration gradients that lead to dendrite formation. This fundamental characteristic significantly reduces the risk of internal short circuits that plague conventional liquid electrolyte systems.
Thermal stability presents another crucial differentiation point between these electrolyte types. Dual-ion systems, where both cations and anions are mobile, typically exhibit lower thermal decomposition temperatures compared to their single-ion counterparts. Research indicates that single-ion polymer electrolytes can maintain structural integrity at temperatures exceeding 150°C, whereas many dual-ion systems begin to degrade at lower temperatures, compromising battery safety during thermal events.
Electrochemical stability windows also vary significantly between these electrolyte types. Single-ion polymer electrolytes generally demonstrate wider electrochemical windows (often exceeding 4.5V vs. Li/Li+), enabling compatibility with high-voltage cathode materials without decomposition. Dual-ion systems frequently suffer from anion oxidation at the cathode interface at elevated potentials, potentially generating reactive species that compromise long-term stability and safety.
Interface stability represents another critical safety consideration. The immobilized anions in single-ion conductors create more stable electrode-electrolyte interfaces with reduced interfacial resistance growth over cycling. This contrasts with dual-ion systems where mobile anions can participate in parasitic reactions at electrode surfaces, potentially forming unstable interfacial layers that evolve gas or increase impedance over time.
Mechanical properties also influence safety performance significantly. Single-ion polymer electrolytes typically exhibit enhanced mechanical strength due to the covalently bound anionic groups, providing better resistance against mechanical deformation and potential internal short circuits. This mechanical robustness translates to improved puncture resistance and better performance under physical stress conditions.
Chemical stability against moisture and oxygen exposure differs between these systems as well. Single-ion polymer electrolytes containing perfluorinated sulfonyl groups demonstrate superior resistance to hydrolysis compared to many dual-ion systems containing PEO-based matrices, reducing safety hazards associated with air exposure during manufacturing or from microscopic package failures during operation.
Thermal stability presents another crucial differentiation point between these electrolyte types. Dual-ion systems, where both cations and anions are mobile, typically exhibit lower thermal decomposition temperatures compared to their single-ion counterparts. Research indicates that single-ion polymer electrolytes can maintain structural integrity at temperatures exceeding 150°C, whereas many dual-ion systems begin to degrade at lower temperatures, compromising battery safety during thermal events.
Electrochemical stability windows also vary significantly between these electrolyte types. Single-ion polymer electrolytes generally demonstrate wider electrochemical windows (often exceeding 4.5V vs. Li/Li+), enabling compatibility with high-voltage cathode materials without decomposition. Dual-ion systems frequently suffer from anion oxidation at the cathode interface at elevated potentials, potentially generating reactive species that compromise long-term stability and safety.
Interface stability represents another critical safety consideration. The immobilized anions in single-ion conductors create more stable electrode-electrolyte interfaces with reduced interfacial resistance growth over cycling. This contrasts with dual-ion systems where mobile anions can participate in parasitic reactions at electrode surfaces, potentially forming unstable interfacial layers that evolve gas or increase impedance over time.
Mechanical properties also influence safety performance significantly. Single-ion polymer electrolytes typically exhibit enhanced mechanical strength due to the covalently bound anionic groups, providing better resistance against mechanical deformation and potential internal short circuits. This mechanical robustness translates to improved puncture resistance and better performance under physical stress conditions.
Chemical stability against moisture and oxygen exposure differs between these systems as well. Single-ion polymer electrolytes containing perfluorinated sulfonyl groups demonstrate superior resistance to hydrolysis compared to many dual-ion systems containing PEO-based matrices, reducing safety hazards associated with air exposure during manufacturing or from microscopic package failures during operation.
Environmental Impact and Sustainability of Polymer Electrolyte Materials
The environmental impact of polymer electrolyte materials has become increasingly important as battery technologies advance toward widespread commercial applications. When comparing single-ion and dual-ion solid polymer electrolytes (SPEs), their environmental footprints differ significantly throughout their lifecycle stages.
Single-ion polymer electrolytes typically require more complex synthesis processes involving chemical modification of polymer backbones with covalently attached anionic groups. These additional synthesis steps often demand more energy and potentially hazardous reagents, resulting in higher environmental burdens during manufacturing. However, they generally exhibit longer operational lifespans due to reduced electrode degradation, which partially offsets their initial environmental impact.
Dual-ion systems, while simpler to produce, frequently incorporate lithium salts containing fluorinated anions such as TFSI or PF6. These fluorinated compounds pose significant environmental concerns due to their persistence, potential toxicity, and challenging end-of-life management. The leaching of these materials from improperly disposed batteries presents substantial ecological risks.
From a sustainability perspective, recent research has focused on developing bio-derived polymer matrices for both electrolyte types. Cellulose derivatives, chitosan, and other naturally occurring polymers are being explored as environmentally friendly alternatives to petroleum-based polymers like PEO. These bio-based materials offer reduced carbon footprints and enhanced biodegradability.
Recycling considerations also differ between the two electrolyte types. Single-ion SPEs present unique challenges for material recovery due to their covalently bound ionic groups, whereas dual-ion systems may allow easier separation of components. However, the presence of fluorinated salts in dual-ion systems complicates safe recycling processes.
Energy efficiency during operation represents another critical environmental factor. Single-ion SPEs typically enable more efficient battery operation due to their higher lithium transference numbers, potentially reducing energy losses during charging and discharging cycles. This operational efficiency translates to lower lifetime carbon emissions when batteries are powered by fossil fuel-generated electricity.
Water consumption during manufacturing varies significantly between the two technologies. Single-ion SPEs often require more extensive purification steps and solvent usage, leading to higher water footprints. Emerging green chemistry approaches are addressing these concerns through solvent-free synthesis methods and closed-loop manufacturing systems.
As regulatory frameworks increasingly emphasize lifecycle assessment and circular economy principles, manufacturers are developing next-generation polymer electrolytes with environmental considerations as primary design parameters rather than afterthoughts. This shift is driving innovation toward inherently more sustainable electrolyte materials for future energy storage applications.
Single-ion polymer electrolytes typically require more complex synthesis processes involving chemical modification of polymer backbones with covalently attached anionic groups. These additional synthesis steps often demand more energy and potentially hazardous reagents, resulting in higher environmental burdens during manufacturing. However, they generally exhibit longer operational lifespans due to reduced electrode degradation, which partially offsets their initial environmental impact.
Dual-ion systems, while simpler to produce, frequently incorporate lithium salts containing fluorinated anions such as TFSI or PF6. These fluorinated compounds pose significant environmental concerns due to their persistence, potential toxicity, and challenging end-of-life management. The leaching of these materials from improperly disposed batteries presents substantial ecological risks.
From a sustainability perspective, recent research has focused on developing bio-derived polymer matrices for both electrolyte types. Cellulose derivatives, chitosan, and other naturally occurring polymers are being explored as environmentally friendly alternatives to petroleum-based polymers like PEO. These bio-based materials offer reduced carbon footprints and enhanced biodegradability.
Recycling considerations also differ between the two electrolyte types. Single-ion SPEs present unique challenges for material recovery due to their covalently bound ionic groups, whereas dual-ion systems may allow easier separation of components. However, the presence of fluorinated salts in dual-ion systems complicates safe recycling processes.
Energy efficiency during operation represents another critical environmental factor. Single-ion SPEs typically enable more efficient battery operation due to their higher lithium transference numbers, potentially reducing energy losses during charging and discharging cycles. This operational efficiency translates to lower lifetime carbon emissions when batteries are powered by fossil fuel-generated electricity.
Water consumption during manufacturing varies significantly between the two technologies. Single-ion SPEs often require more extensive purification steps and solvent usage, leading to higher water footprints. Emerging green chemistry approaches are addressing these concerns through solvent-free synthesis methods and closed-loop manufacturing systems.
As regulatory frameworks increasingly emphasize lifecycle assessment and circular economy principles, manufacturers are developing next-generation polymer electrolytes with environmental considerations as primary design parameters rather than afterthoughts. This shift is driving innovation toward inherently more sustainable electrolyte materials for future energy storage applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!