Research on Solid sorbents for CO2 capture for industrial scale carbon capture and storage
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Capture Technology Background and Objectives
Carbon dioxide capture and storage (CCS) has emerged as a critical technology in the global effort to mitigate climate change by reducing greenhouse gas emissions. The historical development of CO2 capture technologies dates back to the 1930s when absorption processes using amines were first developed for natural gas sweetening. However, the application of these technologies specifically for climate change mitigation gained momentum only in the late 1990s as global awareness of anthropogenic climate impacts increased.
The evolution of CO2 capture technologies has progressed through several generations, from conventional amine scrubbing to more advanced methods including solid sorbents, which represent a promising alternative to liquid-based systems. Solid sorbents offer potential advantages in energy efficiency, reduced corrosion, and lower regeneration energy requirements compared to traditional liquid absorbents.
Current technological trends indicate a shift toward developing materials and systems that can operate at industrial scales with minimal energy penalties. This trend is driven by the recognition that for CCS to make a meaningful impact on global emissions, technologies must be deployable at the scale of major industrial emitters such as power plants, cement factories, and steel mills, which collectively account for approximately 40% of global CO2 emissions.
The primary objective of solid sorbent research for CO2 capture is to develop materials that combine high CO2 selectivity and capacity with rapid adsorption/desorption kinetics, mechanical stability over multiple cycles, and cost-effectiveness for large-scale deployment. Additionally, these materials must perform efficiently under realistic industrial conditions, including the presence of contaminants such as SOx, NOx, and moisture.
Technical goals include reducing the energy penalty of capture to below 1.5 GJ/tonne CO2, achieving sorbent lifetimes of at least 5,000 cycles, and developing systems capable of capturing CO2 at costs below $40/tonne. These targets represent significant improvements over current commercial technologies, which typically require 2.5-3.5 GJ/tonne CO2 and have higher associated costs.
The development pathway for solid sorbents encompasses material design and synthesis, characterization and performance testing, process engineering and integration, and ultimately demonstration at progressively larger scales. This pathway is informed by both fundamental scientific understanding of adsorption mechanisms and practical engineering considerations for industrial implementation.
Achieving these objectives would position solid sorbent-based capture as a viable technology for widespread deployment in the 2030-2040 timeframe, potentially contributing significantly to global decarbonization efforts and the achievement of net-zero emissions targets by mid-century.
The evolution of CO2 capture technologies has progressed through several generations, from conventional amine scrubbing to more advanced methods including solid sorbents, which represent a promising alternative to liquid-based systems. Solid sorbents offer potential advantages in energy efficiency, reduced corrosion, and lower regeneration energy requirements compared to traditional liquid absorbents.
Current technological trends indicate a shift toward developing materials and systems that can operate at industrial scales with minimal energy penalties. This trend is driven by the recognition that for CCS to make a meaningful impact on global emissions, technologies must be deployable at the scale of major industrial emitters such as power plants, cement factories, and steel mills, which collectively account for approximately 40% of global CO2 emissions.
The primary objective of solid sorbent research for CO2 capture is to develop materials that combine high CO2 selectivity and capacity with rapid adsorption/desorption kinetics, mechanical stability over multiple cycles, and cost-effectiveness for large-scale deployment. Additionally, these materials must perform efficiently under realistic industrial conditions, including the presence of contaminants such as SOx, NOx, and moisture.
Technical goals include reducing the energy penalty of capture to below 1.5 GJ/tonne CO2, achieving sorbent lifetimes of at least 5,000 cycles, and developing systems capable of capturing CO2 at costs below $40/tonne. These targets represent significant improvements over current commercial technologies, which typically require 2.5-3.5 GJ/tonne CO2 and have higher associated costs.
The development pathway for solid sorbents encompasses material design and synthesis, characterization and performance testing, process engineering and integration, and ultimately demonstration at progressively larger scales. This pathway is informed by both fundamental scientific understanding of adsorption mechanisms and practical engineering considerations for industrial implementation.
Achieving these objectives would position solid sorbent-based capture as a viable technology for widespread deployment in the 2030-2040 timeframe, potentially contributing significantly to global decarbonization efforts and the achievement of net-zero emissions targets by mid-century.
Market Analysis for Industrial Carbon Capture Solutions
The global carbon capture and storage (CCS) market is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. As of 2023, the industrial carbon capture solutions market was valued at approximately $2.5 billion, with projections indicating a compound annual growth rate of 19.2% through 2030, potentially reaching $7.8 billion by the end of the decade.
The demand for solid sorbent-based carbon capture technologies is particularly strong in power generation, cement production, steel manufacturing, and chemical processing industries, which collectively account for over 60% of global industrial CO2 emissions. These sectors are under mounting pressure to decarbonize operations while maintaining economic viability, creating a substantial market opportunity for advanced capture solutions.
Regional analysis reveals that North America currently leads the market with approximately 35% share, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is expected to demonstrate the fastest growth rate due to rapid industrialization coupled with strengthening environmental policies in China, India, and Southeast Asian nations.
Market segmentation by technology shows that while amine-based liquid solvents currently dominate with about 70% market share, solid sorbents are gaining traction rapidly due to their lower energy requirements and reduced environmental impact. Industry forecasts suggest solid sorbent technologies could capture up to 40% of the market by 2030, representing a significant shift in technological preference.
Key market drivers include tightening carbon regulations across major economies, with carbon pricing mechanisms now covering approximately 23% of global emissions. The European Union's Carbon Border Adjustment Mechanism and similar policies emerging in other regions are creating strong financial incentives for industrial emitters to adopt carbon capture technologies.
Customer demand analysis indicates that large industrial facilities emitting over 500,000 tons of CO2 annually represent the primary market segment, with approximately 7,000 such facilities globally. These high-volume emitters are increasingly viewing carbon capture not merely as a compliance cost but as a strategic investment to future-proof operations against escalating carbon prices.
The competitive landscape features established engineering firms expanding into carbon capture solutions alongside innovative startups specializing in novel sorbent technologies. Recent market trends show increasing interest in modular, scalable systems that can be retrofitted to existing industrial facilities, reducing capital expenditure barriers to adoption.
The demand for solid sorbent-based carbon capture technologies is particularly strong in power generation, cement production, steel manufacturing, and chemical processing industries, which collectively account for over 60% of global industrial CO2 emissions. These sectors are under mounting pressure to decarbonize operations while maintaining economic viability, creating a substantial market opportunity for advanced capture solutions.
Regional analysis reveals that North America currently leads the market with approximately 35% share, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is expected to demonstrate the fastest growth rate due to rapid industrialization coupled with strengthening environmental policies in China, India, and Southeast Asian nations.
Market segmentation by technology shows that while amine-based liquid solvents currently dominate with about 70% market share, solid sorbents are gaining traction rapidly due to their lower energy requirements and reduced environmental impact. Industry forecasts suggest solid sorbent technologies could capture up to 40% of the market by 2030, representing a significant shift in technological preference.
Key market drivers include tightening carbon regulations across major economies, with carbon pricing mechanisms now covering approximately 23% of global emissions. The European Union's Carbon Border Adjustment Mechanism and similar policies emerging in other regions are creating strong financial incentives for industrial emitters to adopt carbon capture technologies.
Customer demand analysis indicates that large industrial facilities emitting over 500,000 tons of CO2 annually represent the primary market segment, with approximately 7,000 such facilities globally. These high-volume emitters are increasingly viewing carbon capture not merely as a compliance cost but as a strategic investment to future-proof operations against escalating carbon prices.
The competitive landscape features established engineering firms expanding into carbon capture solutions alongside innovative startups specializing in novel sorbent technologies. Recent market trends show increasing interest in modular, scalable systems that can be retrofitted to existing industrial facilities, reducing capital expenditure barriers to adoption.
Solid Sorbents: Current Status and Technical Challenges
Solid sorbents have emerged as promising materials for CO2 capture in industrial-scale carbon capture and storage (CCS) systems. Currently, the global landscape of solid sorbent technology shows varying levels of development across different material classes. Amine-functionalized sorbents, particularly those based on silica supports, have reached pilot-scale demonstrations with CO2 capture capacities of 2-4 mmol/g under realistic conditions. Metal-organic frameworks (MOFs) exhibit exceptional theoretical capacities exceeding 5 mmol/g but face stability challenges in industrial environments containing moisture and impurities.
The geographical distribution of solid sorbent research shows concentration in North America, Europe, and East Asia, with the United States, China, and Germany leading patent filings. Academic-industrial partnerships have accelerated in recent years, particularly for zeolite and activated carbon-based solutions that leverage existing manufacturing infrastructure.
Despite promising advances, several critical technical challenges impede widespread industrial implementation. Sorbent durability remains a primary concern, with most materials showing significant capacity degradation after 100-1000 cycles under industrial conditions. This degradation is particularly pronounced in the presence of SOx, NOx, and water vapor commonly found in flue gas streams. Current regeneration processes also impose substantial energy penalties, with temperature swing adsorption (TSA) systems requiring 2.5-3.5 GJ/ton CO2 captured, significantly higher than the theoretical minimum.
Scalability presents another major hurdle, as laboratory-optimized synthesis routes often employ expensive precursors and complex procedures unsuitable for ton-scale production. The cost of high-performance sorbents remains prohibitively high, with current estimates ranging from $20-100/kg for advanced materials like MOFs and covalent organic frameworks (COFs), compared to $2-5/kg for conventional amine solutions.
Process engineering challenges further complicate industrial deployment. Current fixed-bed and fluidized-bed configurations suffer from heat management issues during the exothermic adsorption phase, leading to hotspots that accelerate sorbent degradation. Pressure drop across packed sorbent beds increases operational costs, while attrition in fluidized systems causes material loss estimated at 0.1-0.5% per cycle.
The techno-economic gap between laboratory performance and industrial requirements remains substantial. While laboratory studies often report ideal CO2 capture costs of $30-40/ton, real-world pilot demonstrations indicate costs of $60-90/ton when accounting for sorbent replacement, energy requirements, and system complexity. Bridging this gap requires interdisciplinary approaches combining materials science, process engineering, and system integration to develop next-generation solid sorbent technologies viable for industrial-scale carbon capture.
The geographical distribution of solid sorbent research shows concentration in North America, Europe, and East Asia, with the United States, China, and Germany leading patent filings. Academic-industrial partnerships have accelerated in recent years, particularly for zeolite and activated carbon-based solutions that leverage existing manufacturing infrastructure.
Despite promising advances, several critical technical challenges impede widespread industrial implementation. Sorbent durability remains a primary concern, with most materials showing significant capacity degradation after 100-1000 cycles under industrial conditions. This degradation is particularly pronounced in the presence of SOx, NOx, and water vapor commonly found in flue gas streams. Current regeneration processes also impose substantial energy penalties, with temperature swing adsorption (TSA) systems requiring 2.5-3.5 GJ/ton CO2 captured, significantly higher than the theoretical minimum.
Scalability presents another major hurdle, as laboratory-optimized synthesis routes often employ expensive precursors and complex procedures unsuitable for ton-scale production. The cost of high-performance sorbents remains prohibitively high, with current estimates ranging from $20-100/kg for advanced materials like MOFs and covalent organic frameworks (COFs), compared to $2-5/kg for conventional amine solutions.
Process engineering challenges further complicate industrial deployment. Current fixed-bed and fluidized-bed configurations suffer from heat management issues during the exothermic adsorption phase, leading to hotspots that accelerate sorbent degradation. Pressure drop across packed sorbent beds increases operational costs, while attrition in fluidized systems causes material loss estimated at 0.1-0.5% per cycle.
The techno-economic gap between laboratory performance and industrial requirements remains substantial. While laboratory studies often report ideal CO2 capture costs of $30-40/ton, real-world pilot demonstrations indicate costs of $60-90/ton when accounting for sorbent replacement, energy requirements, and system complexity. Bridging this gap requires interdisciplinary approaches combining materials science, process engineering, and system integration to develop next-generation solid sorbent technologies viable for industrial-scale carbon capture.
Current Solid Sorbent Solutions for Industrial Applications
01 Metal-organic frameworks (MOFs) for CO2 capture
Metal-organic frameworks are porous crystalline materials composed of metal ions or clusters coordinated with organic ligands. These materials have high surface areas and tunable pore sizes, making them effective for selective CO2 adsorption. MOFs can be designed with specific functional groups to enhance CO2 binding affinity and selectivity. Their modular nature allows for customization of properties to optimize capture performance under various conditions.- Metal-organic frameworks (MOFs) for CO2 capture: Metal-organic frameworks are porous crystalline materials composed of metal ions or clusters coordinated with organic ligands. These materials have high surface areas and tunable pore sizes, making them effective for selective CO2 adsorption. MOFs can be designed with specific functional groups to enhance CO2 binding affinity and selectivity. Their modular nature allows for customization to optimize capture performance under various conditions, including temperature and pressure swings.
- Amine-functionalized solid sorbents: Amine-functionalized materials represent a significant class of CO2 sorbents that operate through chemical adsorption mechanisms. These materials incorporate various amine groups onto solid supports such as silica, polymers, or porous carbons. The amine groups react with CO2 to form carbamates or bicarbonates, enabling high selectivity even at low CO2 concentrations. These sorbents can be regenerated through temperature or pressure swing processes, making them suitable for cyclic capture operations in industrial settings.
- Zeolite and molecular sieve-based CO2 capture: Zeolites and molecular sieves are aluminosilicate materials with well-defined pore structures that enable molecular sieving effects for gas separation. These materials can selectively adsorb CO2 based on molecular size and interaction strength. Their thermal stability allows for high-temperature applications and multiple regeneration cycles. Modified zeolites with enhanced hydrophobicity or incorporated functional groups can improve CO2 selectivity in the presence of moisture, addressing a common challenge in flue gas capture applications.
- Carbon-based sorbents for CO2 capture: Carbon-based materials, including activated carbons, carbon nanotubes, and graphene derivatives, offer versatile platforms for CO2 capture. These materials can be produced from various precursors, including biomass and waste products, making them potentially cost-effective. Their high surface area and tunable pore structure facilitate physical adsorption of CO2. Surface modification through nitrogen doping or incorporation of basic functional groups enhances CO2 binding affinity. Carbon-based sorbents typically demonstrate good stability across multiple adsorption-desorption cycles.
- Composite and hybrid sorbent materials: Composite and hybrid materials combine different types of sorbents to leverage complementary properties and overcome limitations of single-component systems. These materials may integrate organic and inorganic components, such as polymer-inorganic hybrids or mixed matrix materials. The synergistic effects can enhance CO2 capacity, selectivity, and stability under various operating conditions. Advanced manufacturing techniques, including 3D printing and controlled deposition methods, enable precise structural control of these composite materials to optimize gas diffusion and adsorption kinetics.
02 Amine-functionalized solid sorbents
Amine-functionalized materials represent a significant class of CO2 sorbents that operate through chemical adsorption mechanisms. These materials incorporate various amine groups onto solid supports such as silica, polymers, or porous carbons. The amine groups react with CO2 to form carbamates or bicarbonates, enabling high selectivity even at low CO2 concentrations. These sorbents can be regenerated through temperature or pressure swing processes, making them suitable for cyclic capture operations.Expand Specific Solutions03 Zeolite and molecular sieve-based CO2 capture
Zeolites and molecular sieves are aluminosilicate materials with well-defined pore structures that can selectively adsorb CO2 based on molecular size and polarity. These materials offer high thermal stability and can be modified with cations to enhance CO2 selectivity. Their rigid framework provides consistent performance over multiple adsorption-desorption cycles, making them suitable for industrial applications. The adsorption mechanism is primarily physical, allowing for relatively low-energy regeneration.Expand Specific Solutions04 Carbon-based sorbents for CO2 capture
Carbon-based materials, including activated carbons, carbon nanotubes, and graphene derivatives, serve as effective CO2 sorbents due to their high surface area and pore volume. These materials can be produced from various precursors, including biomass, polymers, or fossil resources. Surface modification through chemical treatments can enhance CO2 selectivity and capacity. Carbon-based sorbents typically offer good stability under harsh conditions and can be produced at relatively low cost compared to other advanced materials.Expand Specific Solutions05 Regeneration and process integration of solid sorbents
Effective regeneration strategies are crucial for the practical application of solid sorbents in CO2 capture systems. Various approaches include temperature swing adsorption (TSA), pressure swing adsorption (PSA), vacuum swing adsorption (VSA), or combinations thereof. Process integration considerations include heat management, pressure drop minimization, and sorbent stability over multiple cycles. Advanced system designs incorporate heat recovery, optimized flow patterns, and novel contacting methods to improve energy efficiency and reduce operational costs.Expand Specific Solutions
Leading Companies and Research Institutions in CCS
The solid sorbents for CO2 capture market is in a growth phase, driven by increasing global focus on carbon capture and storage (CCS) technologies. The market size is expanding rapidly, projected to reach significant scale as industrial decarbonization efforts intensify worldwide. Technologically, the field shows varying maturity levels, with companies like Sinopec Group, BASF, and Climeworks leading commercial deployment, while research institutions such as Norwegian University of Science & Technology and Arizona State University advance fundamental innovations. Korean power companies (KEPCO and subsidiaries) are actively implementing pilot projects, while Chinese entities like Huadian Electric Power Research Institute and Tianjin University focus on adapting technologies for coal-intensive economies. The competitive landscape features established energy corporations investing in scale-up capabilities alongside specialized startups like CarbonCapture Inc. and Antecy BV developing next-generation sorbent technologies.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced solid sorbent technologies for CO2 capture focusing on metal-organic frameworks (MOFs) and amine-functionalized mesoporous silica materials. Their proprietary MOF-based sorbents demonstrate exceptional CO2 selectivity and capacity under industrial flue gas conditions, achieving capture rates of 90%+ at temperatures between 40-80°C. Sinopec has successfully scaled these materials for pilot testing at their refineries, implementing temperature swing adsorption (TSA) and vacuum swing adsorption (VSA) processes. Their integrated system combines solid sorbent technology with heat integration strategies, reducing regeneration energy requirements to approximately 2.0-2.5 GJ/ton CO2 captured, significantly lower than conventional amine scrubbing methods (3.5-4.0 GJ/ton). Sinopec has demonstrated the technology's durability with sorbents maintaining >85% capacity after 1000+ adsorption-desorption cycles in real industrial environments.
Strengths: Sinopec's solid sorbents demonstrate excellent stability in industrial environments with minimal degradation over numerous cycles. Their integrated process design significantly reduces energy penalties compared to liquid amine systems. Weaknesses: The technology still faces challenges in large-scale manufacturing of consistent quality sorbents and requires further optimization for handling various impurities present in different industrial flue gas streams.
BASF Corp.
Technical Solution: BASF has pioneered advanced metal-organic frameworks (MOFs) and functionalized porous materials for CO2 capture at industrial scale. Their proprietary OASE® blue technology incorporates specially engineered solid sorbents with optimized pore structures and surface chemistry, achieving CO2 capture capacities of 3-5 mmol/g under typical flue gas conditions. BASF's approach combines these high-performance materials with innovative process engineering, utilizing a moving bed temperature swing adsorption system that enables continuous operation while minimizing energy requirements. Their solid sorbent technology reduces regeneration energy to approximately 2.2 GJ/ton CO2, representing a 30-40% improvement over conventional amine scrubbing. BASF has successfully demonstrated this technology at pilot scale (capturing 1-5 tons CO2/day) with partners across power generation and industrial sectors, showing stable performance over thousands of adsorption-desorption cycles with minimal sorbent degradation. The company has also developed specialized manufacturing techniques to produce these advanced materials at commercial scale while maintaining consistent quality and performance characteristics.
Strengths: BASF's extensive chemical engineering expertise enables them to optimize both sorbent materials and process design simultaneously. Their established global manufacturing infrastructure facilitates rapid scaling of promising materials. Weaknesses: Their solid sorbent systems may require significant capital investment for retrofitting existing plants and still face challenges with sensitivity to certain flue gas contaminants that can reduce long-term performance.
Key Patents and Breakthroughs in Solid Sorbent Technology
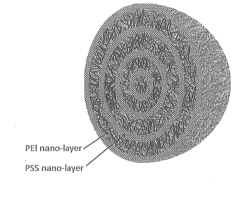
Layered Solid Sorbents For Carbon Dioxide Capture
PatentActiveUS20140127104A1
Innovation
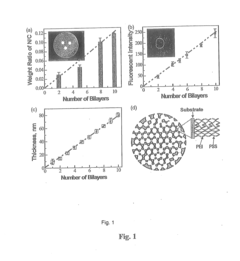
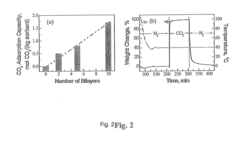
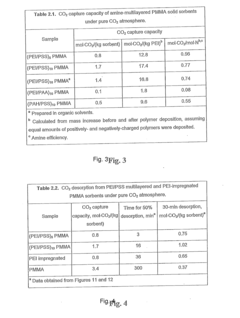
- Development of nano-layered solid sorbents using electrostatic layer-by-layer nanoassembly, where CO2-adsorbing polymers like polyethylenimine and oppositely charged polyelectrolytes are alternately deposited on porous substrates, creating bilayers that enhance CO2 capture and transport efficiency.
Solid sorbents for capturing co 2
PatentWO2023232666A1
Innovation
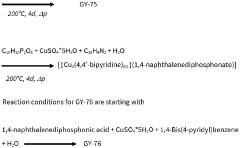
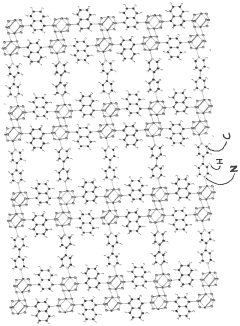
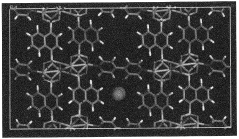
- Development of phosphonate and organoarsonate MOFs with specific molecular formulas, such as [{M2(4,4’-bipyridine)0.5}(l,4-naphthalenediphosphonate)] and [{M2(4,4’-bipyridine)0.5}(l,4-naphthalenediarsonate)], which maintain selectivity and stability under harsh conditions, including high humidity and temperatures up to 360°C, by creating a hydrophobic environment that favors CO2 physisorption over H2O.
Techno-Economic Assessment of Solid Sorbent Systems
The techno-economic assessment of solid sorbent systems for CO2 capture reveals significant potential for cost reduction compared to conventional liquid amine-based technologies. Current economic analyses indicate that solid sorbent systems could achieve capture costs between $40-70 per ton of CO2, representing a 20-35% reduction from first-generation capture technologies.
Capital expenditure (CAPEX) for solid sorbent systems benefits from simpler process configurations and lower equipment corrosion requirements. The absence of large solvent regeneration columns and reduced material costs contribute to an estimated 15-25% lower initial investment compared to liquid amine systems of equivalent capacity. However, these savings must be balanced against the need for specialized solid handling equipment and potentially larger adsorption vessels.
Operational expenditure (OPEX) advantages stem primarily from lower energy requirements for sorbent regeneration. Solid sorbents typically require 2.0-2.8 GJ/ton CO2 for regeneration, compared to 3.0-4.2 GJ/ton CO2 for conventional amine scrubbing. This translates to approximately 25-30% energy savings, significantly reducing operational costs in energy-intensive industries.
Sorbent lifetime and degradation rates represent critical economic factors. Current metal-organic frameworks (MOFs) and amine-functionalized silica sorbents demonstrate stability for 1,000-5,000 cycles under laboratory conditions, but industrial implementation may reduce this performance. Replacement costs are estimated at $5-15 per ton of CO2 captured, depending on sorbent type and operating conditions.
Scale-up considerations reveal that solid sorbent systems maintain favorable economics at industrial scales above 100,000 tons CO2/year. Modeling suggests that the cost advantage over liquid systems increases with scale due to more efficient heat integration opportunities and reduced energy penalties. However, this advantage diminishes at very large scales (>1 million tons CO2/year) where liquid systems benefit from established engineering practices.
Sensitivity analysis identifies regeneration energy requirements and sorbent lifetime as the most influential parameters affecting overall economics. A 10% improvement in either parameter could reduce capture costs by 5-8%, highlighting these as priority areas for continued research and development.
Integration with existing industrial processes presents additional economic benefits through waste heat utilization. Industries with available low-grade heat (100-200°C) could reduce regeneration energy costs by 30-50%, making solid sorbent systems particularly attractive for cement, steel, and certain chemical manufacturing applications.
Capital expenditure (CAPEX) for solid sorbent systems benefits from simpler process configurations and lower equipment corrosion requirements. The absence of large solvent regeneration columns and reduced material costs contribute to an estimated 15-25% lower initial investment compared to liquid amine systems of equivalent capacity. However, these savings must be balanced against the need for specialized solid handling equipment and potentially larger adsorption vessels.
Operational expenditure (OPEX) advantages stem primarily from lower energy requirements for sorbent regeneration. Solid sorbents typically require 2.0-2.8 GJ/ton CO2 for regeneration, compared to 3.0-4.2 GJ/ton CO2 for conventional amine scrubbing. This translates to approximately 25-30% energy savings, significantly reducing operational costs in energy-intensive industries.
Sorbent lifetime and degradation rates represent critical economic factors. Current metal-organic frameworks (MOFs) and amine-functionalized silica sorbents demonstrate stability for 1,000-5,000 cycles under laboratory conditions, but industrial implementation may reduce this performance. Replacement costs are estimated at $5-15 per ton of CO2 captured, depending on sorbent type and operating conditions.
Scale-up considerations reveal that solid sorbent systems maintain favorable economics at industrial scales above 100,000 tons CO2/year. Modeling suggests that the cost advantage over liquid systems increases with scale due to more efficient heat integration opportunities and reduced energy penalties. However, this advantage diminishes at very large scales (>1 million tons CO2/year) where liquid systems benefit from established engineering practices.
Sensitivity analysis identifies regeneration energy requirements and sorbent lifetime as the most influential parameters affecting overall economics. A 10% improvement in either parameter could reduce capture costs by 5-8%, highlighting these as priority areas for continued research and development.
Integration with existing industrial processes presents additional economic benefits through waste heat utilization. Industries with available low-grade heat (100-200°C) could reduce regeneration energy costs by 30-50%, making solid sorbent systems particularly attractive for cement, steel, and certain chemical manufacturing applications.
Environmental Impact and Sustainability Considerations
The environmental impact of solid sorbents for CO2 capture extends far beyond their primary function of carbon sequestration. When evaluating these materials for industrial-scale deployment, a comprehensive life cycle assessment (LCA) becomes essential to ensure that the environmental benefits outweigh the costs. The production of solid sorbents often involves energy-intensive processes and potentially hazardous chemicals, which can generate significant carbon footprints that partially offset their capture benefits.
Water consumption represents another critical environmental consideration. Many solid sorbent systems require substantial water resources for regeneration processes or cooling systems. In regions facing water scarcity, this dependency could exacerbate existing environmental stresses and create competition with agricultural or municipal water needs. Additionally, the disposal or recycling of spent sorbents presents long-term environmental challenges that must be addressed through proper waste management protocols.
Land use implications vary significantly depending on the capture technology deployed. While solid sorbent systems generally have smaller physical footprints compared to liquid solvent facilities, the infrastructure required for industrial-scale operations still demands considerable space. This becomes particularly relevant when considering the entire carbon capture and storage chain, including transportation infrastructure and storage facilities, which may impact natural habitats or compete with other land uses.
The sustainability of solid sorbent technologies also depends heavily on material sourcing. Many advanced sorbents incorporate rare earth elements or specialized compounds that face supply constraints or originate from environmentally sensitive mining operations. Developing sorbents from abundant, renewable, or waste-derived materials represents a promising direction for enhancing sustainability credentials while potentially reducing costs.
Energy requirements for sorbent regeneration remain a significant sustainability challenge. The parasitic energy load of regeneration processes can substantially reduce the net environmental benefit of carbon capture systems. Innovations in low-temperature regeneration techniques and integration with waste heat sources are emerging as vital strategies to improve the overall energy efficiency and environmental performance of solid sorbent systems.
Ultimately, the environmental viability of solid sorbent technologies must be evaluated within specific regional contexts, considering local energy mixes, water availability, and regulatory frameworks. The most sustainable approaches will likely involve customized solutions that optimize material selection, process design, and system integration to minimize environmental impacts while maximizing carbon capture efficiency.
Water consumption represents another critical environmental consideration. Many solid sorbent systems require substantial water resources for regeneration processes or cooling systems. In regions facing water scarcity, this dependency could exacerbate existing environmental stresses and create competition with agricultural or municipal water needs. Additionally, the disposal or recycling of spent sorbents presents long-term environmental challenges that must be addressed through proper waste management protocols.
Land use implications vary significantly depending on the capture technology deployed. While solid sorbent systems generally have smaller physical footprints compared to liquid solvent facilities, the infrastructure required for industrial-scale operations still demands considerable space. This becomes particularly relevant when considering the entire carbon capture and storage chain, including transportation infrastructure and storage facilities, which may impact natural habitats or compete with other land uses.
The sustainability of solid sorbent technologies also depends heavily on material sourcing. Many advanced sorbents incorporate rare earth elements or specialized compounds that face supply constraints or originate from environmentally sensitive mining operations. Developing sorbents from abundant, renewable, or waste-derived materials represents a promising direction for enhancing sustainability credentials while potentially reducing costs.
Energy requirements for sorbent regeneration remain a significant sustainability challenge. The parasitic energy load of regeneration processes can substantially reduce the net environmental benefit of carbon capture systems. Innovations in low-temperature regeneration techniques and integration with waste heat sources are emerging as vital strategies to improve the overall energy efficiency and environmental performance of solid sorbent systems.
Ultimately, the environmental viability of solid sorbent technologies must be evaluated within specific regional contexts, considering local energy mixes, water availability, and regulatory frameworks. The most sustainable approaches will likely involve customized solutions that optimize material selection, process design, and system integration to minimize environmental impacts while maximizing carbon capture efficiency.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!