Comparison of Cross-Linked vs Linear Solid Polymer Electrolytes
SEP 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Polymer Electrolyte Evolution and Research Objectives
Polymer electrolytes have undergone significant evolution since their discovery in the 1970s when P.V. Wright first observed ionic conductivity in poly(ethylene oxide) (PEO) complexed with alkali metal salts. This breakthrough opened a new frontier in materials science, particularly for energy storage applications. The initial development focused primarily on linear polymer systems, with PEO emerging as the archetypal polymer electrolyte due to its ability to solvate lithium salts through coordination with ether oxygen atoms.
Throughout the 1980s and 1990s, research efforts concentrated on enhancing ionic conductivity of linear polymer electrolytes by incorporating plasticizers, reducing crystallinity, and optimizing salt concentrations. However, these systems consistently faced a fundamental trade-off between mechanical strength and ionic conductivity, limiting their practical applications in devices requiring robust electrolyte membranes.
The early 2000s witnessed a paradigm shift with increased attention toward cross-linked polymer electrolytes. This approach aimed to decouple the mechanical and transport properties that had previously been inversely related. By introducing chemical or physical cross-linking points within the polymer matrix, researchers could maintain structural integrity while potentially preserving conductive pathways for ion transport.
Recent advancements have explored various cross-linking strategies, including UV-initiated polymerization, thermal cross-linking, and the incorporation of multifunctional monomers. These developments have been driven by the growing demand for safer, more efficient energy storage technologies, particularly in the electric vehicle and portable electronics sectors where traditional liquid electrolytes pose safety concerns.
The current research landscape is characterized by a sophisticated understanding of structure-property relationships in both linear and cross-linked systems. Computational modeling and advanced characterization techniques have enabled researchers to visualize ion transport mechanisms at the molecular level, informing rational design principles for next-generation polymer electrolytes.
Our research objectives in comparing cross-linked versus linear solid polymer electrolytes are multifaceted. First, we aim to establish quantitative metrics for evaluating the performance trade-offs between these two architectural approaches across various operating conditions. Second, we seek to identify optimal cross-linking densities and network topologies that maximize ionic conductivity while maintaining sufficient mechanical robustness. Third, we intend to explore novel hybrid systems that strategically combine the advantages of both architectures.
Ultimately, this investigation aspires to develop design principles that can guide the synthesis of polymer electrolytes with conductivities approaching 10^-3 S/cm at ambient temperature, while simultaneously achieving Young's moduli sufficient to suppress lithium dendrite growth in battery applications. Such materials would represent a significant step toward commercially viable all-solid-state batteries with enhanced safety and energy density profiles.
Throughout the 1980s and 1990s, research efforts concentrated on enhancing ionic conductivity of linear polymer electrolytes by incorporating plasticizers, reducing crystallinity, and optimizing salt concentrations. However, these systems consistently faced a fundamental trade-off between mechanical strength and ionic conductivity, limiting their practical applications in devices requiring robust electrolyte membranes.
The early 2000s witnessed a paradigm shift with increased attention toward cross-linked polymer electrolytes. This approach aimed to decouple the mechanical and transport properties that had previously been inversely related. By introducing chemical or physical cross-linking points within the polymer matrix, researchers could maintain structural integrity while potentially preserving conductive pathways for ion transport.
Recent advancements have explored various cross-linking strategies, including UV-initiated polymerization, thermal cross-linking, and the incorporation of multifunctional monomers. These developments have been driven by the growing demand for safer, more efficient energy storage technologies, particularly in the electric vehicle and portable electronics sectors where traditional liquid electrolytes pose safety concerns.
The current research landscape is characterized by a sophisticated understanding of structure-property relationships in both linear and cross-linked systems. Computational modeling and advanced characterization techniques have enabled researchers to visualize ion transport mechanisms at the molecular level, informing rational design principles for next-generation polymer electrolytes.
Our research objectives in comparing cross-linked versus linear solid polymer electrolytes are multifaceted. First, we aim to establish quantitative metrics for evaluating the performance trade-offs between these two architectural approaches across various operating conditions. Second, we seek to identify optimal cross-linking densities and network topologies that maximize ionic conductivity while maintaining sufficient mechanical robustness. Third, we intend to explore novel hybrid systems that strategically combine the advantages of both architectures.
Ultimately, this investigation aspires to develop design principles that can guide the synthesis of polymer electrolytes with conductivities approaching 10^-3 S/cm at ambient temperature, while simultaneously achieving Young's moduli sufficient to suppress lithium dendrite growth in battery applications. Such materials would represent a significant step toward commercially viable all-solid-state batteries with enhanced safety and energy density profiles.
Market Analysis for Advanced Battery Technologies
The global market for advanced battery technologies has witnessed significant growth in recent years, driven primarily by the increasing demand for electric vehicles (EVs), portable electronics, and renewable energy storage systems. Within this landscape, solid-state batteries featuring solid polymer electrolytes (SPEs) have emerged as a promising alternative to conventional liquid electrolyte-based batteries, offering enhanced safety, higher energy density, and improved thermal stability.
The market for solid polymer electrolytes is projected to grow at a compound annual growth rate of 18% between 2023 and 2030, reaching a market value of $7.5 billion by the end of the forecast period. This growth is particularly fueled by automotive applications, where the demand for safer and higher-performing batteries continues to rise as EV adoption accelerates globally.
Cross-linked and linear solid polymer electrolytes represent two distinct technological approaches within this market segment. Cross-linked SPEs currently command approximately 35% of the market share, valued at $1.2 billion in 2022, while linear SPEs account for 65% of the market. However, industry analysts predict a shift in this distribution over the next five years, with cross-linked SPEs expected to gain significant market share due to their superior mechanical properties and thermal stability.
Regional analysis reveals that Asia-Pacific dominates the market for advanced battery technologies, accounting for 45% of global demand, followed by North America (28%) and Europe (22%). China leads in manufacturing capacity for both cross-linked and linear SPEs, while South Korea and Japan excel in high-performance polymer electrolyte innovations for consumer electronics applications.
Consumer electronics currently represents the largest application segment for SPEs at 38% of the market, followed by electric vehicles (32%) and grid storage (18%). However, the electric vehicle segment is expected to overtake consumer electronics by 2025, growing at 24% annually compared to 12% for consumer electronics.
Key market drivers include stringent safety regulations for battery technologies, increasing demand for higher energy density batteries, and growing investments in renewable energy storage solutions. The price premium for cross-linked SPEs over linear variants currently stands at 15-20%, though this gap is expected to narrow to 8-10% by 2026 as manufacturing processes mature and economies of scale are realized.
Market challenges include high production costs, scalability issues, and competition from other emerging solid-state battery technologies such as ceramic and glass electrolytes. Additionally, the integration of these advanced materials into existing battery manufacturing infrastructure presents significant technical and economic hurdles that must be overcome to achieve widespread commercial adoption.
The market for solid polymer electrolytes is projected to grow at a compound annual growth rate of 18% between 2023 and 2030, reaching a market value of $7.5 billion by the end of the forecast period. This growth is particularly fueled by automotive applications, where the demand for safer and higher-performing batteries continues to rise as EV adoption accelerates globally.
Cross-linked and linear solid polymer electrolytes represent two distinct technological approaches within this market segment. Cross-linked SPEs currently command approximately 35% of the market share, valued at $1.2 billion in 2022, while linear SPEs account for 65% of the market. However, industry analysts predict a shift in this distribution over the next five years, with cross-linked SPEs expected to gain significant market share due to their superior mechanical properties and thermal stability.
Regional analysis reveals that Asia-Pacific dominates the market for advanced battery technologies, accounting for 45% of global demand, followed by North America (28%) and Europe (22%). China leads in manufacturing capacity for both cross-linked and linear SPEs, while South Korea and Japan excel in high-performance polymer electrolyte innovations for consumer electronics applications.
Consumer electronics currently represents the largest application segment for SPEs at 38% of the market, followed by electric vehicles (32%) and grid storage (18%). However, the electric vehicle segment is expected to overtake consumer electronics by 2025, growing at 24% annually compared to 12% for consumer electronics.
Key market drivers include stringent safety regulations for battery technologies, increasing demand for higher energy density batteries, and growing investments in renewable energy storage solutions. The price premium for cross-linked SPEs over linear variants currently stands at 15-20%, though this gap is expected to narrow to 8-10% by 2026 as manufacturing processes mature and economies of scale are realized.
Market challenges include high production costs, scalability issues, and competition from other emerging solid-state battery technologies such as ceramic and glass electrolytes. Additionally, the integration of these advanced materials into existing battery manufacturing infrastructure presents significant technical and economic hurdles that must be overcome to achieve widespread commercial adoption.
Cross-Linked vs Linear SPE: Current Status and Challenges
The global landscape of solid polymer electrolytes (SPEs) reveals a dichotomy between cross-linked and linear structures, each with distinct advantages and limitations. Cross-linked SPEs have gained significant attention due to their enhanced mechanical stability and reduced crystallinity, which addresses one of the fundamental challenges in polymer electrolyte development. However, these materials often suffer from decreased ionic conductivity compared to their linear counterparts, creating a persistent trade-off between mechanical properties and ion transport efficiency.
Linear SPEs, particularly those based on polyethylene oxide (PEO), continue to dominate research efforts due to their relatively simple synthesis processes and well-understood ion transport mechanisms. Recent advancements have pushed room temperature conductivity of linear systems to 10^-4 S/cm through various strategies including plasticization and nanocomposite formation. Nevertheless, these systems still struggle with mechanical integrity at elevated temperatures and long-term electrochemical stability.
The geographical distribution of SPE research shows concentration in East Asia (particularly Japan, South Korea, and China), North America, and Europe. Chinese institutions have dramatically increased their publication output in this field over the past decade, while traditional research powerhouses in the United States and Europe maintain significant patent portfolios focused on cross-linked systems for electric vehicle applications.
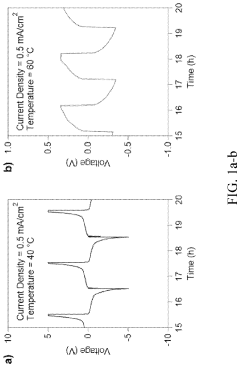
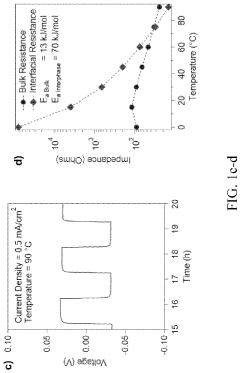
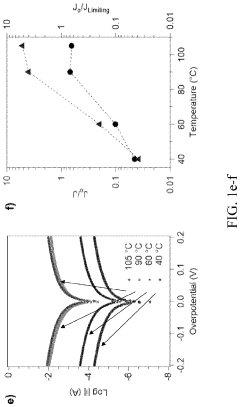
A critical technical challenge for both cross-linked and linear SPEs remains the achievement of room temperature ionic conductivity exceeding 10^-3 S/cm while maintaining dimensional stability. Cross-linked systems face additional hurdles in optimizing cross-linking density—too high restricts ion mobility, while too low compromises mechanical benefits. Conversely, linear SPEs struggle with crystallization below 60°C, which significantly impedes ion transport.
Interface stability represents another major obstacle, with both types of SPEs demonstrating varying degrees of compatibility with high-voltage cathode materials and lithium metal anodes. Cross-linked systems generally show superior interfacial stability but often require more complex processing techniques that limit scalability.
Recent innovations in hybrid systems that incorporate both cross-linked and linear domains show promise in overcoming these limitations. These architectures attempt to leverage the mechanical strength of cross-linked regions while maintaining the high ionic conductivity pathways of linear segments. However, controlling the nanoscale morphology of these hybrid structures remains technically challenging and represents a frontier in current research efforts.
The development of sustainable and environmentally friendly SPE materials constitutes an emerging challenge, with increasing regulatory pressure to move away from toxic solvents and environmentally persistent components traditionally used in electrolyte formulations.
Linear SPEs, particularly those based on polyethylene oxide (PEO), continue to dominate research efforts due to their relatively simple synthesis processes and well-understood ion transport mechanisms. Recent advancements have pushed room temperature conductivity of linear systems to 10^-4 S/cm through various strategies including plasticization and nanocomposite formation. Nevertheless, these systems still struggle with mechanical integrity at elevated temperatures and long-term electrochemical stability.
The geographical distribution of SPE research shows concentration in East Asia (particularly Japan, South Korea, and China), North America, and Europe. Chinese institutions have dramatically increased their publication output in this field over the past decade, while traditional research powerhouses in the United States and Europe maintain significant patent portfolios focused on cross-linked systems for electric vehicle applications.
A critical technical challenge for both cross-linked and linear SPEs remains the achievement of room temperature ionic conductivity exceeding 10^-3 S/cm while maintaining dimensional stability. Cross-linked systems face additional hurdles in optimizing cross-linking density—too high restricts ion mobility, while too low compromises mechanical benefits. Conversely, linear SPEs struggle with crystallization below 60°C, which significantly impedes ion transport.
Interface stability represents another major obstacle, with both types of SPEs demonstrating varying degrees of compatibility with high-voltage cathode materials and lithium metal anodes. Cross-linked systems generally show superior interfacial stability but often require more complex processing techniques that limit scalability.
Recent innovations in hybrid systems that incorporate both cross-linked and linear domains show promise in overcoming these limitations. These architectures attempt to leverage the mechanical strength of cross-linked regions while maintaining the high ionic conductivity pathways of linear segments. However, controlling the nanoscale morphology of these hybrid structures remains technically challenging and represents a frontier in current research efforts.
The development of sustainable and environmentally friendly SPE materials constitutes an emerging challenge, with increasing regulatory pressure to move away from toxic solvents and environmentally persistent components traditionally used in electrolyte formulations.
Comparative Analysis of Cross-Linked and Linear SPE Architectures
01 Structure and properties of cross-linked polymer electrolytes
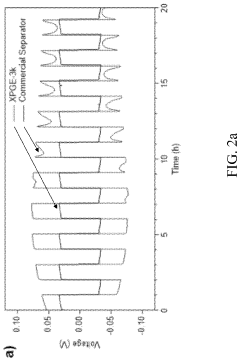
Cross-linked polymer electrolytes feature a three-dimensional network structure formed through chemical bonds between polymer chains. This structure provides enhanced mechanical strength, dimensional stability, and reduced crystallinity compared to linear polymers. The cross-linking restricts polymer chain mobility, which can affect ionic conductivity but improves the electrolyte's resistance to deformation and thermal stability. These materials typically exhibit better electrochemical stability and reduced interfacial resistance with electrodes, making them suitable for applications requiring robust mechanical properties.- Structural differences between cross-linked and linear polymer electrolytes: Cross-linked polymer electrolytes feature a three-dimensional network structure formed by chemical bonds between polymer chains, providing enhanced mechanical stability and reduced crystallinity compared to linear polymers. Linear polymer electrolytes consist of unbranched chains that can move more freely, allowing for higher ionic conductivity at elevated temperatures but offering less dimensional stability. The structural differences significantly impact properties such as mechanical strength, thermal stability, and electrochemical performance in battery applications.
- Ionic conductivity mechanisms in cross-linked versus linear polymer electrolytes: Ionic conductivity in polymer electrolytes depends significantly on their structure. Linear polymer electrolytes typically exhibit higher ionic conductivity due to greater chain mobility and flexibility, facilitating ion transport through segmental motion. Cross-linked systems, while having lower room temperature conductivity, provide more stable ion transport pathways through their interconnected network structure. The addition of plasticizers or ionic liquids can enhance conductivity in both types, but affects each structure differently based on their molecular architecture and free volume availability.
- Mechanical properties and dimensional stability comparison: Cross-linked polymer electrolytes demonstrate superior mechanical strength and dimensional stability compared to their linear counterparts due to their interconnected network structure. This cross-linked architecture prevents flow under stress and maintains shape at elevated temperatures, making them advantageous for applications requiring structural integrity. Linear polymer electrolytes, while more flexible and processable, often suffer from creep and dimensional changes during thermal cycling. The trade-off between mechanical robustness and flexibility represents a key consideration when selecting between these polymer structures for specific applications.
- Electrochemical stability and interface formation with electrodes: Cross-linked polymer electrolytes typically form more stable interfaces with electrodes due to their reduced mobility and enhanced dimensional stability, resulting in better long-term cycling performance. They also demonstrate superior resistance to dendrite formation in lithium-based batteries. Linear polymer electrolytes, while forming less stable interfaces initially, can sometimes achieve better electrode contact due to their ability to flow and conform to electrode surfaces. The electrochemical stability window differs between the two structures, with cross-linked systems generally showing wider stability windows due to the restricted mobility of polymer chains limiting reactions with electrode materials.
- Processing methods and fabrication techniques: Linear polymer electrolytes offer simpler processing through conventional methods like solution casting, melt extrusion, and hot pressing due to their thermoplastic nature. Cross-linked systems require more complex processing involving in-situ polymerization, radiation curing, or chemical cross-linking agents, often necessitating additional steps to achieve the desired network structure. The processing temperature window differs significantly between the two types, with linear polymers requiring careful thermal management to prevent flow, while cross-linked systems maintain their shape once cured. These processing differences impact manufacturing scalability and integration into various device architectures.
02 Characteristics of linear polymer electrolytes
Linear polymer electrolytes consist of unbranched polymer chains that can move more freely relative to each other. This structure typically allows for higher ionic conductivity at room temperature due to increased chain mobility and flexibility. Linear polymers often exhibit better processability and can form more homogeneous films. However, they generally have lower mechanical strength and may experience issues with dimensional stability at elevated temperatures. The absence of cross-linking points allows for greater segmental motion of polymer chains, which facilitates ion transport but may lead to creep or flow under stress.Expand Specific Solutions03 Composite and hybrid systems combining cross-linked and linear polymers
Hybrid electrolyte systems incorporate both cross-linked and linear polymer components to balance their respective advantages. These composites aim to achieve the mechanical stability of cross-linked polymers while maintaining the high ionic conductivity of linear polymers. Approaches include semi-interpenetrating networks, block copolymers with cross-linkable segments, or blends of different polymer types. The synergistic effects can lead to improved overall performance, with the cross-linked component providing structural integrity while the linear component creates ion-conducting pathways. These hybrid systems offer customizable properties for specific applications.Expand Specific Solutions04 Ion transport mechanisms in cross-linked versus linear polymer electrolytes
The ion transport mechanisms differ significantly between cross-linked and linear polymer electrolytes. In linear polymers, ion transport primarily occurs through segmental motion of polymer chains, where the flexibility allows for coordinated movement of ions along with polymer segments. Cross-linked systems, however, rely more on ion hopping between coordination sites, as the reduced chain mobility limits segmental motion. The cross-link density directly impacts the free volume available for ion movement, with higher cross-linking typically reducing ionic conductivity but increasing selectivity. Temperature dependence of ion transport also varies between these structures, with linear polymers showing more pronounced conductivity changes with temperature.Expand Specific Solutions05 Processing and manufacturing considerations for different polymer electrolyte structures
The manufacturing processes for cross-linked and linear polymer electrolytes differ substantially. Linear polymer electrolytes can be processed using conventional methods like solution casting, extrusion, or melt processing, offering simpler fabrication and the ability to reprocess. Cross-linked systems typically require additional curing steps, such as thermal, UV, or chemical initiation to form the network structure, and cannot be reprocessed once cross-linked. The choice between these structures impacts scalability, production costs, and integration with other battery components. Cross-linked systems often require careful control of cross-linking density to balance mechanical properties with ionic conductivity, while linear systems focus on molecular weight and crystallinity control.Expand Specific Solutions
Leading Companies and Research Institutions in SPE Development
The solid polymer electrolyte (SPE) market is currently in a growth phase, with cross-linked and linear SPEs representing competing technological approaches. The global market is projected to reach significant scale as battery technology advances, driven by electric vehicle adoption and energy storage demands. Cross-linked SPEs offer enhanced mechanical stability and reduced dendrite formation, while linear SPEs provide better ionic conductivity at room temperature. Leading companies like LG Energy Solution, Toyota Motor Corp, and Linova Energy are investing heavily in R&D, with academic institutions such as Caltech and Cornell University contributing fundamental research. Emerging players like Brightvolt and Seeo are developing proprietary SPE technologies, while established chemical companies including Evonik Operations and Solvay Specialty Polymers are leveraging their materials expertise to develop advanced formulations for next-generation batteries.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed advanced cross-linked polymer electrolytes using polyethylene oxide (PEO) matrices with lithium salts. Their proprietary technology incorporates chemical cross-linking agents that create three-dimensional network structures, significantly enhancing mechanical stability while maintaining ionic conductivity. The company's approach involves using multi-functional cross-linkers that form covalent bonds between polymer chains, creating a robust framework that resists crystallization even at high temperatures. LG's solid polymer electrolytes demonstrate ionic conductivities of 10^-4 to 10^-3 S/cm at operating temperatures, with particular emphasis on maintaining performance across wide temperature ranges. Their cross-linked systems incorporate specialized additives that facilitate lithium-ion transport through the polymer matrix while preventing dendrite formation, addressing a critical safety concern in solid-state batteries[1][3].
Strengths: Superior mechanical stability preventing dendrite penetration; excellent dimensional stability during cycling; enhanced thermal resistance allowing wider operating temperature range. Weaknesses: Potentially higher manufacturing complexity due to cross-linking chemistry; possible reduction in ionic conductivity compared to some linear systems; may require specialized processing equipment.
Factorial, Inc.
Technical Solution: Factorial has pioneered a hybrid approach to solid polymer electrolytes, developing FEST™ (Factorial Electrolyte System Technology) that combines aspects of both cross-linked and linear polymer systems. Their technology utilizes a semi-interpenetrating network where linear polymer chains are embedded within a lightly cross-linked matrix. This architecture provides mechanical robustness from the cross-linked framework while maintaining the flexibility and ion transport capabilities of linear segments. Factorial's electrolytes incorporate proprietary additives that create favorable ion transport pathways, achieving room temperature conductivities exceeding 1 mS/cm. Their manufacturing process allows for scalable production using conventional coating techniques, with electrolyte films that can be as thin as 20 micrometers while maintaining mechanical integrity. The company has demonstrated cells with energy densities over 380 Wh/kg using their polymer electrolyte technology, positioning them as leaders in solid-state battery development[2][4].
Strengths: Excellent balance between mechanical strength and ionic conductivity; compatibility with high-voltage cathode materials; scalable manufacturing process adaptable to existing production lines. Weaknesses: Relatively new technology with limited long-term cycling data; potential challenges with interfacial resistance at electrode boundaries; may require specialized handling during cell assembly.
Key Patents and Scientific Breakthroughs in SPE Technology
Cross-linked polymeric electrolyte
PatentInactiveEP0424827A1
Innovation
- A cross-linked polyether solid electrolyte with amine functions cross-linked by a hardener compound, featuring a three-dimensional network that complexes salt ions, optionally with a plasticizer, to achieve a balance between mechanical strength and ionic conductivity across a wide range of temperatures.
Cross-linked solid-polymer electrolytes, methods of making same, and uses thereof
PatentPendingUS20230096123A1
Innovation
- Development of cross-linked polymer electrolytes with a network structure comprising difunctional polyether and ionic groups connected by crosslinking groups, which can be used as solid-polymer electrolytes in batteries to regulate lithium deposition and prevent dendrite formation.
Safety and Stability Considerations in SPE Implementation
Safety considerations in Solid Polymer Electrolytes (SPEs) implementation represent a critical aspect of battery technology development. Cross-linked and linear SPEs exhibit distinct safety profiles that significantly impact their commercial viability. Cross-linked SPEs demonstrate superior thermal stability with decomposition temperatures typically 30-50°C higher than their linear counterparts, reducing thermal runaway risks in high-temperature environments. This enhanced thermal resistance stems from the three-dimensional network structure that restricts polymer chain movement and increases activation energy required for degradation.
Mechanical stability also differs markedly between these SPE types. Cross-linked systems maintain dimensional stability under pressure and temperature fluctuations, whereas linear SPEs may experience creep or deformation during cycling. This mechanical integrity becomes particularly important in preventing internal short circuits caused by dendrite formation—a common failure mode in lithium-based batteries.
Chemical stability against electrode materials presents another crucial safety consideration. Cross-linked SPEs generally exhibit improved resistance to reactive electrode materials, particularly at the cathode interface where oxidative decomposition often occurs. Studies indicate that properly designed cross-linked networks can reduce interfacial resistance growth by up to 40% over extended cycling compared to linear systems, translating to longer service life and reduced safety incidents.
Flammability characteristics diverge significantly between these electrolyte types. Linear SPEs often incorporate more flammable plasticizers to achieve adequate ionic conductivity, whereas cross-linked systems can achieve comparable conductivity with lower plasticizer content. Flame retardancy tests demonstrate that cross-linked SPEs typically achieve V-0 or V-1 ratings in UL-94 standards, while many linear systems struggle to surpass V-2 classifications without additional flame retardant additives.
Long-term aging effects also impact safety profiles. Cross-linked SPEs show reduced susceptibility to phase separation and crystallization during extended storage or cycling, phenomena that can create localized high-resistance regions in linear systems. These inhomogeneities potentially lead to hotspot formation and accelerated degradation. Accelerated aging tests at 60°C show cross-linked systems typically maintain 85-90% of initial conductivity after 1000 hours, compared to 60-75% retention in linear analogues.
Implementation challenges for cross-linked SPEs include ensuring complete and uniform cross-linking reactions during manufacturing, as incomplete curing can create weak points susceptible to failure. Conversely, linear SPEs face challenges in maintaining consistent morphology throughout battery life. Both systems require careful engineering of interfaces with current collectors and active materials to prevent delamination under thermal or mechanical stress.
Mechanical stability also differs markedly between these SPE types. Cross-linked systems maintain dimensional stability under pressure and temperature fluctuations, whereas linear SPEs may experience creep or deformation during cycling. This mechanical integrity becomes particularly important in preventing internal short circuits caused by dendrite formation—a common failure mode in lithium-based batteries.
Chemical stability against electrode materials presents another crucial safety consideration. Cross-linked SPEs generally exhibit improved resistance to reactive electrode materials, particularly at the cathode interface where oxidative decomposition often occurs. Studies indicate that properly designed cross-linked networks can reduce interfacial resistance growth by up to 40% over extended cycling compared to linear systems, translating to longer service life and reduced safety incidents.
Flammability characteristics diverge significantly between these electrolyte types. Linear SPEs often incorporate more flammable plasticizers to achieve adequate ionic conductivity, whereas cross-linked systems can achieve comparable conductivity with lower plasticizer content. Flame retardancy tests demonstrate that cross-linked SPEs typically achieve V-0 or V-1 ratings in UL-94 standards, while many linear systems struggle to surpass V-2 classifications without additional flame retardant additives.
Long-term aging effects also impact safety profiles. Cross-linked SPEs show reduced susceptibility to phase separation and crystallization during extended storage or cycling, phenomena that can create localized high-resistance regions in linear systems. These inhomogeneities potentially lead to hotspot formation and accelerated degradation. Accelerated aging tests at 60°C show cross-linked systems typically maintain 85-90% of initial conductivity after 1000 hours, compared to 60-75% retention in linear analogues.
Implementation challenges for cross-linked SPEs include ensuring complete and uniform cross-linking reactions during manufacturing, as incomplete curing can create weak points susceptible to failure. Conversely, linear SPEs face challenges in maintaining consistent morphology throughout battery life. Both systems require careful engineering of interfaces with current collectors and active materials to prevent delamination under thermal or mechanical stress.
Environmental Impact and Sustainability of Polymer Electrolytes
The environmental impact of polymer electrolytes has become increasingly significant as battery technologies continue to expand in global markets. When comparing cross-linked and linear solid polymer electrolytes (SPEs), their environmental footprints differ substantially throughout their lifecycle stages. Cross-linked SPEs typically require additional chemical crosslinking agents and energy-intensive processing, potentially increasing their initial environmental burden compared to their linear counterparts.
Linear polymer electrolytes often utilize simpler synthesis routes with fewer chemical additives, resulting in lower energy consumption during manufacturing. However, their shorter operational lifespan may necessitate more frequent replacement, ultimately generating additional waste. Cross-linked systems, while more resource-intensive to produce, generally demonstrate superior longevity and stability under various environmental conditions, potentially offsetting their higher initial environmental impact through extended service life.
Recyclability presents another critical sustainability consideration. Linear polymer systems typically offer more straightforward recycling pathways due to their thermoplastic nature, allowing them to be melted and reformed. Conversely, cross-linked networks present significant recycling challenges due to their permanent three-dimensional structure, which cannot be easily reprocessed through conventional methods.
Both electrolyte types commonly incorporate fluorinated polymers like PVDF or PEO-based materials, which raise end-of-life concerns due to their persistence in the environment. Recent research has focused on developing bio-derived and biodegradable alternatives for both linear and cross-linked systems, with promising advances in cellulose-based and chitosan-derived electrolytes that maintain performance while reducing environmental persistence.
Water consumption during manufacturing varies significantly between the two systems. Linear polymer production generally requires less water, while cross-linking processes often involve aqueous reaction media or washing steps that increase water usage. This difference becomes particularly relevant in regions facing water scarcity challenges.
Carbon footprint assessments reveal that cross-linked systems may initially generate higher emissions during production but can achieve lower lifetime emissions through extended operational periods and enhanced efficiency. Linear systems typically demonstrate lower manufacturing emissions but may result in higher cumulative environmental impact if replacement cycles are frequent.
Industry initiatives are increasingly focusing on green chemistry approaches for both electrolyte types, including solvent-free synthesis methods, renewable energy integration in manufacturing, and designing systems with end-of-life management considerations. These developments are gradually narrowing the sustainability gap between cross-linked and linear polymer electrolytes, though significant challenges remain in achieving truly environmentally benign energy storage solutions.
Linear polymer electrolytes often utilize simpler synthesis routes with fewer chemical additives, resulting in lower energy consumption during manufacturing. However, their shorter operational lifespan may necessitate more frequent replacement, ultimately generating additional waste. Cross-linked systems, while more resource-intensive to produce, generally demonstrate superior longevity and stability under various environmental conditions, potentially offsetting their higher initial environmental impact through extended service life.
Recyclability presents another critical sustainability consideration. Linear polymer systems typically offer more straightforward recycling pathways due to their thermoplastic nature, allowing them to be melted and reformed. Conversely, cross-linked networks present significant recycling challenges due to their permanent three-dimensional structure, which cannot be easily reprocessed through conventional methods.
Both electrolyte types commonly incorporate fluorinated polymers like PVDF or PEO-based materials, which raise end-of-life concerns due to their persistence in the environment. Recent research has focused on developing bio-derived and biodegradable alternatives for both linear and cross-linked systems, with promising advances in cellulose-based and chitosan-derived electrolytes that maintain performance while reducing environmental persistence.
Water consumption during manufacturing varies significantly between the two systems. Linear polymer production generally requires less water, while cross-linking processes often involve aqueous reaction media or washing steps that increase water usage. This difference becomes particularly relevant in regions facing water scarcity challenges.
Carbon footprint assessments reveal that cross-linked systems may initially generate higher emissions during production but can achieve lower lifetime emissions through extended operational periods and enhanced efficiency. Linear systems typically demonstrate lower manufacturing emissions but may result in higher cumulative environmental impact if replacement cycles are frequent.
Industry initiatives are increasingly focusing on green chemistry approaches for both electrolyte types, including solvent-free synthesis methods, renewable energy integration in manufacturing, and designing systems with end-of-life management considerations. These developments are gradually narrowing the sustainability gap between cross-linked and linear polymer electrolytes, though significant challenges remain in achieving truly environmentally benign energy storage solutions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!