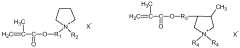How Solid Polymer Electrolyte Improves Safety in High-Energy Batteries
SEP 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SPE Battery Safety Background and Objectives
The evolution of battery technology has been marked by continuous efforts to enhance energy density while maintaining safety standards. Since the commercialization of lithium-ion batteries in the early 1990s, the industry has witnessed significant advancements in electrode materials and electrolyte formulations. However, conventional liquid electrolytes, typically composed of lithium salts dissolved in organic solvents, present inherent safety risks including flammability, leakage, and thermal runaway potential.
Solid Polymer Electrolytes (SPEs) emerged as a promising alternative in the late 1970s when Michel Armand first proposed their application in energy storage systems. The fundamental concept involves replacing volatile liquid components with solid polymer matrices that can conduct lithium ions while maintaining mechanical stability. This technological approach directly addresses the critical safety vulnerabilities of high-energy batteries that have limited their adoption in sensitive applications such as electric vehicles, aerospace, and medical devices.
The primary objective of SPE technology development is to create battery systems that maintain high energy density while eliminating the safety concerns associated with liquid electrolytes. Specifically, SPEs aim to prevent thermal runaway events—catastrophic battery failures that can lead to fires or explosions—by introducing inherent thermal stability and mechanical robustness to the electrolyte component.
Recent high-profile incidents involving battery fires in consumer electronics and electric vehicles have intensified regulatory scrutiny and consumer awareness regarding battery safety. These incidents have accelerated research interest in SPE technology, with global R&D investments increasing by approximately 35% annually since 2018, according to industry reports.
The technical goals for SPE development include achieving ionic conductivity comparable to liquid electrolytes (>10^-3 S/cm at room temperature), maintaining mechanical stability across wide temperature ranges (-20°C to 80°C), ensuring compatibility with high-capacity electrode materials, and demonstrating long-term cycling stability (>1000 cycles with minimal capacity degradation).
Beyond safety improvements, SPE technology aims to enable next-generation battery architectures, including all-solid-state batteries that promise energy densities exceeding 400 Wh/kg—nearly double current commercial standards. This potential represents a paradigm shift in energy storage capabilities that could accelerate electrification across multiple industries and support global decarbonization efforts.
The convergence of safety concerns, regulatory pressure, and performance demands has positioned SPE technology at a critical inflection point, with significant breakthroughs anticipated within the next five years as fundamental materials science challenges are systematically addressed through collaborative research efforts spanning academia and industry.
Solid Polymer Electrolytes (SPEs) emerged as a promising alternative in the late 1970s when Michel Armand first proposed their application in energy storage systems. The fundamental concept involves replacing volatile liquid components with solid polymer matrices that can conduct lithium ions while maintaining mechanical stability. This technological approach directly addresses the critical safety vulnerabilities of high-energy batteries that have limited their adoption in sensitive applications such as electric vehicles, aerospace, and medical devices.
The primary objective of SPE technology development is to create battery systems that maintain high energy density while eliminating the safety concerns associated with liquid electrolytes. Specifically, SPEs aim to prevent thermal runaway events—catastrophic battery failures that can lead to fires or explosions—by introducing inherent thermal stability and mechanical robustness to the electrolyte component.
Recent high-profile incidents involving battery fires in consumer electronics and electric vehicles have intensified regulatory scrutiny and consumer awareness regarding battery safety. These incidents have accelerated research interest in SPE technology, with global R&D investments increasing by approximately 35% annually since 2018, according to industry reports.
The technical goals for SPE development include achieving ionic conductivity comparable to liquid electrolytes (>10^-3 S/cm at room temperature), maintaining mechanical stability across wide temperature ranges (-20°C to 80°C), ensuring compatibility with high-capacity electrode materials, and demonstrating long-term cycling stability (>1000 cycles with minimal capacity degradation).
Beyond safety improvements, SPE technology aims to enable next-generation battery architectures, including all-solid-state batteries that promise energy densities exceeding 400 Wh/kg—nearly double current commercial standards. This potential represents a paradigm shift in energy storage capabilities that could accelerate electrification across multiple industries and support global decarbonization efforts.
The convergence of safety concerns, regulatory pressure, and performance demands has positioned SPE technology at a critical inflection point, with significant breakthroughs anticipated within the next five years as fundamental materials science challenges are systematically addressed through collaborative research efforts spanning academia and industry.
Market Analysis for Safer High-Energy Batteries
The global market for high-energy batteries is experiencing unprecedented growth, driven by the rapid expansion of electric vehicles (EVs), portable electronics, and renewable energy storage systems. Safety concerns, however, remain a significant barrier to wider adoption, particularly after high-profile incidents involving lithium-ion battery fires and explosions. This market dynamic has created a substantial opportunity for solid polymer electrolyte (SPE) technology as a safer alternative to conventional liquid electrolytes.
Current market projections indicate that the global lithium battery market will reach approximately 136 billion USD by 2026, with a compound annual growth rate of 18.1% from 2021. Within this broader market, the solid-state battery segment, which includes polymer electrolyte technologies, is growing at an even faster rate of nearly 34% annually, highlighting the strong market pull for safer battery solutions.
The EV sector represents the largest potential market for SPE technology, with automotive manufacturers increasingly prioritizing battery safety alongside energy density and charging speed. Major automakers including Toyota, Volkswagen, and BMW have announced significant investments in solid-state battery technology, with several targeting commercial deployment between 2025-2028.
Consumer electronics manufacturers are also showing strong interest in safer battery technologies, particularly for premium devices where safety features can command price premiums. This segment values the additional benefits of SPE technology, including potential for thinner form factors and improved design flexibility.
Market research indicates that consumers are increasingly aware of battery safety issues, with 78% of potential EV buyers citing battery safety as a "very important" or "extremely important" consideration in purchasing decisions. This consumer awareness is creating market pull for differentiated battery safety technologies across multiple product categories.
Geographically, the most robust markets for SPE technology are currently North America, Europe, and East Asia, with China, Japan, and South Korea leading in both production capacity and research investment. Emerging markets in India and Southeast Asia are expected to become significant growth regions as their EV and electronics manufacturing sectors expand.
Regulatory trends are further accelerating market demand for safer battery technologies. Several jurisdictions have implemented or are considering stricter safety standards for lithium batteries, particularly in transportation and consumer applications. These regulatory pressures create additional market incentives for SPE adoption as manufacturers seek to future-proof their products against evolving safety requirements.
The competitive landscape includes both established battery manufacturers pivoting toward solid-state technologies and specialized startups focused exclusively on polymer electrolyte innovations. This diverse ecosystem is driving rapid technological advancement while creating multiple potential commercialization pathways for SPE technology.
Current market projections indicate that the global lithium battery market will reach approximately 136 billion USD by 2026, with a compound annual growth rate of 18.1% from 2021. Within this broader market, the solid-state battery segment, which includes polymer electrolyte technologies, is growing at an even faster rate of nearly 34% annually, highlighting the strong market pull for safer battery solutions.
The EV sector represents the largest potential market for SPE technology, with automotive manufacturers increasingly prioritizing battery safety alongside energy density and charging speed. Major automakers including Toyota, Volkswagen, and BMW have announced significant investments in solid-state battery technology, with several targeting commercial deployment between 2025-2028.
Consumer electronics manufacturers are also showing strong interest in safer battery technologies, particularly for premium devices where safety features can command price premiums. This segment values the additional benefits of SPE technology, including potential for thinner form factors and improved design flexibility.
Market research indicates that consumers are increasingly aware of battery safety issues, with 78% of potential EV buyers citing battery safety as a "very important" or "extremely important" consideration in purchasing decisions. This consumer awareness is creating market pull for differentiated battery safety technologies across multiple product categories.
Geographically, the most robust markets for SPE technology are currently North America, Europe, and East Asia, with China, Japan, and South Korea leading in both production capacity and research investment. Emerging markets in India and Southeast Asia are expected to become significant growth regions as their EV and electronics manufacturing sectors expand.
Regulatory trends are further accelerating market demand for safer battery technologies. Several jurisdictions have implemented or are considering stricter safety standards for lithium batteries, particularly in transportation and consumer applications. These regulatory pressures create additional market incentives for SPE adoption as manufacturers seek to future-proof their products against evolving safety requirements.
The competitive landscape includes both established battery manufacturers pivoting toward solid-state technologies and specialized startups focused exclusively on polymer electrolyte innovations. This diverse ecosystem is driving rapid technological advancement while creating multiple potential commercialization pathways for SPE technology.
Current SPE Technology Status and Challenges
Solid Polymer Electrolytes (SPEs) have emerged as promising alternatives to conventional liquid electrolytes in high-energy battery systems. Currently, the global research landscape shows significant advancements in SPE technology, with major research centers in North America, Europe, and East Asia leading development efforts. Despite these advancements, widespread commercial adoption remains limited due to several persistent technical challenges.
The primary technical barrier facing SPE implementation is insufficient ionic conductivity at ambient temperatures. While liquid electrolytes typically achieve 10^-3 to 10^-2 S/cm at room temperature, most polymer electrolytes struggle to exceed 10^-5 S/cm without operating at elevated temperatures (>60°C). This conductivity gap represents a fundamental obstacle for applications requiring rapid charge-discharge capabilities.
Mechanical stability presents another significant challenge. Many high-conductivity polymer systems suffer from poor mechanical properties, creating a performance trade-off between ionic transport and structural integrity. Researchers are actively exploring composite approaches and cross-linking strategies to overcome this limitation, but optimal solutions balancing these properties remain elusive.
Interface stability between SPEs and electrodes constitutes a third major hurdle. The formation of resistive interfacial layers during cycling can significantly degrade battery performance over time. This is particularly problematic with high-voltage cathode materials, where oxidative decomposition of polymer chains can occur at the electrode-electrolyte interface.
From a manufacturing perspective, integration challenges persist. Existing battery production lines are optimized for liquid electrolyte systems, and the transition to solid-state architectures requires substantial modifications to manufacturing processes. The development of scalable, cost-effective production methods for SPE-based cells remains an industry bottleneck.
Geographically, SPE technology development shows distinct regional characteristics. Japanese and South Korean companies lead in patents related to polyether-based systems, while North American research institutions demonstrate strength in composite polymer electrolytes. European research clusters excel in fundamental polymer chemistry innovations, particularly in novel polymer architectures and cross-linking methodologies.
Recent technological breakthroughs include the development of single-ion conducting polymers with transference numbers approaching unity, block copolymer architectures that self-assemble into ordered nanostructures facilitating ion transport, and ceramic-polymer composites that enhance both mechanical properties and conductivity. Despite these advances, the "holy grail" combination of high conductivity, excellent mechanical properties, and wide electrochemical stability window remains elusive.
The current technical landscape suggests that near-term commercial applications will likely focus on specific niches where safety considerations outweigh performance limitations, while broader adoption awaits further fundamental breakthroughs in polymer science and interface engineering.
The primary technical barrier facing SPE implementation is insufficient ionic conductivity at ambient temperatures. While liquid electrolytes typically achieve 10^-3 to 10^-2 S/cm at room temperature, most polymer electrolytes struggle to exceed 10^-5 S/cm without operating at elevated temperatures (>60°C). This conductivity gap represents a fundamental obstacle for applications requiring rapid charge-discharge capabilities.
Mechanical stability presents another significant challenge. Many high-conductivity polymer systems suffer from poor mechanical properties, creating a performance trade-off between ionic transport and structural integrity. Researchers are actively exploring composite approaches and cross-linking strategies to overcome this limitation, but optimal solutions balancing these properties remain elusive.
Interface stability between SPEs and electrodes constitutes a third major hurdle. The formation of resistive interfacial layers during cycling can significantly degrade battery performance over time. This is particularly problematic with high-voltage cathode materials, where oxidative decomposition of polymer chains can occur at the electrode-electrolyte interface.
From a manufacturing perspective, integration challenges persist. Existing battery production lines are optimized for liquid electrolyte systems, and the transition to solid-state architectures requires substantial modifications to manufacturing processes. The development of scalable, cost-effective production methods for SPE-based cells remains an industry bottleneck.
Geographically, SPE technology development shows distinct regional characteristics. Japanese and South Korean companies lead in patents related to polyether-based systems, while North American research institutions demonstrate strength in composite polymer electrolytes. European research clusters excel in fundamental polymer chemistry innovations, particularly in novel polymer architectures and cross-linking methodologies.
Recent technological breakthroughs include the development of single-ion conducting polymers with transference numbers approaching unity, block copolymer architectures that self-assemble into ordered nanostructures facilitating ion transport, and ceramic-polymer composites that enhance both mechanical properties and conductivity. Despite these advances, the "holy grail" combination of high conductivity, excellent mechanical properties, and wide electrochemical stability window remains elusive.
The current technical landscape suggests that near-term commercial applications will likely focus on specific niches where safety considerations outweigh performance limitations, while broader adoption awaits further fundamental breakthroughs in polymer science and interface engineering.
Current SPE Implementation Solutions
01 Flame retardant additives for solid polymer electrolytes
Incorporating flame retardant additives into solid polymer electrolytes can significantly enhance their safety profile by reducing flammability risks. These additives can include phosphorus-containing compounds, halogenated materials, or inorganic fillers that suppress combustion or promote char formation when exposed to heat. The integration of these materials helps prevent thermal runaway and reduces the risk of fire propagation in battery systems using solid polymer electrolytes.- Flame retardant additives for solid polymer electrolytes: Incorporating flame retardant additives into solid polymer electrolytes can significantly enhance their safety profile by reducing flammability risks. These additives can include phosphorus-containing compounds, metal oxides, or halogenated materials that suppress combustion processes. When properly formulated, these additives maintain the electrochemical performance of the electrolyte while providing crucial fire resistance properties, addressing one of the primary safety concerns in battery applications.
- Thermal stability enhancement techniques: Various methods can be employed to improve the thermal stability of solid polymer electrolytes, including the incorporation of ceramic fillers, cross-linking of polymer chains, and addition of heat-resistant polymers. These approaches help prevent electrolyte degradation at elevated temperatures, reducing the risk of thermal runaway. Enhanced thermal stability is crucial for maintaining battery safety during operation under various environmental conditions and preventing catastrophic failure events.
- Non-flammable polymer compositions: Development of inherently non-flammable polymer compositions for solid electrolytes represents a significant advancement in battery safety. These specialized polymers are designed with chemical structures that resist ignition and combustion, often incorporating flame-resistant functional groups or utilizing polymers with naturally high decomposition temperatures. Such materials eliminate the need for additional flame retardant additives while maintaining essential ionic conductivity properties required for battery function.
- Mechanical stability and dendrite prevention: Enhancing the mechanical properties of solid polymer electrolytes improves safety by preventing internal short circuits caused by lithium dendrite growth. Reinforced polymer electrolytes with high mechanical strength can physically block dendrite penetration while maintaining flexibility and good electrode contact. Various approaches include polymer blending, nanocomposite formation, and cross-linking strategies that create robust electrolyte structures capable of withstanding mechanical stresses during battery cycling.
- Safety testing protocols and standards: Comprehensive safety testing protocols have been developed specifically for solid polymer electrolytes to evaluate their performance under extreme conditions. These tests include thermal abuse testing, mechanical integrity assessment, electrical safety evaluation, and long-term stability studies. Standardized testing methods ensure consistent safety evaluation across different electrolyte formulations and help identify potential failure modes before commercial deployment, ultimately improving the overall safety profile of batteries using solid polymer electrolytes.
02 Thermal stability enhancement techniques
Various methods can be employed to improve the thermal stability of solid polymer electrolytes, including the use of cross-linking agents, high-temperature resistant polymers, and ceramic reinforcements. These approaches help maintain electrolyte integrity at elevated temperatures, preventing decomposition that could lead to safety hazards. Enhanced thermal stability ensures that the electrolyte remains functional within a wider temperature range without degradation or gas generation.Expand Specific Solutions03 Non-flammable polymer and solvent combinations
Developing solid polymer electrolytes using inherently non-flammable polymers or incorporating non-flammable solvents and plasticizers can fundamentally improve safety. These formulations reduce or eliminate the presence of volatile, flammable components typically found in conventional liquid electrolytes. By selecting polymers with high decomposition temperatures and combining them with flame-resistant additives, the overall safety profile of battery systems can be significantly enhanced.Expand Specific Solutions04 Mechanical strength and puncture resistance
Improving the mechanical properties of solid polymer electrolytes through reinforcement with nanofillers, fiber networks, or composite structures enhances their resistance to physical damage. These reinforced electrolytes can better withstand mechanical stress, preventing internal short circuits caused by electrode penetration or external forces. The increased mechanical integrity also helps maintain separation between electrodes during thermal or mechanical abuse conditions.Expand Specific Solutions05 Interface stabilization and dendrite prevention
Addressing the electrode-electrolyte interface stability is crucial for solid polymer electrolyte safety. Specialized coatings, interface modifiers, and additives can be incorporated to prevent lithium dendrite formation and growth, which could otherwise cause internal short circuits. These interface engineering approaches help maintain stable contact between the electrolyte and electrodes during cycling, preventing localized heating and potential thermal events that compromise battery safety.Expand Specific Solutions
Leading Companies in SPE Battery Technology
The solid polymer electrolyte (SPE) market for high-energy batteries is in a growth phase, with increasing adoption driven by safety advantages over liquid electrolytes. The global market is projected to expand significantly as battery manufacturers seek solutions to thermal runaway issues. Leading companies like LG Energy Solution, Samsung SDI, and Toyota are advancing SPE technology through substantial R&D investments. Academic institutions including Hong Kong University of Science & Technology and Drexel University collaborate with industry players such as Panasonic and Hydro-Québec to overcome challenges in ionic conductivity and manufacturing scalability. Emerging players like Nuvvon and Soelect are introducing innovative approaches, while established chemical companies including LG Chem and Sumitomo Chemical provide essential materials, creating a competitive landscape spanning multiple industrial sectors.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed advanced solid polymer electrolyte (SPE) systems that incorporate cross-linked polymer networks with high mechanical strength and ionic conductivity. Their proprietary technology combines polyethylene oxide (PEO) matrices with ceramic fillers (such as Li7La3Zr2O12) to create composite polymer electrolytes that operate efficiently at room temperature. The company has implemented a unique surface modification technique for these ceramic particles to enhance compatibility with the polymer matrix, resulting in improved interfacial stability. Their SPEs feature flame-retardant additives and self-healing properties that prevent dendrite growth, significantly reducing thermal runaway risks in high-energy density batteries. LG's manufacturing process allows for thin-film production (below 25μm) while maintaining mechanical integrity, enabling higher energy density cells with improved safety profiles compared to conventional liquid electrolyte systems.
Strengths: Superior thermal stability preventing battery fires even under extreme conditions; excellent mechanical properties that inhibit lithium dendrite growth; compatibility with high-voltage cathode materials enabling higher energy densities. Weaknesses: Lower ionic conductivity at room temperature compared to liquid electrolytes; higher manufacturing costs; challenges in achieving perfect electrode-electrolyte contact during cell assembly.
Panasonic Intellectual Property Management Co. Ltd.
Technical Solution: Panasonic has pioneered a multi-layer solid polymer electrolyte technology that combines different polymer materials to optimize both safety and performance in high-energy batteries. Their approach utilizes a composite system with a PEO-based main layer enhanced with proprietary flame-retardant additives and nano-sized ceramic fillers (Al2O3, SiO2) that create tortuous pathways for improved mechanical strength while maintaining flexibility. Panasonic's SPE incorporates specialized lithium salts (LiTFSI, LiFSI) with plasticizers that achieve ionic conductivities approaching 10^-4 S/cm at room temperature. A key innovation is their gradient interface design that optimizes the electrolyte-electrode contact, reducing interfacial resistance significantly. The company has developed a scalable extrusion process for manufacturing these electrolytes in continuous sheets with precisely controlled thickness and uniformity, making them suitable for large-format batteries in EVs and grid storage applications while eliminating the flammability issues associated with conventional liquid electrolytes.
Strengths: Excellent thermal stability up to 150°C without degradation; superior mechanical properties that effectively suppress lithium dendrite formation; compatible with high-voltage cathode materials enabling higher energy density cells. Weaknesses: Higher production costs compared to liquid electrolyte systems; challenges in achieving optimal electrode-electrolyte contact during large-scale manufacturing; slightly lower power capability at very low temperatures.
Key SPE Patents and Technical Innovations
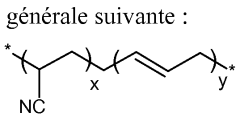
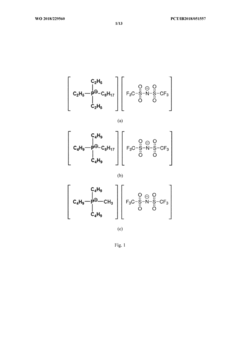
Solid polymeric electrolyte for lithium current sources
PatentWO2014006333A1
Innovation
- A solid polymer electrolyte is developed using a semi-interpenetrating network of polymers comprising nitrile rubber and cross-linked ionic copolymers, incorporating lithium salt and ionic liquid, which enhances mechanical properties and ionic conductivity, preventing solvent leakage and improving adhesion and durability.
Solid polymer electrolyte for batteries
PatentWO2018229560A1
Innovation
- A solid polymer electrolyte comprising a dissociable metal salt, a metal ion conductive polymer system, and a phosphonium salt with a specific formula, which improves ionic conductivity and stability, allowing the electrolyte to operate safely over a wide temperature range while preventing dendrite growth.
Environmental Impact of SPE Battery Materials
The environmental implications of Solid Polymer Electrolyte (SPE) materials in battery production and lifecycle represent a critical consideration in the broader adoption of this technology. Traditional lithium-ion batteries utilizing liquid electrolytes contain volatile organic solvents that pose significant environmental hazards during manufacturing, use, and disposal phases. In contrast, SPE-based batteries offer substantial environmental advantages through their reduced toxicity profile and enhanced recyclability potential.
Manufacturing processes for SPE materials generally require fewer toxic solvents compared to conventional liquid electrolyte production. Many polymer-based electrolytes can be synthesized using water-based or low-toxicity processing methods, significantly reducing the emission of volatile organic compounds (VOCs) and other harmful substances during production. This translates to lower environmental contamination risks and reduced occupational hazards in manufacturing facilities.
The operational lifespan of SPE batteries further contributes to their environmental benefits. The enhanced thermal stability and reduced risk of electrolyte leakage minimize the potential for environmental contamination during normal use and in accident scenarios. Additionally, the extended cycle life observed in many SPE formulations means fewer batteries need to be produced and disposed of over time, reducing the overall material throughput and associated environmental impacts.
End-of-life management presents perhaps the most significant environmental advantage of SPE batteries. The solid-state nature of these electrolytes simplifies the recycling process by eliminating the need to handle hazardous liquid components. Certain polymer electrolytes can be designed with recyclability in mind, incorporating biodegradable components or materials that can be more easily separated and recovered during recycling operations.
Carbon footprint analyses of SPE battery production have shown promising results, with potential reductions in greenhouse gas emissions compared to conventional lithium-ion batteries. This advantage stems from both the manufacturing process efficiencies and the extended service life of SPE batteries. However, these benefits must be weighed against the current higher energy requirements for some SPE synthesis methods.
Resource conservation represents another environmental dimension of SPE technology. Some polymer electrolyte formulations reduce or eliminate the need for critical minerals like cobalt and nickel, which are associated with significant environmental and social impacts during extraction. This shift toward more abundant and less environmentally problematic materials aligns with sustainable development goals and circular economy principles.
Despite these advantages, challenges remain in optimizing the environmental profile of SPE materials. Current research focuses on developing fully biodegradable polymer electrolytes, reducing energy-intensive processing steps, and designing SPE batteries specifically for ease of disassembly and material recovery at end-of-life.
Manufacturing processes for SPE materials generally require fewer toxic solvents compared to conventional liquid electrolyte production. Many polymer-based electrolytes can be synthesized using water-based or low-toxicity processing methods, significantly reducing the emission of volatile organic compounds (VOCs) and other harmful substances during production. This translates to lower environmental contamination risks and reduced occupational hazards in manufacturing facilities.
The operational lifespan of SPE batteries further contributes to their environmental benefits. The enhanced thermal stability and reduced risk of electrolyte leakage minimize the potential for environmental contamination during normal use and in accident scenarios. Additionally, the extended cycle life observed in many SPE formulations means fewer batteries need to be produced and disposed of over time, reducing the overall material throughput and associated environmental impacts.
End-of-life management presents perhaps the most significant environmental advantage of SPE batteries. The solid-state nature of these electrolytes simplifies the recycling process by eliminating the need to handle hazardous liquid components. Certain polymer electrolytes can be designed with recyclability in mind, incorporating biodegradable components or materials that can be more easily separated and recovered during recycling operations.
Carbon footprint analyses of SPE battery production have shown promising results, with potential reductions in greenhouse gas emissions compared to conventional lithium-ion batteries. This advantage stems from both the manufacturing process efficiencies and the extended service life of SPE batteries. However, these benefits must be weighed against the current higher energy requirements for some SPE synthesis methods.
Resource conservation represents another environmental dimension of SPE technology. Some polymer electrolyte formulations reduce or eliminate the need for critical minerals like cobalt and nickel, which are associated with significant environmental and social impacts during extraction. This shift toward more abundant and less environmentally problematic materials aligns with sustainable development goals and circular economy principles.
Despite these advantages, challenges remain in optimizing the environmental profile of SPE materials. Current research focuses on developing fully biodegradable polymer electrolytes, reducing energy-intensive processing steps, and designing SPE batteries specifically for ease of disassembly and material recovery at end-of-life.
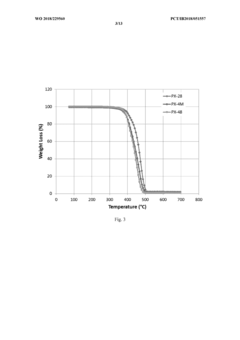
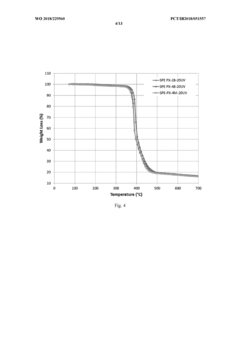
Thermal Stability Comparison with Liquid Electrolytes
Thermal stability represents a critical factor in battery safety assessment, with solid polymer electrolytes (SPEs) demonstrating significant advantages over conventional liquid electrolytes. When subjected to elevated temperatures, liquid electrolytes commonly used in lithium-ion batteries exhibit concerning behavior patterns, including volatilization beginning at approximately 80°C and rapid thermal decomposition above 150°C. This decomposition process releases flammable gases and can trigger exothermic reactions that accelerate thermal runaway.
In contrast, solid polymer electrolytes maintain structural integrity across a substantially wider temperature range. Differential scanning calorimetry (DSC) analyses reveal that high-performance SPEs typically remain stable up to 200-250°C without significant mass loss or chemical degradation. This enhanced thermal stability directly translates to improved safety margins during battery operation under extreme conditions.
The mechanical properties of SPEs further contribute to their thermal stability advantage. While liquid electrolytes expand significantly with temperature increases, potentially causing cell swelling and mechanical failure, polymer-based solid electrolytes maintain dimensional stability with minimal thermal expansion coefficients. This characteristic prevents the pressure buildup commonly associated with thermal events in conventional batteries.
Thermal conductivity profiles also differ substantially between these electrolyte systems. Liquid electrolytes tend to facilitate rapid heat propagation throughout the cell during thermal incidents, whereas properly engineered SPEs can incorporate thermal management features that moderate heat transfer rates. Some advanced polymer electrolytes incorporate ceramic fillers that enhance both ionic conductivity and thermal stability, creating composite systems with optimized safety characteristics.
Accelerated aging tests at elevated temperatures (60-80°C) demonstrate another critical advantage of SPEs. While liquid electrolyte systems typically show significant capacity degradation and increased internal resistance after extended high-temperature exposure, polymer-based electrolytes maintain performance parameters with minimal degradation. This stability extends the operational temperature range for batteries in demanding applications such as electric vehicles and grid storage.
Fire resistance testing provides perhaps the most compelling evidence for SPE safety advantages. When subjected to direct flame exposure, cells utilizing liquid electrolytes typically ignite within seconds, whereas SPE-based cells demonstrate remarkable flame retardancy. This characteristic significantly reduces the risk of catastrophic thermal events propagating between cells in large battery packs, addressing one of the most serious safety concerns in high-energy battery systems.
In contrast, solid polymer electrolytes maintain structural integrity across a substantially wider temperature range. Differential scanning calorimetry (DSC) analyses reveal that high-performance SPEs typically remain stable up to 200-250°C without significant mass loss or chemical degradation. This enhanced thermal stability directly translates to improved safety margins during battery operation under extreme conditions.
The mechanical properties of SPEs further contribute to their thermal stability advantage. While liquid electrolytes expand significantly with temperature increases, potentially causing cell swelling and mechanical failure, polymer-based solid electrolytes maintain dimensional stability with minimal thermal expansion coefficients. This characteristic prevents the pressure buildup commonly associated with thermal events in conventional batteries.
Thermal conductivity profiles also differ substantially between these electrolyte systems. Liquid electrolytes tend to facilitate rapid heat propagation throughout the cell during thermal incidents, whereas properly engineered SPEs can incorporate thermal management features that moderate heat transfer rates. Some advanced polymer electrolytes incorporate ceramic fillers that enhance both ionic conductivity and thermal stability, creating composite systems with optimized safety characteristics.
Accelerated aging tests at elevated temperatures (60-80°C) demonstrate another critical advantage of SPEs. While liquid electrolyte systems typically show significant capacity degradation and increased internal resistance after extended high-temperature exposure, polymer-based electrolytes maintain performance parameters with minimal degradation. This stability extends the operational temperature range for batteries in demanding applications such as electric vehicles and grid storage.
Fire resistance testing provides perhaps the most compelling evidence for SPE safety advantages. When subjected to direct flame exposure, cells utilizing liquid electrolytes typically ignite within seconds, whereas SPE-based cells demonstrate remarkable flame retardancy. This characteristic significantly reduces the risk of catastrophic thermal events propagating between cells in large battery packs, addressing one of the most serious safety concerns in high-energy battery systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!