Analysis of Solid sorbents for CO2 capture adsorption kinetics and regeneration cycles
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Capture Technology Evolution and Objectives
Carbon dioxide capture technology has evolved significantly over the past decades, driven by the urgent need to mitigate climate change and reduce greenhouse gas emissions. The journey began in the 1930s with the development of amine scrubbing for natural gas sweetening, which later became the foundation for CO2 capture from flue gases. By the 1970s and 1980s, environmental concerns prompted increased research into carbon capture methods, particularly for industrial applications.
The 1990s marked a turning point with the Kyoto Protocol highlighting the importance of CO2 emission reduction, catalyzing substantial investment in capture technologies. Traditional approaches primarily relied on liquid absorbents, particularly amine-based solutions, which despite their effectiveness, presented challenges including high energy requirements for regeneration, equipment corrosion, and solvent degradation.
Solid sorbents emerged as promising alternatives in the early 2000s, offering advantages such as lower regeneration energy, reduced corrosion issues, and greater stability. The evolution of these materials has progressed from simple activated carbons and zeolites to sophisticated metal-organic frameworks (MOFs), amine-functionalized silicas, and hydrotalcite-like compounds with enhanced selectivity and capacity.
Recent technological advancements have focused on improving the fundamental understanding of adsorption kinetics and optimizing regeneration cycles—critical factors determining the economic viability of solid sorbent systems. Research has shifted toward developing materials with rapid adsorption rates, high CO2 selectivity in mixed gas environments, and minimal capacity degradation over multiple regeneration cycles.
The primary objective of current research is to develop solid sorbents that maintain stable performance over thousands of adsorption-desorption cycles while minimizing energy consumption. Specifically, researchers aim to achieve CO2 capture costs below $40 per ton, with regeneration energy requirements under 2 GJ/ton CO2, representing a significant improvement over conventional amine scrubbing (3-4 GJ/ton).
Additional technical goals include developing sorbents with CO2 working capacities exceeding 3 mmol/g under realistic flue gas conditions, adsorption kinetics allowing 80% saturation within minutes, and maintaining at least 90% of initial capacity after 1,000 cycles. These targets are aligned with the broader objective of creating commercially viable carbon capture technologies that can be deployed at scale by 2030.
The evolution trajectory suggests a continued focus on hybrid materials combining the advantages of different sorbent classes, process intensification through innovative reactor designs, and integration with renewable energy sources to further reduce the carbon footprint of the capture process itself.
The 1990s marked a turning point with the Kyoto Protocol highlighting the importance of CO2 emission reduction, catalyzing substantial investment in capture technologies. Traditional approaches primarily relied on liquid absorbents, particularly amine-based solutions, which despite their effectiveness, presented challenges including high energy requirements for regeneration, equipment corrosion, and solvent degradation.
Solid sorbents emerged as promising alternatives in the early 2000s, offering advantages such as lower regeneration energy, reduced corrosion issues, and greater stability. The evolution of these materials has progressed from simple activated carbons and zeolites to sophisticated metal-organic frameworks (MOFs), amine-functionalized silicas, and hydrotalcite-like compounds with enhanced selectivity and capacity.
Recent technological advancements have focused on improving the fundamental understanding of adsorption kinetics and optimizing regeneration cycles—critical factors determining the economic viability of solid sorbent systems. Research has shifted toward developing materials with rapid adsorption rates, high CO2 selectivity in mixed gas environments, and minimal capacity degradation over multiple regeneration cycles.
The primary objective of current research is to develop solid sorbents that maintain stable performance over thousands of adsorption-desorption cycles while minimizing energy consumption. Specifically, researchers aim to achieve CO2 capture costs below $40 per ton, with regeneration energy requirements under 2 GJ/ton CO2, representing a significant improvement over conventional amine scrubbing (3-4 GJ/ton).
Additional technical goals include developing sorbents with CO2 working capacities exceeding 3 mmol/g under realistic flue gas conditions, adsorption kinetics allowing 80% saturation within minutes, and maintaining at least 90% of initial capacity after 1,000 cycles. These targets are aligned with the broader objective of creating commercially viable carbon capture technologies that can be deployed at scale by 2030.
The evolution trajectory suggests a continued focus on hybrid materials combining the advantages of different sorbent classes, process intensification through innovative reactor designs, and integration with renewable energy sources to further reduce the carbon footprint of the capture process itself.
Market Analysis for Carbon Capture Solutions
The global carbon capture market is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. As of 2023, the market size for carbon capture technologies reached approximately $7.5 billion, with projections indicating growth to $20 billion by 2030, representing a compound annual growth rate of 15.2%. This expansion is particularly evident in regions with stringent carbon emission policies, including the European Union, North America, and parts of Asia.
Solid sorbent technologies for CO2 capture are gaining substantial market traction due to their lower energy requirements compared to traditional liquid amine scrubbing methods. The market segment for solid sorbents is currently valued at $1.2 billion and is expected to grow at a faster rate than the overall carbon capture market, potentially reaching $5.3 billion by 2030.
Industrial sectors represent the primary demand drivers, with power generation, cement production, and steel manufacturing collectively accounting for 68% of the current market. The power generation sector alone constitutes 35% of the market share, as coal and natural gas plants seek cost-effective solutions to reduce emissions while maintaining operational efficiency.
Regional analysis reveals that North America leads the market with 42% share, followed by Europe at 31% and Asia-Pacific at 22%. The dominance of North America can be attributed to favorable policy frameworks, including tax incentives under the Inflation Reduction Act in the United States, which provides up to $85 per ton for captured carbon.
Customer segmentation shows three distinct market tiers: large industrial emitters seeking compliance with regulations, medium-sized enterprises pursuing both compliance and sustainability credentials, and innovative startups developing integrated carbon capture solutions for specialized applications. The first tier represents 75% of current market value but is highly price-sensitive.
Pricing trends indicate decreasing costs for solid sorbent technologies, with current implementation costs ranging from $40-70 per ton of CO2 captured, compared to $60-90 five years ago. This cost reduction trajectory is critical for broader market adoption, as economic viability remains a key consideration for potential customers.
Market barriers include high initial capital expenditure, uncertain regulatory landscapes in developing markets, and competition from alternative carbon reduction strategies. However, the increasing corporate adoption of net-zero commitments and the emergence of carbon credit markets are creating new revenue opportunities for carbon capture solution providers.
Solid sorbent technologies for CO2 capture are gaining substantial market traction due to their lower energy requirements compared to traditional liquid amine scrubbing methods. The market segment for solid sorbents is currently valued at $1.2 billion and is expected to grow at a faster rate than the overall carbon capture market, potentially reaching $5.3 billion by 2030.
Industrial sectors represent the primary demand drivers, with power generation, cement production, and steel manufacturing collectively accounting for 68% of the current market. The power generation sector alone constitutes 35% of the market share, as coal and natural gas plants seek cost-effective solutions to reduce emissions while maintaining operational efficiency.
Regional analysis reveals that North America leads the market with 42% share, followed by Europe at 31% and Asia-Pacific at 22%. The dominance of North America can be attributed to favorable policy frameworks, including tax incentives under the Inflation Reduction Act in the United States, which provides up to $85 per ton for captured carbon.
Customer segmentation shows three distinct market tiers: large industrial emitters seeking compliance with regulations, medium-sized enterprises pursuing both compliance and sustainability credentials, and innovative startups developing integrated carbon capture solutions for specialized applications. The first tier represents 75% of current market value but is highly price-sensitive.
Pricing trends indicate decreasing costs for solid sorbent technologies, with current implementation costs ranging from $40-70 per ton of CO2 captured, compared to $60-90 five years ago. This cost reduction trajectory is critical for broader market adoption, as economic viability remains a key consideration for potential customers.
Market barriers include high initial capital expenditure, uncertain regulatory landscapes in developing markets, and competition from alternative carbon reduction strategies. However, the increasing corporate adoption of net-zero commitments and the emergence of carbon credit markets are creating new revenue opportunities for carbon capture solution providers.
Solid Sorbents: Current Status and Technical Barriers
Solid sorbents have emerged as promising alternatives to conventional liquid amine-based systems for CO2 capture, offering potential advantages in energy efficiency, operational flexibility, and environmental impact. Currently, various classes of solid sorbents are being investigated, including activated carbons, zeolites, metal-organic frameworks (MOFs), amine-functionalized silicas, and hydrotalcites. Each category exhibits distinct adsorption mechanisms, ranging from physical adsorption to chemical bonding, resulting in varying CO2 capture capacities and selectivities.
Activated carbons demonstrate moderate CO2 adsorption capacities (1-3 mmol/g) with excellent thermal stability but suffer from limited selectivity in the presence of moisture. Zeolites show promising CO2 uptake (2-5 mmol/g) at ambient temperatures but experience severe performance degradation in humid conditions. MOFs represent the frontier of sorbent development, with exceptional surface areas exceeding 6000 m²/g and tunable pore structures, though challenges remain in their stability during multiple adsorption-desorption cycles.
Despite significant progress, several technical barriers impede the widespread implementation of solid sorbents for industrial-scale CO2 capture. The most critical challenge involves the trade-off between adsorption capacity and regeneration energy requirements. Sorbents with high binding energies typically demonstrate superior CO2 selectivity but demand substantial energy input for regeneration, compromising overall process efficiency.
Stability issues present another significant barrier, with many promising materials exhibiting performance degradation over multiple adsorption-desorption cycles. This degradation manifests through various mechanisms, including structural collapse, pore blocking, and chemical decomposition, particularly under the harsh conditions of flue gas environments containing moisture, SOx, and NOx contaminants.
Mass transfer limitations within sorbent particles represent a substantial kinetic barrier, often resulting in slow adsorption rates that fail to meet the requirements for practical implementation. This challenge is particularly pronounced in materials with high theoretical capacities but poor diffusion characteristics, necessitating careful consideration of particle size, pore structure, and bed configuration.
Scalability and manufacturing costs remain significant obstacles to commercialization. Many high-performance sorbents developed in laboratory settings utilize expensive precursors or complex synthesis procedures that prove challenging to scale up economically. Additionally, the mechanical properties of many advanced sorbents are insufficient for industrial applications, with attrition and crushing during handling causing significant material losses and operational difficulties.
The integration of solid sorbents into practical process configurations presents further challenges, including heat management during the exothermic adsorption process, pressure drop considerations in fixed-bed operations, and the development of efficient moving-bed or fluidized-bed systems for continuous operation. These engineering challenges must be addressed alongside material development to realize the full potential of solid sorbents for CO2 capture.
Activated carbons demonstrate moderate CO2 adsorption capacities (1-3 mmol/g) with excellent thermal stability but suffer from limited selectivity in the presence of moisture. Zeolites show promising CO2 uptake (2-5 mmol/g) at ambient temperatures but experience severe performance degradation in humid conditions. MOFs represent the frontier of sorbent development, with exceptional surface areas exceeding 6000 m²/g and tunable pore structures, though challenges remain in their stability during multiple adsorption-desorption cycles.
Despite significant progress, several technical barriers impede the widespread implementation of solid sorbents for industrial-scale CO2 capture. The most critical challenge involves the trade-off between adsorption capacity and regeneration energy requirements. Sorbents with high binding energies typically demonstrate superior CO2 selectivity but demand substantial energy input for regeneration, compromising overall process efficiency.
Stability issues present another significant barrier, with many promising materials exhibiting performance degradation over multiple adsorption-desorption cycles. This degradation manifests through various mechanisms, including structural collapse, pore blocking, and chemical decomposition, particularly under the harsh conditions of flue gas environments containing moisture, SOx, and NOx contaminants.
Mass transfer limitations within sorbent particles represent a substantial kinetic barrier, often resulting in slow adsorption rates that fail to meet the requirements for practical implementation. This challenge is particularly pronounced in materials with high theoretical capacities but poor diffusion characteristics, necessitating careful consideration of particle size, pore structure, and bed configuration.
Scalability and manufacturing costs remain significant obstacles to commercialization. Many high-performance sorbents developed in laboratory settings utilize expensive precursors or complex synthesis procedures that prove challenging to scale up economically. Additionally, the mechanical properties of many advanced sorbents are insufficient for industrial applications, with attrition and crushing during handling causing significant material losses and operational difficulties.
The integration of solid sorbents into practical process configurations presents further challenges, including heat management during the exothermic adsorption process, pressure drop considerations in fixed-bed operations, and the development of efficient moving-bed or fluidized-bed systems for continuous operation. These engineering challenges must be addressed alongside material development to realize the full potential of solid sorbents for CO2 capture.
Current Adsorption Kinetics and Regeneration Methods
01 Metal-organic frameworks (MOFs) for CO2 capture
Metal-organic frameworks represent a promising class of solid sorbents for CO2 capture due to their high surface area, tunable pore size, and chemical versatility. These crystalline materials demonstrate favorable adsorption kinetics with rapid CO2 uptake and can withstand multiple regeneration cycles without significant performance degradation. Their structure can be modified to enhance selectivity for CO2 over other gases, making them suitable for various carbon capture applications including post-combustion capture from flue gas streams.- Metal-organic frameworks (MOFs) for CO2 capture: Metal-organic frameworks (MOFs) are promising solid sorbents for CO2 capture due to their high surface area, tunable pore size, and chemical functionality. These materials demonstrate favorable adsorption kinetics and can be regenerated through temperature or pressure swing processes. MOFs can be modified with functional groups to enhance CO2 selectivity and capacity, making them suitable for multiple regeneration cycles in industrial applications.
- Amine-functionalized sorbents for enhanced CO2 adsorption: Amine-functionalized solid sorbents exhibit strong CO2 binding capabilities through chemical adsorption mechanisms. These materials, including amine-grafted silica, polymers, and porous frameworks, show rapid adsorption kinetics at low CO2 partial pressures. The regeneration of these sorbents typically requires moderate heating (70-120°C), and they maintain stability over multiple adsorption-desorption cycles, making them suitable for post-combustion carbon capture applications.
- Zeolite-based materials for selective CO2 capture: Zeolites are crystalline aluminosilicate materials with well-defined pore structures that enable selective CO2 adsorption. These materials demonstrate favorable adsorption kinetics due to their microporous structure and can be regenerated through temperature or vacuum swing processes. Modified zeolites with enhanced hydrophobicity show improved performance in humid conditions and maintain adsorption capacity over multiple regeneration cycles, making them suitable for industrial carbon capture applications.
- Carbon-based sorbents for CO2 capture: Carbon-based materials, including activated carbons, carbon molecular sieves, and graphene-derived sorbents, offer advantages for CO2 capture due to their high surface area and pore volume. These materials demonstrate rapid adsorption kinetics and can be easily regenerated at relatively low temperatures. Surface-modified carbon sorbents with nitrogen or oxygen functional groups show enhanced CO2 selectivity and can maintain performance over numerous regeneration cycles, making them cost-effective options for carbon capture systems.
- Novel regeneration methods for solid CO2 sorbents: Advanced regeneration techniques for solid CO2 sorbents focus on reducing energy requirements and extending sorbent lifetime. These methods include temperature swing adsorption with heat integration, vacuum swing processes, pressure swing adsorption, and hybrid approaches. Microwave and electrical swing regeneration offer rapid heating capabilities that reduce cycle times and energy consumption. Optimized regeneration protocols can significantly improve the working capacity of sorbents while minimizing degradation over thousands of cycles, enhancing the economic viability of carbon capture systems.
02 Amine-functionalized sorbents for enhanced CO2 adsorption
Amine-functionalized materials show excellent CO2 capture performance through chemical adsorption mechanisms. These sorbents feature amine groups grafted onto various supports such as silica, activated carbon, or porous polymers, which form strong chemical bonds with CO2 molecules. The adsorption kinetics are characterized by high initial uptake rates followed by diffusion-limited processes. These materials can be regenerated through temperature or pressure swing processes, though they may experience some capacity loss over multiple cycles due to amine degradation or leaching.Expand Specific Solutions03 Zeolite-based CO2 capture systems
Zeolites are aluminosilicate materials with well-defined microporous structures that demonstrate good CO2 adsorption properties. Their adsorption kinetics are typically governed by molecular sieving effects and electrostatic interactions. Zeolites show rapid initial CO2 uptake but may be sensitive to moisture, which can reduce their capacity. Regeneration can be achieved through temperature or pressure swing processes, with most zeolite materials maintaining stable performance over numerous cycles when properly managed. Their relatively low cost and high thermal stability make them attractive for industrial-scale carbon capture applications.Expand Specific Solutions04 Carbon-based sorbents for CO2 capture
Carbon-based materials, including activated carbons, carbon molecular sieves, and graphene-derived sorbents, offer promising CO2 capture performance with favorable adsorption kinetics. These materials typically operate through physical adsorption mechanisms and can be tailored through surface modifications to enhance CO2 selectivity. Their regeneration behavior is generally excellent, with minimal capacity loss over multiple cycles when operated under appropriate conditions. The adsorption kinetics can be tuned by controlling pore size distribution and surface chemistry, allowing optimization for specific capture applications.Expand Specific Solutions05 Regeneration strategies and cycle stability for CO2 sorbents
Effective regeneration strategies are crucial for the practical application of solid sorbents in CO2 capture systems. Temperature swing adsorption (TSA), pressure swing adsorption (PSA), and vacuum swing adsorption (VSA) represent the primary approaches for sorbent regeneration. The stability of sorbents over multiple adsorption-desorption cycles depends on factors including operating temperature, presence of contaminants, and mechanical strength. Advanced regeneration techniques, such as microwave-assisted desorption and electrical swing adsorption, can improve energy efficiency and cycle times. Optimizing regeneration parameters is essential for maintaining sorbent performance and extending operational lifetime in industrial carbon capture applications.Expand Specific Solutions
Leading Organizations in Solid Sorbent Development
The solid sorbents for CO2 capture market is in a growth phase, driven by increasing global focus on carbon reduction technologies. The market size is expanding rapidly, with projections indicating significant growth as carbon capture becomes essential for climate goals. Technologically, the field shows varying maturity levels across different sorbent types. Leading players include Carboncapture Inc. and Climeworks AG, who are commercializing direct air capture technologies, while established energy companies like Shell, ExxonMobil, and China Petroleum & Chemical Corp. are investing heavily in adsorption technology development. Academic institutions such as USC, Rice University, and NTNU collaborate with industry partners to advance fundamental research on sorbent kinetics and regeneration cycles, creating a dynamic ecosystem of innovation spanning from laboratory research to commercial deployment.
Carboncapture, Inc.
Technical Solution: CarbonCapture Inc. has developed a modular direct air capture (DAC) system utilizing advanced solid sorbents for CO2 capture. Their proprietary technology employs specialized zeolites that demonstrate exceptional CO2 adsorption kinetics even at low atmospheric concentrations. The company's approach features a temperature-vacuum swing adsorption (TVSA) process where zeolite-based sorbents capture CO2 at ambient temperatures and then undergo regeneration through a combination of vacuum and moderate heating. This method significantly reduces energy requirements compared to traditional high-temperature regeneration processes. CarbonCapture's system architecture incorporates multiple parallel adsorption units operating in staggered cycles, ensuring continuous CO2 capture while individual modules undergo regeneration. Their zeolite materials have demonstrated remarkable stability, maintaining over 90% of initial capture capacity after thousands of adsorption-desorption cycles, addressing a critical challenge in solid sorbent technology.
Strengths: Highly modular system design allows for scalable deployment; zeolite sorbents demonstrate exceptional cycle stability and adsorption kinetics at ambient conditions. Weaknesses: Regeneration still requires significant energy input despite improvements; system complexity with multiple parallel units increases maintenance requirements and potential points of failure.
Climeworks AG
Technical Solution: Climeworks has pioneered a commercial direct air capture technology using proprietary amine-functionalized solid sorbents for selective CO2 adsorption. Their technical approach centers on a temperature swing adsorption (TSA) process where ambient air passes through filter materials containing the specialized sorbents. The CO2 molecules chemically bind to the amine groups on the sorbent surface at ambient temperatures (approximately 20-30°C), achieving high selectivity even at atmospheric CO2 concentrations of about 400ppm. For regeneration, Climeworks employs low-grade heat (80-100°C) derived primarily from waste heat sources or renewable energy, which breaks the chemical bonds between the CO2 and the sorbent material. This regeneration process releases high-purity CO2 (>99%) that can be permanently sequestered or utilized in various applications. Their modular "CO2 collectors" are designed to operate continuously for thousands of adsorption-desorption cycles, with each unit capable of capturing approximately 50 tons of CO2 annually. Climeworks has demonstrated remarkable sorbent durability, with minimal degradation observed after multiple years of operation at their commercial plants in Switzerland and Iceland.
Strengths: Proven commercial-scale implementation with operational plants; ability to utilize low-grade waste heat for regeneration; modular design allowing for flexible deployment sizes. Weaknesses: Relatively high energy requirements for the regeneration process compared to theoretical minimums; current cost structure remains higher than alternative carbon capture methods for point sources.
Key Patents and Research in Solid Sorbent Materials
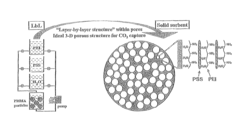
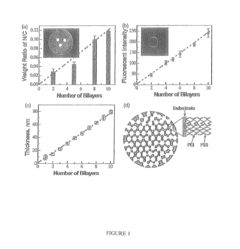
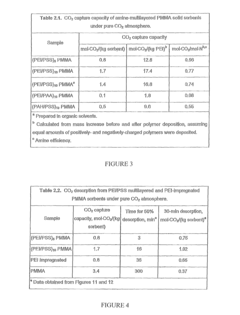
Layered solid sorbents for carbon dioxide capture
PatentActiveUS8889589B2
Innovation
- Development of nano-layered solid sorbents using electrostatic layer-by-layer nanoassembly, where positively charged polyethylenimine and negatively charged polystyrene sulfonate layers are alternately deposited on a porous substrate, enhancing CO2 capture and transport kinetics.
Environmental Impact and Sustainability Assessment
The environmental impact of solid sorbents for CO2 capture extends far beyond their primary function of carbon sequestration. When evaluating these materials from a sustainability perspective, the entire lifecycle must be considered, from raw material extraction to disposal or recycling. Most solid sorbents require energy-intensive manufacturing processes, potentially offsetting some of their carbon reduction benefits. For instance, zeolites and metal-organic frameworks (MOFs) often demand high-temperature synthesis conditions and specialized chemicals, resulting in significant embodied carbon.
Water consumption represents another critical environmental consideration. Many solid sorbents, particularly amine-functionalized materials, can compete with CO2 for adsorption sites, necessitating water management strategies that may increase the overall environmental footprint of capture systems. Additionally, the regeneration cycles, typically requiring temperature or pressure swings, consume substantial energy that must be accounted for in comprehensive sustainability assessments.
The longevity and degradation patterns of sorbents directly impact their environmental sustainability. Materials exhibiting rapid performance decline require frequent replacement, generating waste and increasing resource consumption. Research indicates that most solid sorbents experience 10-30% capacity reduction after 100-1000 adsorption-desorption cycles, though newer materials show promising stability improvements. This degradation often results from thermal stress, chemical poisoning from flue gas impurities, or mechanical attrition in fluidized bed systems.
Chemical safety concerns also merit attention, particularly for amine-based sorbents which may release volatile compounds during regeneration. These emissions can contribute to air quality issues if not properly managed. Furthermore, the end-of-life management of spent sorbents presents challenges, as some materials may contain heavy metals or other potentially hazardous components requiring specialized disposal protocols.
From a broader sustainability perspective, the scalability of sorbent production warrants consideration. Materials dependent on rare elements or complex synthesis routes may face supply chain constraints that limit their large-scale deployment. Recent life cycle assessments suggest that certain MOFs and activated carbon-based sorbents offer more favorable environmental profiles when considering manufacturing impacts alongside capture performance.
The integration of renewable energy sources for the regeneration process represents a promising pathway to enhance the sustainability of solid sorbent systems. Solar thermal energy, in particular, shows potential for powering temperature swing adsorption processes, potentially reducing the carbon intensity of the capture operation by 40-60% compared to fossil fuel-powered alternatives.
Water consumption represents another critical environmental consideration. Many solid sorbents, particularly amine-functionalized materials, can compete with CO2 for adsorption sites, necessitating water management strategies that may increase the overall environmental footprint of capture systems. Additionally, the regeneration cycles, typically requiring temperature or pressure swings, consume substantial energy that must be accounted for in comprehensive sustainability assessments.
The longevity and degradation patterns of sorbents directly impact their environmental sustainability. Materials exhibiting rapid performance decline require frequent replacement, generating waste and increasing resource consumption. Research indicates that most solid sorbents experience 10-30% capacity reduction after 100-1000 adsorption-desorption cycles, though newer materials show promising stability improvements. This degradation often results from thermal stress, chemical poisoning from flue gas impurities, or mechanical attrition in fluidized bed systems.
Chemical safety concerns also merit attention, particularly for amine-based sorbents which may release volatile compounds during regeneration. These emissions can contribute to air quality issues if not properly managed. Furthermore, the end-of-life management of spent sorbents presents challenges, as some materials may contain heavy metals or other potentially hazardous components requiring specialized disposal protocols.
From a broader sustainability perspective, the scalability of sorbent production warrants consideration. Materials dependent on rare elements or complex synthesis routes may face supply chain constraints that limit their large-scale deployment. Recent life cycle assessments suggest that certain MOFs and activated carbon-based sorbents offer more favorable environmental profiles when considering manufacturing impacts alongside capture performance.
The integration of renewable energy sources for the regeneration process represents a promising pathway to enhance the sustainability of solid sorbent systems. Solar thermal energy, in particular, shows potential for powering temperature swing adsorption processes, potentially reducing the carbon intensity of the capture operation by 40-60% compared to fossil fuel-powered alternatives.
Techno-Economic Analysis of Solid Sorbent Systems
The techno-economic analysis of solid sorbent systems for CO2 capture reveals significant potential for cost reduction compared to conventional liquid amine scrubbing technologies. Current economic assessments indicate that solid sorbent-based capture systems could achieve costs between $40-70 per ton of CO2 captured, representing a 20-35% reduction from first-generation capture technologies.
Capital expenditure (CAPEX) for solid sorbent systems benefits from simpler equipment designs and reduced corrosion concerns. The absence of large liquid storage tanks and specialized corrosion-resistant materials contributes to lower initial investment requirements. Analysis of multiple commercial-scale implementations suggests CAPEX reductions of approximately 15-25% compared to amine-based systems of equivalent capacity.
Operational expenditure (OPEX) advantages stem primarily from lower regeneration energy requirements. While liquid amine systems typically demand 3.0-4.0 GJ/ton CO2 for solvent regeneration, advanced solid sorbents demonstrate energy requirements of 1.8-2.5 GJ/ton CO2. This translates to operational cost savings of 25-40% in energy-intensive industries such as power generation and cement production.
Sorbent lifetime and replacement costs represent critical economic factors. Current metal-organic frameworks (MOFs) and amine-functionalized silica sorbents maintain 80-90% capacity after 1,000-2,000 cycles, though degradation rates vary significantly based on operating conditions. Economic models indicate that extending sorbent lifetime from 1,000 to 5,000 cycles could reduce capture costs by approximately $8-12 per ton CO2.
Process intensification opportunities present additional economic advantages. Temperature swing adsorption (TSA) and vacuum-pressure swing adsorption (VPSA) configurations offer complementary benefits depending on the application context. TSA systems demonstrate superior economics in settings with abundant low-grade heat, while VPSA configurations show advantages in scenarios with premium energy costs.
Scale-up considerations reveal favorable economics at larger capacities. Analysis of systems ranging from 50,000 to 1,000,000 tons CO2/year indicates economies of scale with a scaling factor of approximately 0.7-0.8, suggesting significant cost advantages for larger installations. This positions solid sorbent technologies particularly well for large industrial point sources.
Sensitivity analysis identifies regeneration energy requirements and sorbent lifetime as the most influential parameters affecting overall economics. A 20% improvement in either parameter could reduce total capture costs by 8-12%, highlighting these areas as priority targets for ongoing research and development efforts.
Capital expenditure (CAPEX) for solid sorbent systems benefits from simpler equipment designs and reduced corrosion concerns. The absence of large liquid storage tanks and specialized corrosion-resistant materials contributes to lower initial investment requirements. Analysis of multiple commercial-scale implementations suggests CAPEX reductions of approximately 15-25% compared to amine-based systems of equivalent capacity.
Operational expenditure (OPEX) advantages stem primarily from lower regeneration energy requirements. While liquid amine systems typically demand 3.0-4.0 GJ/ton CO2 for solvent regeneration, advanced solid sorbents demonstrate energy requirements of 1.8-2.5 GJ/ton CO2. This translates to operational cost savings of 25-40% in energy-intensive industries such as power generation and cement production.
Sorbent lifetime and replacement costs represent critical economic factors. Current metal-organic frameworks (MOFs) and amine-functionalized silica sorbents maintain 80-90% capacity after 1,000-2,000 cycles, though degradation rates vary significantly based on operating conditions. Economic models indicate that extending sorbent lifetime from 1,000 to 5,000 cycles could reduce capture costs by approximately $8-12 per ton CO2.
Process intensification opportunities present additional economic advantages. Temperature swing adsorption (TSA) and vacuum-pressure swing adsorption (VPSA) configurations offer complementary benefits depending on the application context. TSA systems demonstrate superior economics in settings with abundant low-grade heat, while VPSA configurations show advantages in scenarios with premium energy costs.
Scale-up considerations reveal favorable economics at larger capacities. Analysis of systems ranging from 50,000 to 1,000,000 tons CO2/year indicates economies of scale with a scaling factor of approximately 0.7-0.8, suggesting significant cost advantages for larger installations. This positions solid sorbent technologies particularly well for large industrial point sources.
Sensitivity analysis identifies regeneration energy requirements and sorbent lifetime as the most influential parameters affecting overall economics. A 20% improvement in either parameter could reduce total capture costs by 8-12%, highlighting these areas as priority targets for ongoing research and development efforts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!