Solid Polymer Electrolyte Optimization for High-Temperature Battery Operation
SEP 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SPE Development Background and Objectives
Solid Polymer Electrolytes (SPEs) have emerged as a promising alternative to conventional liquid electrolytes in battery systems over the past four decades. The development trajectory of SPEs began in the 1970s with the discovery of ionic conductivity in polymer-salt complexes, followed by significant advancements in the 1980s and 1990s that established their potential for electrochemical applications. The evolution of SPE technology has been driven by the increasing demand for safer, more efficient, and environmentally friendly energy storage solutions.
The fundamental challenge that SPEs address is the inherent safety risk associated with traditional liquid electrolytes, which are typically flammable and prone to leakage. This safety concern becomes particularly critical in high-temperature operating environments where thermal stability is paramount. Additionally, the growing emphasis on sustainable energy solutions has accelerated research into SPEs as they offer potential advantages in terms of recyclability and reduced environmental impact.
Recent technological trends indicate a shift towards hybrid and composite polymer electrolyte systems that combine the mechanical stability of solid polymers with enhanced ionic conductivity. The integration of nanomaterials and the development of block copolymer architectures represent significant innovations in this field. These advancements aim to overcome the historical limitations of SPEs, particularly their relatively low ionic conductivity at ambient temperatures.
The primary technical objective for SPE optimization in high-temperature battery applications is to develop materials that maintain structural integrity and electrochemical performance under elevated temperature conditions (typically above 60°C). This involves enhancing ionic conductivity without compromising mechanical properties or interfacial stability with electrode materials. Achieving a balance between these often competing requirements remains a central challenge.
Secondary objectives include extending the electrochemical stability window to enable compatibility with high-voltage cathode materials, improving the lithium transference number to enhance battery efficiency, and developing scalable manufacturing processes that can transition laboratory successes to commercial production. The long-term vision encompasses the creation of all-solid-state batteries that can operate reliably across a wide temperature range.
The technological trajectory suggests that future SPE development will increasingly focus on multifunctional materials that can simultaneously address multiple performance parameters. This includes self-healing capabilities to mitigate degradation over repeated thermal cycles, adaptive properties that respond to environmental changes, and integration with smart battery management systems for optimized performance in diverse operating conditions.
The fundamental challenge that SPEs address is the inherent safety risk associated with traditional liquid electrolytes, which are typically flammable and prone to leakage. This safety concern becomes particularly critical in high-temperature operating environments where thermal stability is paramount. Additionally, the growing emphasis on sustainable energy solutions has accelerated research into SPEs as they offer potential advantages in terms of recyclability and reduced environmental impact.
Recent technological trends indicate a shift towards hybrid and composite polymer electrolyte systems that combine the mechanical stability of solid polymers with enhanced ionic conductivity. The integration of nanomaterials and the development of block copolymer architectures represent significant innovations in this field. These advancements aim to overcome the historical limitations of SPEs, particularly their relatively low ionic conductivity at ambient temperatures.
The primary technical objective for SPE optimization in high-temperature battery applications is to develop materials that maintain structural integrity and electrochemical performance under elevated temperature conditions (typically above 60°C). This involves enhancing ionic conductivity without compromising mechanical properties or interfacial stability with electrode materials. Achieving a balance between these often competing requirements remains a central challenge.
Secondary objectives include extending the electrochemical stability window to enable compatibility with high-voltage cathode materials, improving the lithium transference number to enhance battery efficiency, and developing scalable manufacturing processes that can transition laboratory successes to commercial production. The long-term vision encompasses the creation of all-solid-state batteries that can operate reliably across a wide temperature range.
The technological trajectory suggests that future SPE development will increasingly focus on multifunctional materials that can simultaneously address multiple performance parameters. This includes self-healing capabilities to mitigate degradation over repeated thermal cycles, adaptive properties that respond to environmental changes, and integration with smart battery management systems for optimized performance in diverse operating conditions.
High-Temperature Battery Market Analysis
The high-temperature battery market has experienced significant growth over the past decade, driven primarily by increasing demand in automotive, aerospace, and industrial applications. The global market value for high-temperature batteries reached approximately $4.6 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 8.7% through 2030, potentially reaching $9.2 billion by the end of the forecast period.
Key market segments driving this growth include electric vehicles operating in extreme environments, aerospace and defense applications, industrial equipment in high-temperature settings, and grid-scale energy storage systems. The automotive sector represents the largest market share at 38%, followed by industrial applications at 27%, and aerospace at 19%.
Geographically, North America currently leads the market with 35% share, followed closely by Asia-Pacific at 32%, which is experiencing the fastest growth rate due to rapid industrialization and electric vehicle adoption in China, Japan, and South Korea. Europe accounts for 25% of the market, with particular strength in automotive applications.
Consumer demand patterns indicate a growing preference for batteries that can maintain performance integrity at temperatures exceeding 60°C, with optimal performance desired up to 120°C for specialized applications. This has created significant market pull for solid polymer electrolyte solutions that can address the thermal stability limitations of conventional liquid electrolytes.
Market research indicates that customers are willing to pay a premium of 15-20% for batteries with enhanced high-temperature performance, particularly in safety-critical applications. However, price sensitivity remains high in consumer electronics and entry-level electric vehicle segments.
The competitive landscape is characterized by both established battery manufacturers expanding their high-temperature offerings and specialized startups focusing exclusively on thermal management innovations. Major players include Panasonic, LG Energy Solution, and Samsung SDI, who collectively control approximately 45% of the high-temperature battery market.
Supply chain analysis reveals potential vulnerabilities in raw material sourcing for advanced polymer electrolytes, with 67% of key materials concentrated in regions susceptible to geopolitical tensions. This has prompted increased investment in alternative material research and supply chain diversification strategies among leading manufacturers.
Customer feedback consistently highlights three primary pain points: capacity degradation at elevated temperatures, safety concerns related to thermal runaway, and cycle life reduction in high-temperature environments. These market signals provide clear direction for solid polymer electrolyte optimization efforts, suggesting that solutions addressing these specific challenges will capture significant market share.
Key market segments driving this growth include electric vehicles operating in extreme environments, aerospace and defense applications, industrial equipment in high-temperature settings, and grid-scale energy storage systems. The automotive sector represents the largest market share at 38%, followed by industrial applications at 27%, and aerospace at 19%.
Geographically, North America currently leads the market with 35% share, followed closely by Asia-Pacific at 32%, which is experiencing the fastest growth rate due to rapid industrialization and electric vehicle adoption in China, Japan, and South Korea. Europe accounts for 25% of the market, with particular strength in automotive applications.
Consumer demand patterns indicate a growing preference for batteries that can maintain performance integrity at temperatures exceeding 60°C, with optimal performance desired up to 120°C for specialized applications. This has created significant market pull for solid polymer electrolyte solutions that can address the thermal stability limitations of conventional liquid electrolytes.
Market research indicates that customers are willing to pay a premium of 15-20% for batteries with enhanced high-temperature performance, particularly in safety-critical applications. However, price sensitivity remains high in consumer electronics and entry-level electric vehicle segments.
The competitive landscape is characterized by both established battery manufacturers expanding their high-temperature offerings and specialized startups focusing exclusively on thermal management innovations. Major players include Panasonic, LG Energy Solution, and Samsung SDI, who collectively control approximately 45% of the high-temperature battery market.
Supply chain analysis reveals potential vulnerabilities in raw material sourcing for advanced polymer electrolytes, with 67% of key materials concentrated in regions susceptible to geopolitical tensions. This has prompted increased investment in alternative material research and supply chain diversification strategies among leading manufacturers.
Customer feedback consistently highlights three primary pain points: capacity degradation at elevated temperatures, safety concerns related to thermal runaway, and cycle life reduction in high-temperature environments. These market signals provide clear direction for solid polymer electrolyte optimization efforts, suggesting that solutions addressing these specific challenges will capture significant market share.
Current SPE Technologies and Thermal Challenges
Solid polymer electrolytes (SPEs) have emerged as promising alternatives to conventional liquid electrolytes in lithium-ion batteries, offering enhanced safety and stability. Current SPE technologies primarily fall into three categories: polyethylene oxide (PEO)-based systems, polycarbonate-based electrolytes, and composite polymer electrolytes. PEO-based SPEs remain the most widely studied due to their excellent lithium-ion conductivity and compatibility with lithium metal anodes. However, their performance is severely limited at temperatures above 60°C, where crystallization and mechanical softening lead to decreased ionic conductivity and increased interfacial resistance.
Polycarbonate-based electrolytes offer improved thermal stability compared to PEO systems, maintaining structural integrity at temperatures up to 80°C. These materials exhibit lower crystallinity and higher glass transition temperatures, which contribute to their enhanced performance at elevated temperatures. Nevertheless, they typically demonstrate lower room-temperature ionic conductivity, necessitating operation at higher temperatures to achieve practical performance levels.
Composite polymer electrolytes represent the current state-of-the-art approach, incorporating inorganic fillers such as Al2O3, SiO2, and ceramic particles into polymer matrices. These fillers serve multiple functions: they disrupt polymer crystallization, create additional ion transport pathways, and improve mechanical properties. Recent advancements include the development of hybrid organic-inorganic systems utilizing functionalized nanoparticles that chemically interact with both the polymer matrix and lithium salts.
The primary thermal challenges facing current SPE technologies include thermal degradation, interfacial stability at high temperatures, and maintaining consistent mechanical properties across wide temperature ranges. Above 80°C, many polymer systems begin to experience chemical degradation, leading to the formation of undesirable byproducts that can interfere with ion transport and electrode reactions. This degradation accelerates with increasing temperature, severely limiting the upper operating temperature of most current SPE systems.
Interfacial resistance presents another significant challenge, particularly at the electrolyte-electrode interface. As temperatures rise, differences in thermal expansion coefficients between the polymer electrolyte and electrodes can create mechanical stress, leading to delamination and increased contact resistance. This phenomenon is especially problematic during thermal cycling, where repeated expansion and contraction can progressively degrade battery performance.
Recent research has focused on developing cross-linked polymer networks and thermally resistant polymer blends to address these challenges. Cross-linking enhances mechanical stability at elevated temperatures while maintaining adequate ionic conductivity. Additionally, the incorporation of flame-retardant additives and thermally stable ionic liquids has shown promise in extending the upper temperature limit of SPE systems, though often at the expense of other performance metrics such as ionic conductivity or electrochemical stability window.
Polycarbonate-based electrolytes offer improved thermal stability compared to PEO systems, maintaining structural integrity at temperatures up to 80°C. These materials exhibit lower crystallinity and higher glass transition temperatures, which contribute to their enhanced performance at elevated temperatures. Nevertheless, they typically demonstrate lower room-temperature ionic conductivity, necessitating operation at higher temperatures to achieve practical performance levels.
Composite polymer electrolytes represent the current state-of-the-art approach, incorporating inorganic fillers such as Al2O3, SiO2, and ceramic particles into polymer matrices. These fillers serve multiple functions: they disrupt polymer crystallization, create additional ion transport pathways, and improve mechanical properties. Recent advancements include the development of hybrid organic-inorganic systems utilizing functionalized nanoparticles that chemically interact with both the polymer matrix and lithium salts.
The primary thermal challenges facing current SPE technologies include thermal degradation, interfacial stability at high temperatures, and maintaining consistent mechanical properties across wide temperature ranges. Above 80°C, many polymer systems begin to experience chemical degradation, leading to the formation of undesirable byproducts that can interfere with ion transport and electrode reactions. This degradation accelerates with increasing temperature, severely limiting the upper operating temperature of most current SPE systems.
Interfacial resistance presents another significant challenge, particularly at the electrolyte-electrode interface. As temperatures rise, differences in thermal expansion coefficients between the polymer electrolyte and electrodes can create mechanical stress, leading to delamination and increased contact resistance. This phenomenon is especially problematic during thermal cycling, where repeated expansion and contraction can progressively degrade battery performance.
Recent research has focused on developing cross-linked polymer networks and thermally resistant polymer blends to address these challenges. Cross-linking enhances mechanical stability at elevated temperatures while maintaining adequate ionic conductivity. Additionally, the incorporation of flame-retardant additives and thermally stable ionic liquids has shown promise in extending the upper temperature limit of SPE systems, though often at the expense of other performance metrics such as ionic conductivity or electrochemical stability window.
Current High-Temperature SPE Solutions
01 Polymer composition for high-temperature stability
Specific polymer compositions can be engineered to maintain stability and conductivity at elevated temperatures. These compositions often include fluorinated polymers, cross-linked structures, or thermally resistant polymer backbones that prevent degradation when exposed to high temperatures. The incorporation of specific monomers and polymer architectures can significantly improve the thermal stability threshold of solid polymer electrolytes, allowing them to function effectively in high-temperature applications without losing their mechanical or electrochemical properties.- High-temperature resistant polymer materials: Specific polymer materials that can withstand high temperatures are essential for solid polymer electrolytes in high-temperature applications. These materials include fluoropolymers, polyethylene oxide derivatives, and thermally stable aromatic polymers that maintain structural integrity and ionic conductivity at elevated temperatures. These polymers are often modified with cross-linking agents or reinforcing additives to enhance their thermal stability while preserving electrochemical performance.
- Ceramic-polymer composite electrolytes: Incorporating ceramic fillers into polymer matrices creates composite electrolytes with enhanced thermal stability and mechanical strength for high-temperature operation. These ceramic additives, such as aluminum oxide, silicon dioxide, or lithium-containing ceramics, help maintain dimensional stability and prevent polymer degradation at elevated temperatures. The ceramic particles also create additional pathways for ion transport, which can compensate for the decreased polymer chain mobility at high temperatures.
- Ionic liquid incorporation techniques: Ionic liquids can be integrated into solid polymer electrolytes to improve ionic conductivity at high temperatures. These non-volatile, thermally stable salts provide additional charge carriers and plasticize the polymer matrix, enabling efficient ion transport even when conventional electrolytes would degrade. The selection of appropriate ionic liquid structures with high decomposition temperatures and compatibility with the polymer host is crucial for maintaining performance in extreme thermal conditions.
- Cross-linking and network formation strategies: Cross-linking techniques create three-dimensional polymer networks that resist softening or melting at high temperatures. These methods include chemical cross-linking using multifunctional reagents, radiation-induced cross-linking, or the incorporation of self-cross-linking monomers. The resulting network structure maintains mechanical integrity while allowing sufficient local chain mobility for ion transport, creating solid polymer electrolytes that can function effectively at elevated temperatures without dimensional instability.
- Flame-retardant and thermally stable additives: Specialized additives can be incorporated into solid polymer electrolytes to enhance safety and performance at high temperatures. These include phosphorus-containing compounds, metal hydroxides, and nitrogen-based flame retardants that suppress combustion while maintaining electrochemical stability. Other thermal stabilizers prevent polymer degradation through mechanisms such as free radical scavenging or decomposition pathway interruption, extending the operational temperature range of the electrolyte system.
02 Inorganic-organic composite electrolytes
Hybrid electrolyte systems combining inorganic components (such as ceramic particles, metal oxides, or nanofillers) with polymer matrices create composite materials with enhanced thermal stability. These composites leverage the high-temperature resistance of inorganic materials while maintaining the flexibility and processability of polymers. The inorganic components can act as heat-resistant reinforcements, creating thermally stable pathways for ion transport even at elevated temperatures, while also potentially reducing polymer crystallinity that might otherwise impede ion movement at high temperatures.Expand Specific Solutions03 Ionic liquid incorporation for thermal stability
Incorporating ionic liquids into solid polymer electrolytes can significantly improve their high-temperature performance. Ionic liquids have negligible vapor pressure, high thermal stability, and excellent ionic conductivity, making them ideal additives for high-temperature applications. When blended with polymer matrices, they can create systems that maintain conductivity and electrochemical stability at elevated temperatures. The ionic liquids can also act as plasticizers, improving chain mobility and ion transport while simultaneously enhancing the thermal resistance of the overall electrolyte system.Expand Specific Solutions04 Cross-linking and network structures
Cross-linked polymer networks offer superior dimensional stability and mechanical strength at high temperatures compared to linear polymers. These three-dimensional networks resist flow and deformation when heated, maintaining critical electrolyte properties. Various cross-linking methods, including chemical cross-linking, radiation-induced cross-linking, or the use of multifunctional monomers, can be employed to create these thermally resistant structures. The degree of cross-linking can be optimized to balance mechanical stability with sufficient chain mobility for ion transport at elevated temperatures.Expand Specific Solutions05 Salt selection and concentration optimization
The choice of conducting salt and its concentration significantly impacts the high-temperature performance of solid polymer electrolytes. Thermally stable salts with low lattice energy and good compatibility with the polymer matrix are essential for maintaining conductivity at elevated temperatures. Optimizing salt concentration can create a balance between ion availability and polymer-salt interactions that maintains conductivity while preventing salt precipitation or polymer degradation at high temperatures. Some advanced formulations incorporate multiple salts to achieve synergistic effects that enhance thermal stability and electrochemical performance.Expand Specific Solutions
Leading Companies in SPE Research and Development
The solid polymer electrolyte (SPE) optimization for high-temperature battery operation market is currently in an early growth phase, characterized by intensive R&D activities across academic institutions and commercial entities. The global market size for advanced battery electrolytes is projected to reach $8.5 billion by 2027, with SPEs representing a rapidly expanding segment. Technical maturity varies significantly among key players, with companies like SAMSUNG SDI, Blue Solutions, and Beijing WeLion New Energy Technology leading commercial development. Research institutions including CNRS, Aix-Marseille University, and HKUST are advancing fundamental innovations, while established manufacturers such as Murata, Toshiba, and BASF are leveraging their materials expertise to develop proprietary SPE solutions. The competitive landscape reflects a balance between specialized startups focused solely on SPE technology and diversified corporations integrating SPEs into broader energy storage portfolios.
Ionic Materials Inc.
Technical Solution: Ionic Materials has developed a revolutionary solid polymer electrolyte platform specifically designed for high-temperature battery operation. Their proprietary technology centers on a polymer matrix that conducts ions at room temperature without liquid components, maintaining stability at elevated temperatures up to 150°C. The polymer electrolyte incorporates specialized additives that enhance ionic conductivity while preserving mechanical integrity under thermal stress. Their approach involves a cross-linked polymer network with strategically positioned functional groups that facilitate lithium-ion transport through coordinated hopping mechanisms[1]. The material demonstrates conductivity values exceeding 1 mS/cm at elevated temperatures, comparable to liquid electrolytes but without their safety concerns. Ionic Materials' solid polymer electrolyte also forms stable interfaces with both lithium metal anodes and high-voltage cathodes, enabling higher energy density batteries suitable for extreme operating conditions[3].
Strengths: Superior thermal stability compared to conventional electrolytes, enabling safe operation at high temperatures without thermal runaway risks. Compatible with lithium metal anodes, allowing for higher energy density battery designs. Weaknesses: Manufacturing scalability remains challenging due to specialized polymer synthesis requirements. May exhibit reduced ionic conductivity at lower temperatures compared to gel polymer alternatives.
SAMSUNG SDI CO LTD
Technical Solution: Samsung SDI has engineered an advanced solid polymer electrolyte system optimized for high-temperature battery applications, focusing on electric vehicle and energy storage markets. Their technology utilizes a composite approach combining polyethylene oxide (PEO) matrices with ceramic fillers (primarily Al2O3 and SiO2 nanoparticles) to enhance mechanical properties and thermal stability. The company's proprietary cross-linking methodology creates three-dimensional polymer networks that maintain structural integrity at temperatures exceeding 100°C while facilitating lithium-ion transport[2]. Samsung's formulation incorporates flame-retardant additives and specialized lithium salts (LiTFSI and LiFSI) that remain stable at elevated temperatures. Their manufacturing process employs solvent-free extrusion techniques that enable production of thin, uniform electrolyte films with consistent performance characteristics. Recent developments include gradient-structured polymer electrolytes that optimize the interface between electrodes and electrolyte, reducing interfacial resistance at high temperatures[4].
Strengths: Extensive manufacturing infrastructure allows for large-scale production capabilities. Comprehensive integration with existing battery cell designs enables faster commercialization pathway. Weaknesses: Higher cost compared to conventional liquid electrolytes due to specialized materials and processing requirements. Performance at extreme temperature cycling (repeated heating/cooling) still presents challenges for long-term stability.
Key Patents and Innovations in Thermal-Stable Polymers
Solid polymer electrolyte membrane, method for manufacturing the same, and fuel cell using the solid polymer electrolyte membrane
PatentActiveUS7879506B2
Innovation
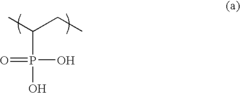
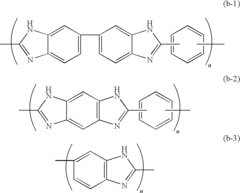
- A solid polymer electrolyte membrane is developed using a polymer compound with a side chain unit represented by Formula (a) at a heterocyclic nitrogen atom of polybenzimidazole, which is doped with vinylphosphonic acid through addition polymerization, enhancing proton conductivity and thermal resistance.
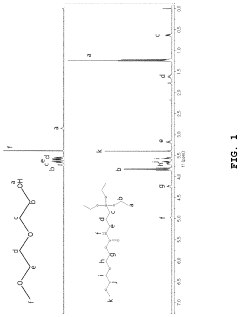
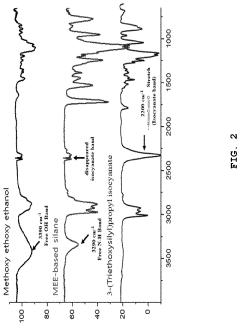
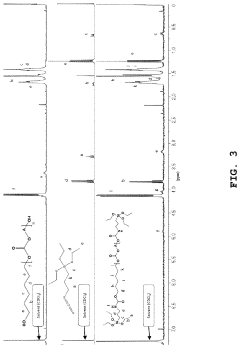
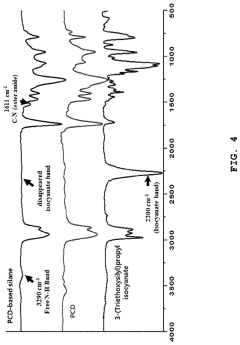
Solid polymer electrolyte comprising an alkoxysilane compound having a urethane bond, a method of preparing the electrolyte, and a lithium secondary battery including
PatentActiveUS10797346B2
Innovation
- A solid polymer electrolyte composition is developed, comprising an alkoxysilane compound with a urethane bond, a silica precursor, and a polycarbonate-based diol polymer, which undergoes a sol-gel reaction to form a matrix with a lithium salt, enhancing compatibility, stability, and ionic conductivity.
Material Safety and Environmental Impact Assessment
The safety profile of solid polymer electrolytes (SPEs) represents a significant advantage over conventional liquid electrolytes in high-temperature battery applications. Most polymer-based electrolytes exhibit inherently lower flammability compared to their organic liquid counterparts, substantially reducing fire hazards in battery systems. Polymers such as polyethylene oxide (PEO), polyvinylidene fluoride (PVDF), and polyacrylonitrile (PAN) demonstrate thermal stability without releasing volatile organic compounds at elevated temperatures, making them particularly suitable for high-temperature operations.
Environmental considerations for SPE materials extend throughout their lifecycle. During production, many polymer electrolytes require fewer toxic solvents than liquid electrolyte manufacturing processes. The synthesis of fluorinated polymers, however, presents environmental challenges due to the persistence of fluorinated compounds in the environment. Non-fluorinated alternatives like bio-derived cellulose-based polymers offer promising eco-friendly options with reduced environmental footprint.
Toxicity assessments of common SPE materials indicate generally favorable profiles. Most polymer matrices exhibit low acute toxicity and minimal bioaccumulation potential. However, certain additives like lithium salts require careful handling due to their reactivity with moisture and potential health impacts. Nano-sized ceramic fillers incorporated into composite SPEs warrant additional safety considerations regarding potential inhalation risks during manufacturing.
End-of-life management presents both challenges and opportunities for SPE-based batteries. The recyclability of solid-state batteries potentially exceeds that of conventional lithium-ion batteries due to easier separation of components. Research indicates that up to 90% of materials from SPE batteries could be recovered through appropriate recycling processes. Biodegradable polymer options are emerging as environmentally superior alternatives, though their high-temperature performance characteristics require further optimization.
Regulatory frameworks governing SPE safety are evolving globally. The European Union's REACH regulations and Battery Directive specifically address polymer-based battery components, while the United States Environmental Protection Agency provides guidelines for polymer material safety assessment. International standards organizations are developing specialized protocols for solid-state battery safety evaluation, including thermal runaway resistance testing at elevated temperatures.
Risk mitigation strategies for SPE implementation include comprehensive material safety data documentation, controlled manufacturing environments, and specialized recycling infrastructure development. Manufacturers are increasingly adopting green chemistry principles in SPE formulation, prioritizing non-toxic catalysts and environmentally benign processing methods to enhance overall sustainability profiles.
Environmental considerations for SPE materials extend throughout their lifecycle. During production, many polymer electrolytes require fewer toxic solvents than liquid electrolyte manufacturing processes. The synthesis of fluorinated polymers, however, presents environmental challenges due to the persistence of fluorinated compounds in the environment. Non-fluorinated alternatives like bio-derived cellulose-based polymers offer promising eco-friendly options with reduced environmental footprint.
Toxicity assessments of common SPE materials indicate generally favorable profiles. Most polymer matrices exhibit low acute toxicity and minimal bioaccumulation potential. However, certain additives like lithium salts require careful handling due to their reactivity with moisture and potential health impacts. Nano-sized ceramic fillers incorporated into composite SPEs warrant additional safety considerations regarding potential inhalation risks during manufacturing.
End-of-life management presents both challenges and opportunities for SPE-based batteries. The recyclability of solid-state batteries potentially exceeds that of conventional lithium-ion batteries due to easier separation of components. Research indicates that up to 90% of materials from SPE batteries could be recovered through appropriate recycling processes. Biodegradable polymer options are emerging as environmentally superior alternatives, though their high-temperature performance characteristics require further optimization.
Regulatory frameworks governing SPE safety are evolving globally. The European Union's REACH regulations and Battery Directive specifically address polymer-based battery components, while the United States Environmental Protection Agency provides guidelines for polymer material safety assessment. International standards organizations are developing specialized protocols for solid-state battery safety evaluation, including thermal runaway resistance testing at elevated temperatures.
Risk mitigation strategies for SPE implementation include comprehensive material safety data documentation, controlled manufacturing environments, and specialized recycling infrastructure development. Manufacturers are increasingly adopting green chemistry principles in SPE formulation, prioritizing non-toxic catalysts and environmentally benign processing methods to enhance overall sustainability profiles.
Scalability and Manufacturing Considerations
The scalability of solid polymer electrolyte (SPE) manufacturing represents a critical factor in the widespread adoption of high-temperature battery technologies. Current laboratory-scale production methods often involve solution casting or hot-pressing techniques that yield excellent results for research purposes but face significant challenges when transitioning to industrial-scale production. These challenges include maintaining consistent electrolyte thickness, ensuring uniform distribution of additives, and preserving the desired mechanical properties across large-area films.
Manufacturing considerations for SPE optimization must address several key aspects. First, the selection of polymer materials must balance not only electrochemical performance but also processability characteristics such as melt flow index, thermal stability during processing, and compatibility with existing manufacturing equipment. Polymers like PVDF-HFP and PEO offer promising electrochemical properties but require different processing approaches that impact production throughput and cost structures.
Roll-to-roll manufacturing emerges as the most promising approach for large-scale SPE production, potentially reducing production costs by 40-60% compared to batch processes. This continuous manufacturing method allows for inline quality control and can achieve production speeds of 5-20 meters per minute depending on the polymer system. However, the high temperatures required for processing certain polymer electrolytes (150-200°C) necessitate specialized equipment and careful thermal management to prevent degradation of temperature-sensitive components.
Crosslinking processes, essential for enhancing the mechanical and thermal stability of SPEs at elevated temperatures, introduce additional manufacturing complexities. UV-initiated crosslinking offers rapid processing capabilities but may result in depth-dependent crosslinking density, while thermal crosslinking provides more uniform properties but extends production cycle times significantly. Recent innovations in electron-beam crosslinking show promise for balancing these considerations.
The integration of ceramic fillers into polymer matrices for composite electrolytes presents another manufacturing challenge. Achieving homogeneous dispersion of nano-sized ceramic particles (typically 1-10 wt%) requires specialized mixing equipment and often necessitates surface modification of particles to prevent agglomeration. Scalable methods for ensuring consistent filler distribution remain an active area of research and development.
Economic analysis indicates that material costs currently dominate SPE production expenses, with specialty polymers and high-purity ceramic fillers accounting for 40-60% of total manufacturing costs. Process optimization and economies of scale could potentially reduce these costs by 30-50% over the next five years, making high-temperature SPE batteries more commercially viable across various applications including electric vehicles and grid storage systems.
Manufacturing considerations for SPE optimization must address several key aspects. First, the selection of polymer materials must balance not only electrochemical performance but also processability characteristics such as melt flow index, thermal stability during processing, and compatibility with existing manufacturing equipment. Polymers like PVDF-HFP and PEO offer promising electrochemical properties but require different processing approaches that impact production throughput and cost structures.
Roll-to-roll manufacturing emerges as the most promising approach for large-scale SPE production, potentially reducing production costs by 40-60% compared to batch processes. This continuous manufacturing method allows for inline quality control and can achieve production speeds of 5-20 meters per minute depending on the polymer system. However, the high temperatures required for processing certain polymer electrolytes (150-200°C) necessitate specialized equipment and careful thermal management to prevent degradation of temperature-sensitive components.
Crosslinking processes, essential for enhancing the mechanical and thermal stability of SPEs at elevated temperatures, introduce additional manufacturing complexities. UV-initiated crosslinking offers rapid processing capabilities but may result in depth-dependent crosslinking density, while thermal crosslinking provides more uniform properties but extends production cycle times significantly. Recent innovations in electron-beam crosslinking show promise for balancing these considerations.
The integration of ceramic fillers into polymer matrices for composite electrolytes presents another manufacturing challenge. Achieving homogeneous dispersion of nano-sized ceramic particles (typically 1-10 wt%) requires specialized mixing equipment and often necessitates surface modification of particles to prevent agglomeration. Scalable methods for ensuring consistent filler distribution remain an active area of research and development.
Economic analysis indicates that material costs currently dominate SPE production expenses, with specialty polymers and high-purity ceramic fillers accounting for 40-60% of total manufacturing costs. Process optimization and economies of scale could potentially reduce these costs by 30-50% over the next five years, making high-temperature SPE batteries more commercially viable across various applications including electric vehicles and grid storage systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!