Solid Polymer Electrolyte Market Analysis for Energy Storage Applications
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SPE Technology Background and Objectives
Solid polymer electrolytes (SPEs) have emerged as a transformative technology in the energy storage sector, particularly for advanced battery systems. The evolution of SPEs can be traced back to the 1970s when the first polymer-salt complexes were discovered to exhibit ionic conductivity. Since then, research has progressed through several generations of materials, from simple PEO-salt complexes to sophisticated composite and block copolymer systems that address the inherent limitations of early formulations.
The technological trajectory of SPEs has been driven by the growing demand for safer, higher-energy-density batteries that can meet the requirements of electric vehicles, portable electronics, and grid-scale energy storage. Traditional liquid electrolytes, while offering high ionic conductivity, present significant safety concerns due to their flammability and potential for leakage. SPEs offer a compelling alternative by eliminating these risks while potentially enabling the use of high-capacity electrode materials that are incompatible with conventional liquid systems.
Current SPE development focuses on overcoming the fundamental challenge of achieving sufficient ionic conductivity at ambient temperatures while maintaining mechanical stability. This represents a critical trade-off, as the segmental motion of polymer chains that facilitates ion transport typically compromises mechanical integrity. The field has seen significant breakthroughs in recent years through the incorporation of ceramic fillers, plasticizers, and the design of novel polymer architectures.
The global push toward electrification and renewable energy integration has accelerated SPE research, with particular emphasis on enabling next-generation battery chemistries such as lithium-sulfur and solid-state lithium metal batteries. These technologies promise energy densities 2-3 times higher than current lithium-ion systems but require the unique properties that advanced SPEs can provide.
The primary technical objectives in SPE development include achieving room temperature ionic conductivity exceeding 10^-3 S/cm, maintaining a wide electrochemical stability window (>4.5V), ensuring good interfacial contact with electrodes, and demonstrating long-term cycling stability. Secondary objectives focus on cost-effective manufacturing processes, environmental sustainability, and compatibility with existing battery production infrastructure.
As the energy storage landscape continues to evolve, SPEs are positioned to play a pivotal role in enabling safer, higher-performance batteries. The technology stands at an inflection point where fundamental materials science advances are beginning to translate into commercially viable products, with several major battery manufacturers and startups actively pursuing SPE-based energy storage solutions.
The technological trajectory of SPEs has been driven by the growing demand for safer, higher-energy-density batteries that can meet the requirements of electric vehicles, portable electronics, and grid-scale energy storage. Traditional liquid electrolytes, while offering high ionic conductivity, present significant safety concerns due to their flammability and potential for leakage. SPEs offer a compelling alternative by eliminating these risks while potentially enabling the use of high-capacity electrode materials that are incompatible with conventional liquid systems.
Current SPE development focuses on overcoming the fundamental challenge of achieving sufficient ionic conductivity at ambient temperatures while maintaining mechanical stability. This represents a critical trade-off, as the segmental motion of polymer chains that facilitates ion transport typically compromises mechanical integrity. The field has seen significant breakthroughs in recent years through the incorporation of ceramic fillers, plasticizers, and the design of novel polymer architectures.
The global push toward electrification and renewable energy integration has accelerated SPE research, with particular emphasis on enabling next-generation battery chemistries such as lithium-sulfur and solid-state lithium metal batteries. These technologies promise energy densities 2-3 times higher than current lithium-ion systems but require the unique properties that advanced SPEs can provide.
The primary technical objectives in SPE development include achieving room temperature ionic conductivity exceeding 10^-3 S/cm, maintaining a wide electrochemical stability window (>4.5V), ensuring good interfacial contact with electrodes, and demonstrating long-term cycling stability. Secondary objectives focus on cost-effective manufacturing processes, environmental sustainability, and compatibility with existing battery production infrastructure.
As the energy storage landscape continues to evolve, SPEs are positioned to play a pivotal role in enabling safer, higher-performance batteries. The technology stands at an inflection point where fundamental materials science advances are beginning to translate into commercially viable products, with several major battery manufacturers and startups actively pursuing SPE-based energy storage solutions.
Market Demand Analysis for SPE in Energy Storage
The global market for Solid Polymer Electrolytes (SPEs) in energy storage applications is experiencing robust growth, driven primarily by the increasing demand for safer, higher energy density batteries across multiple sectors. The electric vehicle (EV) industry represents the largest demand driver, with projections indicating that EVs will account for approximately 30% of new vehicle sales by 2030, creating substantial demand for advanced battery technologies incorporating SPEs.
Consumer electronics constitutes another significant market segment, with manufacturers seeking batteries that offer improved safety profiles and higher energy densities to support increasingly power-hungry devices. The portable electronics market alone is expected to grow at a compound annual growth rate of 7% through 2028, further accelerating demand for SPE-based energy storage solutions.
Grid-scale energy storage represents an emerging but rapidly expanding application area for SPEs. As renewable energy integration increases globally, the need for efficient, safe, and long-duration energy storage solutions grows proportionally. Market analysis indicates that grid storage capacity will triple by 2030, with solid-state batteries potentially capturing a significant portion of this growth.
Regional market assessment reveals Asia-Pacific as the dominant manufacturing hub, with Japan, South Korea, and China leading SPE research and production. North America and Europe follow as significant markets, with substantial investments in research and manufacturing capabilities. Particularly noteworthy is the European Battery Alliance's strategic focus on developing solid-state battery technology as part of its energy transition initiatives.
Market penetration analysis indicates that while liquid electrolyte systems currently dominate commercial applications, SPEs are gaining traction in premium segments where safety and performance advantages justify higher costs. Industry forecasts suggest SPEs could capture 15% of the energy storage market by 2028, with accelerating adoption thereafter as manufacturing scales and costs decrease.
Customer requirement analysis reveals that industrial users prioritize cycle life and safety, while consumer applications emphasize energy density and fast charging capabilities. This divergence in requirements is driving the development of specialized SPE formulations optimized for specific application domains.
Price sensitivity analysis demonstrates that while current SPE solutions command a premium over conventional technologies, the cost differential is narrowing as production scales and material innovations reduce manufacturing complexity. The market is approaching several critical price thresholds that, once crossed, could trigger rapid adoption across multiple application segments.
Consumer electronics constitutes another significant market segment, with manufacturers seeking batteries that offer improved safety profiles and higher energy densities to support increasingly power-hungry devices. The portable electronics market alone is expected to grow at a compound annual growth rate of 7% through 2028, further accelerating demand for SPE-based energy storage solutions.
Grid-scale energy storage represents an emerging but rapidly expanding application area for SPEs. As renewable energy integration increases globally, the need for efficient, safe, and long-duration energy storage solutions grows proportionally. Market analysis indicates that grid storage capacity will triple by 2030, with solid-state batteries potentially capturing a significant portion of this growth.
Regional market assessment reveals Asia-Pacific as the dominant manufacturing hub, with Japan, South Korea, and China leading SPE research and production. North America and Europe follow as significant markets, with substantial investments in research and manufacturing capabilities. Particularly noteworthy is the European Battery Alliance's strategic focus on developing solid-state battery technology as part of its energy transition initiatives.
Market penetration analysis indicates that while liquid electrolyte systems currently dominate commercial applications, SPEs are gaining traction in premium segments where safety and performance advantages justify higher costs. Industry forecasts suggest SPEs could capture 15% of the energy storage market by 2028, with accelerating adoption thereafter as manufacturing scales and costs decrease.
Customer requirement analysis reveals that industrial users prioritize cycle life and safety, while consumer applications emphasize energy density and fast charging capabilities. This divergence in requirements is driving the development of specialized SPE formulations optimized for specific application domains.
Price sensitivity analysis demonstrates that while current SPE solutions command a premium over conventional technologies, the cost differential is narrowing as production scales and material innovations reduce manufacturing complexity. The market is approaching several critical price thresholds that, once crossed, could trigger rapid adoption across multiple application segments.
Current State and Technical Challenges of SPE
Solid Polymer Electrolytes (SPEs) have emerged as promising alternatives to liquid electrolytes in energy storage applications, particularly in lithium-ion batteries. Currently, the global market for SPEs is experiencing significant growth, with a compound annual growth rate (CAGR) of approximately 7.8% projected through 2028. This growth is primarily driven by increasing demand for safer, more efficient energy storage solutions across multiple sectors including electric vehicles, consumer electronics, and grid storage systems.
The current state of SPE technology represents a complex landscape of achievements and limitations. Commercial implementations predominantly utilize polyethylene oxide (PEO)-based systems, which have demonstrated reasonable ionic conductivity at elevated temperatures (>60°C). Several major battery manufacturers have begun incorporating SPEs into specialized product lines, though widespread adoption remains limited due to performance constraints at ambient temperatures.
From a technical perspective, the primary challenge facing SPE development is achieving sufficient ionic conductivity at room temperature. Current state-of-the-art polymer electrolytes typically exhibit conductivities in the range of 10^-5 to 10^-4 S/cm at 25°C, which falls short of the 10^-3 S/cm threshold generally considered necessary for practical applications. This limitation stems from the semi-crystalline nature of many polymer hosts, which restricts ion mobility through the material matrix.
Another significant challenge is the mechanical stability of SPEs during cycling. As lithium-ion batteries charge and discharge, dimensional changes in the electrodes can stress the electrolyte interface, potentially creating microcracks that compromise performance and safety. Current SPE formulations often struggle to maintain consistent contact with electrodes throughout extended cycling regimes.
The interfacial resistance between SPEs and electrodes presents an additional hurdle. Unlike liquid electrolytes that can readily wet electrode surfaces, solid polymer systems frequently exhibit high interfacial impedance, limiting power density and rate capability. Research efforts have focused on surface modification strategies and composite approaches to address this issue, though optimal solutions remain elusive.
Geographically, SPE technology development is concentrated in East Asia (particularly Japan and South Korea), North America, and Western Europe. These regions host both academic research centers and industrial R&D facilities dedicated to advancing polymer electrolyte science. China has recently emerged as a significant player, with substantial government investment in solid-state battery technologies including SPE development.
Manufacturing scalability represents a final critical challenge. Current laboratory-scale production methods for high-performance SPEs often involve complex synthesis procedures that are difficult to translate to industrial scales. The development of cost-effective, high-throughput manufacturing processes remains essential for broader commercial adoption of SPE technology in energy storage applications.
The current state of SPE technology represents a complex landscape of achievements and limitations. Commercial implementations predominantly utilize polyethylene oxide (PEO)-based systems, which have demonstrated reasonable ionic conductivity at elevated temperatures (>60°C). Several major battery manufacturers have begun incorporating SPEs into specialized product lines, though widespread adoption remains limited due to performance constraints at ambient temperatures.
From a technical perspective, the primary challenge facing SPE development is achieving sufficient ionic conductivity at room temperature. Current state-of-the-art polymer electrolytes typically exhibit conductivities in the range of 10^-5 to 10^-4 S/cm at 25°C, which falls short of the 10^-3 S/cm threshold generally considered necessary for practical applications. This limitation stems from the semi-crystalline nature of many polymer hosts, which restricts ion mobility through the material matrix.
Another significant challenge is the mechanical stability of SPEs during cycling. As lithium-ion batteries charge and discharge, dimensional changes in the electrodes can stress the electrolyte interface, potentially creating microcracks that compromise performance and safety. Current SPE formulations often struggle to maintain consistent contact with electrodes throughout extended cycling regimes.
The interfacial resistance between SPEs and electrodes presents an additional hurdle. Unlike liquid electrolytes that can readily wet electrode surfaces, solid polymer systems frequently exhibit high interfacial impedance, limiting power density and rate capability. Research efforts have focused on surface modification strategies and composite approaches to address this issue, though optimal solutions remain elusive.
Geographically, SPE technology development is concentrated in East Asia (particularly Japan and South Korea), North America, and Western Europe. These regions host both academic research centers and industrial R&D facilities dedicated to advancing polymer electrolyte science. China has recently emerged as a significant player, with substantial government investment in solid-state battery technologies including SPE development.
Manufacturing scalability represents a final critical challenge. Current laboratory-scale production methods for high-performance SPEs often involve complex synthesis procedures that are difficult to translate to industrial scales. The development of cost-effective, high-throughput manufacturing processes remains essential for broader commercial adoption of SPE technology in energy storage applications.
Current SPE Solutions for Energy Storage
01 Polymer matrix compositions for solid electrolytes
Solid polymer electrolytes utilize various polymer matrices as hosts for ionic conductivity. These matrices include polyethylene oxide (PEO), polyvinylidene fluoride (PVDF), and other polymers that provide mechanical stability while allowing ion transport. The polymer backbone structure significantly influences the electrolyte's conductivity, mechanical properties, and electrochemical stability. These matrices can be modified with plasticizers or cross-linking agents to optimize performance for battery applications.- Polymer matrix compositions for solid electrolytes: Various polymer matrices are used as the foundation for solid polymer electrolytes, providing mechanical stability and ion transport pathways. Common polymers include polyethylene oxide (PEO), polyvinylidene fluoride (PVDF), and their copolymers. These polymers are selected for their ability to dissolve lithium salts and facilitate ion movement while maintaining structural integrity. The polymer matrix composition significantly affects the electrolyte's conductivity, mechanical properties, and electrochemical stability.
- Ionic conductivity enhancement strategies: Various approaches are employed to enhance the ionic conductivity of solid polymer electrolytes. These include incorporating ceramic fillers like Al2O3, SiO2, or TiO2 to create composite polymer electrolytes, using ionic liquids as plasticizers, and developing polymer blends with optimized chain mobility. Nanostructured additives can create additional ion transport pathways, while specialized salt mixtures can increase the concentration of mobile charge carriers. These strategies aim to achieve room-temperature conductivities comparable to liquid electrolytes while maintaining the safety advantages of solid systems.
- Interface engineering and electrode compatibility: Interface engineering is crucial for solid polymer electrolytes to ensure good contact with electrodes and minimize interfacial resistance. Techniques include surface modification of electrodes, gradient composition electrolytes, and specialized coating layers. Addressing the challenges of electrode-electrolyte interfaces is essential for preventing capacity fade and ensuring stable cycling performance. Compatibility with both cathode and anode materials must be considered, particularly for high-voltage cathodes and lithium metal anodes where chemical and electrochemical stability are critical concerns.
- Cross-linked and gel polymer electrolyte systems: Cross-linked and gel polymer electrolyte systems offer improved mechanical properties while maintaining good ionic conductivity. Cross-linking creates a three-dimensional network that enhances dimensional stability and reduces crystallinity, which can impede ion transport. Gel polymer electrolytes incorporate liquid components within a polymer matrix, combining the safety of solids with the conductivity of liquids. UV, thermal, or chemical cross-linking methods are employed to optimize the balance between mechanical strength and ion mobility. These systems are particularly valuable for flexible and shape-conforming battery designs.
- Temperature stability and safety enhancements: Solid polymer electrolytes offer significant safety advantages over liquid electrolytes, particularly in terms of thermal stability and reduced flammability. Research focuses on expanding the temperature range for stable operation, with specialized additives and polymer designs that maintain conductivity at low temperatures while preventing degradation at elevated temperatures. Flame-retardant components and self-extinguishing properties are incorporated to enhance safety. These electrolytes eliminate the risk of electrolyte leakage and help prevent thermal runaway, making them particularly valuable for applications with strict safety requirements.
02 Ionic salt additives for enhanced conductivity
Lithium salts and other ionic compounds are incorporated into solid polymer electrolytes to provide charge carriers for ionic conductivity. Common salts include LiPF6, LiTFSI, and LiBF4, which dissociate within the polymer matrix. The concentration and type of salt significantly impact the electrolyte's conductivity, electrochemical stability window, and interfacial properties with electrodes. Optimizing salt composition and concentration is crucial for achieving high ionic conductivity at ambient temperatures.Expand Specific Solutions03 Ceramic fillers and nanocomposite approaches
Inorganic ceramic fillers such as Al2O3, SiO2, and TiO2 are incorporated into polymer electrolytes to create composite systems with enhanced properties. These fillers improve mechanical strength, thermal stability, and often increase ionic conductivity by creating additional pathways for ion transport. Nanoparticle fillers can also help suppress crystallization of the polymer matrix, maintaining amorphous regions favorable for ion conduction even at lower temperatures.Expand Specific Solutions04 Interface engineering and electrode compatibility
Solid polymer electrolytes require careful interface engineering to ensure good contact with electrodes and minimize interfacial resistance. Surface modifications, additives, and specialized coating techniques are employed to improve the electrolyte-electrode interface. These approaches address challenges such as interfacial impedance, dendrite formation, and chemical compatibility between the electrolyte and electrode materials, which are critical for long-term battery performance and safety.Expand Specific Solutions05 Cross-linked and gel polymer electrolyte systems
Cross-linked polymer networks and gel polymer electrolytes represent advanced approaches to solid electrolyte design. Cross-linking improves mechanical properties while maintaining ionic conductivity. Gel polymer electrolytes incorporate liquid components within a polymer matrix, offering a balance between the safety of solids and the conductivity of liquid electrolytes. These systems often employ UV or thermal curing methods to create three-dimensional networks that can accommodate high salt concentrations while maintaining dimensional stability.Expand Specific Solutions
Key Industry Players in SPE Development
The Solid Polymer Electrolyte (SPE) market for energy storage applications is currently in a growth phase, with increasing adoption driven by the demand for safer, more efficient energy storage solutions. The global market size is projected to expand significantly, fueled by the electric vehicle revolution and stationary storage requirements. Technologically, SPEs are advancing from early commercial stages toward maturity, with key players demonstrating varying levels of innovation. Companies like LG Energy Solution and Toyota are leading commercial deployment, while BMW and Bosch focus on automotive applications. Academic institutions including University of Yamanashi and Johns Hopkins University contribute fundamental research, while specialized firms like Soelect develop next-generation materials. Chemical conglomerates such as Solvay and RESONAC provide essential materials expertise, creating a diverse competitive landscape spanning multiple industrial sectors.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed advanced solid polymer electrolyte (SPE) systems based on polyethylene oxide (PEO) matrices combined with lithium salts. Their proprietary technology incorporates ceramic fillers like Al2O3 and SiO2 nanoparticles to enhance ionic conductivity and mechanical stability. The company has implemented a cross-linking strategy that improves the dimensional stability of their SPEs while maintaining flexibility. Their latest generation SPEs achieve ionic conductivities of 10^-4 S/cm at room temperature, addressing one of the key limitations of traditional polymer electrolytes. LG has also developed composite polymer electrolytes that integrate with their existing battery manufacturing infrastructure, allowing for scalable production of solid-state batteries with energy densities exceeding 400 Wh/kg. Their SPE technology has been successfully demonstrated in pouch cells with capacities ranging from 5-20 Ah for various energy storage applications.
Strengths: Superior integration with existing manufacturing processes, allowing cost-effective scaling; excellent compatibility with high-voltage cathode materials (>4.3V); and demonstrated cycle life exceeding 1000 cycles with minimal capacity fade. Weaknesses: Room temperature ionic conductivity still lower than liquid electrolytes; requires operation at elevated temperatures (>60°C) for optimal performance in high-power applications.
Hitachi Ltd.
Technical Solution: Hitachi has developed a sophisticated solid polymer electrolyte platform based on polyether-polyester block copolymers with precisely controlled morphology. Their SPE system incorporates specially designed lithium salts with delocalized anions that enhance dissociation and improve ionic conductivity to levels approaching 10^-3 S/cm at operating temperatures. Hitachi's technology features a unique cross-linking approach that maintains flexibility while providing sufficient mechanical strength to prevent dendrite penetration. A distinguishing aspect of their SPE is the incorporation of functionalized nanoparticles that create favorable Lewis acid-base interactions with lithium ions, enhancing transport properties. Hitachi has also developed specialized interface modification techniques that minimize resistance at the electrode-electrolyte boundaries, a critical factor for high-power applications. Their manufacturing process utilizes solvent-free extrusion techniques that enable cost-effective production at scale. Hitachi has demonstrated this technology in various energy storage applications, from consumer electronics to grid-scale systems, with particular success in applications requiring high safety standards and long service life.
Strengths: Excellent thermal stability with minimal dimensional changes across wide temperature ranges; superior compatibility with various electrode materials; and environmentally friendly solvent-free manufacturing process. Weaknesses: Complex synthesis process for specialized block copolymers increases production costs; requires careful moisture control during manufacturing and assembly; and shows performance limitations under extremely high discharge rates.
Critical Patents and Technical Literature Review
Polymer solid electrolyte and application
PatentActiveEP4398362A1
Innovation
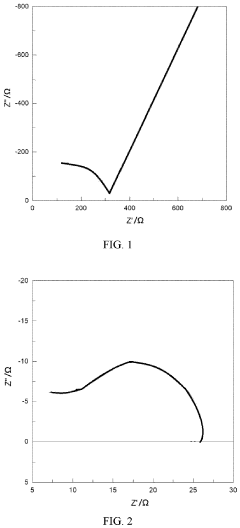
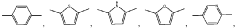
- A polymer solid electrolyte composed of conjugated and non-conjugated units, with a general formula R-(A n -B m ) k -R, where A n is a conjugated unit and B m is a non-conjugated unit, is developed, enhancing ionic conductivity and processability while reducing electronic conductivity, allowing for the creation of a high-performance solid-state lithium battery.
Solid-state polymer electrolyte for an energy storage device
PatentPendingUS20230378529A1
Innovation
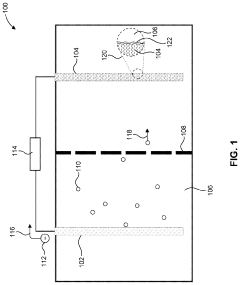
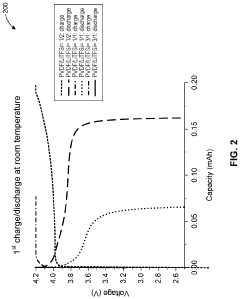
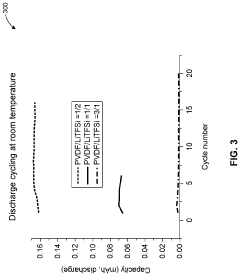
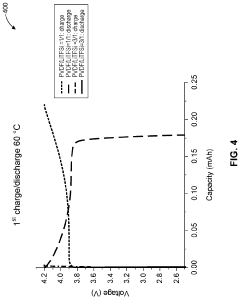
- A solid polymer electrolyte composition based on polyvinylidene fluoride (PVDF) and lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) with a higher mass content of LiTFSI, potentially combined with lithium lanthanum zirconate oxide (LLZO) and plasticizers like succinonitrile, is used to enhance ion conductivity and mechanical stability, forming a solid medium that can be processed from a solution with a solid content of approximately 0.19 or greater.
Environmental Impact and Sustainability Assessment
The environmental footprint of solid polymer electrolytes (SPEs) represents a critical consideration in their adoption for energy storage applications. Compared to conventional liquid electrolytes, SPEs demonstrate significant sustainability advantages through the elimination of volatile organic compounds and toxic materials commonly found in liquid counterparts. This reduction in hazardous substances translates to decreased environmental contamination risks during manufacturing, operation, and end-of-life disposal phases.
Life cycle assessment (LCA) studies indicate that SPE-based batteries can achieve up to 25-30% lower carbon emissions compared to conventional lithium-ion batteries with liquid electrolytes. This reduction stems primarily from simplified manufacturing processes, enhanced safety features that eliminate the need for complex thermal management systems, and extended operational lifespans that reduce replacement frequency.
The raw material sourcing for SPEs presents both challenges and opportunities from a sustainability perspective. While some polymer systems rely on petroleum-derived precursors, significant research advances have been made in developing bio-based polymers from renewable feedstocks. These bio-derived alternatives, including cellulose derivatives and chitosan-based systems, demonstrate comparable performance while reducing dependence on fossil resources by up to 40-60% according to recent industry analyses.
End-of-life management represents another dimension where SPEs offer sustainability advantages. The solid-state nature of these electrolytes facilitates more straightforward recycling processes compared to liquid systems that require complex separation techniques. Recovery rates for critical materials from SPE-based batteries can reach 85-90%, significantly higher than conventional systems, thereby supporting circular economy principles and reducing primary resource extraction demands.
Water consumption metrics also favor SPE manufacturing, with production processes requiring approximately 35-45% less water compared to conventional electrolyte production. This reduction becomes particularly significant in regions facing water scarcity challenges, where battery manufacturing facilities may compete with other essential water uses.
Despite these advantages, challenges remain in scaling sustainable SPE production. Current manufacturing techniques for high-performance SPEs often involve energy-intensive processes, particularly in achieving the necessary molecular architecture for optimal ionic conductivity. Industry estimates suggest that energy consumption during manufacturing represents the largest environmental impact category for SPEs, accounting for approximately 40-50% of their total ecological footprint.
Future sustainability improvements will likely emerge from process optimization, renewable energy integration in manufacturing, and continued development of bio-based polymer systems that maintain or exceed performance benchmarks of petroleum-derived alternatives.
Life cycle assessment (LCA) studies indicate that SPE-based batteries can achieve up to 25-30% lower carbon emissions compared to conventional lithium-ion batteries with liquid electrolytes. This reduction stems primarily from simplified manufacturing processes, enhanced safety features that eliminate the need for complex thermal management systems, and extended operational lifespans that reduce replacement frequency.
The raw material sourcing for SPEs presents both challenges and opportunities from a sustainability perspective. While some polymer systems rely on petroleum-derived precursors, significant research advances have been made in developing bio-based polymers from renewable feedstocks. These bio-derived alternatives, including cellulose derivatives and chitosan-based systems, demonstrate comparable performance while reducing dependence on fossil resources by up to 40-60% according to recent industry analyses.
End-of-life management represents another dimension where SPEs offer sustainability advantages. The solid-state nature of these electrolytes facilitates more straightforward recycling processes compared to liquid systems that require complex separation techniques. Recovery rates for critical materials from SPE-based batteries can reach 85-90%, significantly higher than conventional systems, thereby supporting circular economy principles and reducing primary resource extraction demands.
Water consumption metrics also favor SPE manufacturing, with production processes requiring approximately 35-45% less water compared to conventional electrolyte production. This reduction becomes particularly significant in regions facing water scarcity challenges, where battery manufacturing facilities may compete with other essential water uses.
Despite these advantages, challenges remain in scaling sustainable SPE production. Current manufacturing techniques for high-performance SPEs often involve energy-intensive processes, particularly in achieving the necessary molecular architecture for optimal ionic conductivity. Industry estimates suggest that energy consumption during manufacturing represents the largest environmental impact category for SPEs, accounting for approximately 40-50% of their total ecological footprint.
Future sustainability improvements will likely emerge from process optimization, renewable energy integration in manufacturing, and continued development of bio-based polymer systems that maintain or exceed performance benchmarks of petroleum-derived alternatives.
Manufacturing Scalability and Cost Analysis
The manufacturing scalability of solid polymer electrolytes (SPEs) represents a critical factor in their commercial viability for energy storage applications. Current production methods for SPEs vary significantly in terms of scalability, with solution casting and hot-pressing techniques being the most widely adopted in laboratory settings. However, these methods face substantial challenges when transitioning to industrial-scale production, particularly in maintaining consistent material properties across large batches.
Roll-to-roll processing has emerged as a promising approach for large-scale SPE manufacturing, offering continuous production capabilities that significantly reduce unit costs. This technique has demonstrated throughput rates up to 100 times faster than conventional batch processes, though material uniformity remains a persistent challenge. Several industry leaders, including LG Chem and Solid Power, have made substantial investments in adapting roll-to-roll technologies specifically for SPE production.
From a cost perspective, raw material expenses currently constitute approximately 40-60% of total SPE production costs. Polymer matrices like PEO and PVDF-HFP remain relatively affordable at $5-15/kg, while specialty additives and lithium salts represent the most significant cost drivers, with prices ranging from $80-500/kg depending on purity requirements. Recent innovations in polymer synthesis have shown potential to reduce these costs by 15-25% through more efficient chemical pathways.
Equipment capital expenditure presents another substantial barrier to market entry. Industrial-scale SPE production lines typically require investments of $5-20 million, with depreciation costs adding $0.50-2.00 per square meter of produced electrolyte. This high initial investment necessitates significant production volumes to achieve competitive unit economics, creating a challenging environment for smaller market entrants.
Energy consumption during manufacturing represents another critical cost factor, with drying and annealing processes being particularly energy-intensive. Current production methods require 2-5 kWh per square meter of electrolyte, though recent process innovations have demonstrated potential reductions of up to 30% through optimized thermal management systems and ambient processing techniques.
Yield rates significantly impact overall production economics, with current industrial processes achieving 75-85% yields. Material waste during production not only increases direct costs but also creates environmental challenges related to solvent recovery and disposal. Closed-loop manufacturing systems have shown promise in addressing these issues, potentially improving yield rates to over 90% while reducing environmental impact.
Roll-to-roll processing has emerged as a promising approach for large-scale SPE manufacturing, offering continuous production capabilities that significantly reduce unit costs. This technique has demonstrated throughput rates up to 100 times faster than conventional batch processes, though material uniformity remains a persistent challenge. Several industry leaders, including LG Chem and Solid Power, have made substantial investments in adapting roll-to-roll technologies specifically for SPE production.
From a cost perspective, raw material expenses currently constitute approximately 40-60% of total SPE production costs. Polymer matrices like PEO and PVDF-HFP remain relatively affordable at $5-15/kg, while specialty additives and lithium salts represent the most significant cost drivers, with prices ranging from $80-500/kg depending on purity requirements. Recent innovations in polymer synthesis have shown potential to reduce these costs by 15-25% through more efficient chemical pathways.
Equipment capital expenditure presents another substantial barrier to market entry. Industrial-scale SPE production lines typically require investments of $5-20 million, with depreciation costs adding $0.50-2.00 per square meter of produced electrolyte. This high initial investment necessitates significant production volumes to achieve competitive unit economics, creating a challenging environment for smaller market entrants.
Energy consumption during manufacturing represents another critical cost factor, with drying and annealing processes being particularly energy-intensive. Current production methods require 2-5 kWh per square meter of electrolyte, though recent process innovations have demonstrated potential reductions of up to 30% through optimized thermal management systems and ambient processing techniques.
Yield rates significantly impact overall production economics, with current industrial processes achieving 75-85% yields. Material waste during production not only increases direct costs but also creates environmental challenges related to solvent recovery and disposal. Closed-loop manufacturing systems have shown promise in addressing these issues, potentially improving yield rates to over 90% while reducing environmental impact.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!