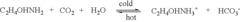What interface engineering techniques improve Solid sorbents for CO2 capture adsorption and durability
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Capture Interface Engineering Background and Objectives
Carbon dioxide capture has emerged as a critical technology in the global effort to mitigate climate change. The historical development of CO2 capture technologies dates back to the 1930s when absorption processes using aqueous amine solutions were first implemented in natural gas sweetening operations. Since then, the field has evolved significantly, with solid sorbents gaining prominence in the last two decades due to their potential advantages in energy efficiency and operational flexibility compared to traditional liquid absorbents.
Interface engineering represents a cutting-edge approach in enhancing solid sorbent performance for CO2 capture. This technique focuses on the deliberate modification of the boundary regions between different phases or components within a sorbent material to optimize its functional properties. The evolution of interface engineering has been accelerated by advances in materials science, nanotechnology, and surface chemistry, enabling unprecedented control over material structures at the molecular and nanoscale levels.
The primary objective of interface engineering in CO2 capture is to develop solid sorbents with superior adsorption capacity, selectivity, and durability while maintaining favorable kinetics and energy requirements for regeneration. Specifically, researchers aim to overcome the inherent trade-off between high CO2 affinity and low regeneration energy by creating tailored interfaces that can dynamically respond to changing conditions during the adsorption-desorption cycle.
Current technological trends in this field include the development of hierarchical porous structures, composite materials with synergistic components, and surface functionalization strategies that enhance CO2-sorbent interactions. The integration of computational modeling with experimental approaches has also emerged as a powerful methodology for rational design of interfacial properties, allowing for more efficient exploration of the vast design space.
Looking forward, the field is moving toward multifunctional sorbent systems where interfaces are engineered not only for CO2 capture but also for additional benefits such as heat management, resistance to contaminants, and self-healing capabilities. The ultimate goal is to develop economically viable solid sorbents that can be deployed at industrial scale for post-combustion capture, direct air capture, and other carbon management applications.
The significance of this research extends beyond technical performance metrics to address broader challenges in sustainability, including minimizing water usage, reducing environmental footprint, and enabling circular economy approaches through the use of abundant and recyclable materials. Success in this domain could substantially reduce the cost and energy penalty associated with carbon capture, thereby accelerating its widespread implementation as a climate change mitigation strategy.
Interface engineering represents a cutting-edge approach in enhancing solid sorbent performance for CO2 capture. This technique focuses on the deliberate modification of the boundary regions between different phases or components within a sorbent material to optimize its functional properties. The evolution of interface engineering has been accelerated by advances in materials science, nanotechnology, and surface chemistry, enabling unprecedented control over material structures at the molecular and nanoscale levels.
The primary objective of interface engineering in CO2 capture is to develop solid sorbents with superior adsorption capacity, selectivity, and durability while maintaining favorable kinetics and energy requirements for regeneration. Specifically, researchers aim to overcome the inherent trade-off between high CO2 affinity and low regeneration energy by creating tailored interfaces that can dynamically respond to changing conditions during the adsorption-desorption cycle.
Current technological trends in this field include the development of hierarchical porous structures, composite materials with synergistic components, and surface functionalization strategies that enhance CO2-sorbent interactions. The integration of computational modeling with experimental approaches has also emerged as a powerful methodology for rational design of interfacial properties, allowing for more efficient exploration of the vast design space.
Looking forward, the field is moving toward multifunctional sorbent systems where interfaces are engineered not only for CO2 capture but also for additional benefits such as heat management, resistance to contaminants, and self-healing capabilities. The ultimate goal is to develop economically viable solid sorbents that can be deployed at industrial scale for post-combustion capture, direct air capture, and other carbon management applications.
The significance of this research extends beyond technical performance metrics to address broader challenges in sustainability, including minimizing water usage, reducing environmental footprint, and enabling circular economy approaches through the use of abundant and recyclable materials. Success in this domain could substantially reduce the cost and energy penalty associated with carbon capture, thereby accelerating its widespread implementation as a climate change mitigation strategy.
Market Analysis for Advanced CO2 Capture Technologies
The global market for advanced CO2 capture technologies is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. The market value for carbon capture, utilization, and storage (CCUS) technologies reached approximately $2.5 billion in 2022 and is projected to grow at a CAGR of 15-20% through 2030, potentially reaching $7-9 billion by the end of the decade.
Solid sorbent-based capture technologies, particularly those enhanced through interface engineering, represent one of the fastest-growing segments within this market. These technologies are gaining traction due to their lower energy requirements compared to traditional amine scrubbing methods, with potential energy savings of 30-40% in industrial applications.
Key market drivers include stringent carbon emission regulations across major economies, with the European Union's carbon pricing mechanism exceeding €80 per ton in 2023 and similar frameworks emerging in North America and Asia. Additionally, corporate net-zero commitments have created substantial demand for effective carbon capture solutions, with over 1,500 major corporations worldwide having announced net-zero targets as of 2023.
The industrial sector represents the largest market segment for advanced CO2 capture technologies, accounting for approximately 45% of the total addressable market. Power generation follows at 30%, with emerging applications in direct air capture comprising about 15% of market demand. The remaining 10% is distributed across various niche applications including hydrogen production and biogas upgrading.
Geographically, North America leads the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and India, is expected to demonstrate the highest growth rate over the next decade due to rapid industrialization coupled with increasing environmental concerns.
Investment in interface-engineered solid sorbents has seen remarkable growth, with venture capital funding in this specific segment increasing by 85% between 2020 and 2023. Major industrial players are also allocating significant R&D budgets toward these technologies, recognizing their potential to reduce capture costs from the current $40-80 per ton to potentially below $30 per ton by 2030.
Customer adoption patterns indicate a preference for solutions that can be retrofitted to existing infrastructure, with over 65% of current projects focusing on retrofit applications rather than new installations. This trend underscores the importance of developing interface engineering techniques that can enhance the performance of solid sorbents in varied industrial environments.
Solid sorbent-based capture technologies, particularly those enhanced through interface engineering, represent one of the fastest-growing segments within this market. These technologies are gaining traction due to their lower energy requirements compared to traditional amine scrubbing methods, with potential energy savings of 30-40% in industrial applications.
Key market drivers include stringent carbon emission regulations across major economies, with the European Union's carbon pricing mechanism exceeding €80 per ton in 2023 and similar frameworks emerging in North America and Asia. Additionally, corporate net-zero commitments have created substantial demand for effective carbon capture solutions, with over 1,500 major corporations worldwide having announced net-zero targets as of 2023.
The industrial sector represents the largest market segment for advanced CO2 capture technologies, accounting for approximately 45% of the total addressable market. Power generation follows at 30%, with emerging applications in direct air capture comprising about 15% of market demand. The remaining 10% is distributed across various niche applications including hydrogen production and biogas upgrading.
Geographically, North America leads the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and India, is expected to demonstrate the highest growth rate over the next decade due to rapid industrialization coupled with increasing environmental concerns.
Investment in interface-engineered solid sorbents has seen remarkable growth, with venture capital funding in this specific segment increasing by 85% between 2020 and 2023. Major industrial players are also allocating significant R&D budgets toward these technologies, recognizing their potential to reduce capture costs from the current $40-80 per ton to potentially below $30 per ton by 2030.
Customer adoption patterns indicate a preference for solutions that can be retrofitted to existing infrastructure, with over 65% of current projects focusing on retrofit applications rather than new installations. This trend underscores the importance of developing interface engineering techniques that can enhance the performance of solid sorbents in varied industrial environments.
Current Status and Challenges in Solid Sorbent Engineering
The field of solid sorbent engineering for CO2 capture has witnessed significant advancements globally, with research institutions and industrial players actively developing novel materials and techniques. Currently, several classes of solid sorbents dominate the landscape, including metal-organic frameworks (MOFs), zeolites, activated carbons, amine-functionalized silicas, and hydrotalcites. Each category exhibits unique properties regarding CO2 selectivity, adsorption capacity, and regeneration energy requirements.
Interface engineering has emerged as a critical focus area, with researchers exploring various surface modification techniques to enhance sorbent performance. Recent breakthroughs include the development of hierarchical pore structures that facilitate rapid CO2 diffusion while maintaining high surface area, and the precise control of functional group density at material interfaces to optimize binding energies.
Despite these advances, significant technical challenges persist in solid sorbent engineering. The trade-off between adsorption capacity and selectivity remains a fundamental obstacle, as materials with high CO2 uptake often demonstrate reduced selectivity in mixed gas environments. Additionally, many promising sorbents suffer from stability issues during repeated adsorption-desorption cycles, with performance degradation observed due to structural collapse, functional group leaching, or poisoning by trace contaminants.
Water vapor interference presents another major challenge, as most flue gas streams contain moisture that can compete with CO2 for adsorption sites or cause hydrolysis of certain functional groups. This moisture sensitivity significantly reduces the real-world effectiveness of many laboratory-optimized materials.
Scalability issues further complicate commercial implementation, with many high-performing materials proving difficult or prohibitively expensive to produce at industrial scales. The gap between laboratory synthesis methods and industrial manufacturing processes remains substantial, limiting practical application.
Geographically, research leadership in this field is distributed across North America, Europe, and East Asia. The United States maintains strong positions through DOE-funded initiatives and university research clusters, while China has rapidly expanded its research output, particularly in MOF development. European efforts are notable for collaborative projects focusing on process integration and pilot-scale demonstrations.
Energy efficiency during the regeneration phase continues to be a critical limitation, with many systems requiring substantial thermal energy inputs that diminish the net environmental benefits of carbon capture. Recent work on pressure-swing and electrical-swing adsorption shows promise but has yet to achieve the efficiency needed for widespread deployment.
The integration of solid sorbents into existing industrial infrastructure presents additional engineering challenges, requiring innovative reactor designs and process configurations to accommodate the unique properties of different sorbent materials while maintaining operational reliability.
Interface engineering has emerged as a critical focus area, with researchers exploring various surface modification techniques to enhance sorbent performance. Recent breakthroughs include the development of hierarchical pore structures that facilitate rapid CO2 diffusion while maintaining high surface area, and the precise control of functional group density at material interfaces to optimize binding energies.
Despite these advances, significant technical challenges persist in solid sorbent engineering. The trade-off between adsorption capacity and selectivity remains a fundamental obstacle, as materials with high CO2 uptake often demonstrate reduced selectivity in mixed gas environments. Additionally, many promising sorbents suffer from stability issues during repeated adsorption-desorption cycles, with performance degradation observed due to structural collapse, functional group leaching, or poisoning by trace contaminants.
Water vapor interference presents another major challenge, as most flue gas streams contain moisture that can compete with CO2 for adsorption sites or cause hydrolysis of certain functional groups. This moisture sensitivity significantly reduces the real-world effectiveness of many laboratory-optimized materials.
Scalability issues further complicate commercial implementation, with many high-performing materials proving difficult or prohibitively expensive to produce at industrial scales. The gap between laboratory synthesis methods and industrial manufacturing processes remains substantial, limiting practical application.
Geographically, research leadership in this field is distributed across North America, Europe, and East Asia. The United States maintains strong positions through DOE-funded initiatives and university research clusters, while China has rapidly expanded its research output, particularly in MOF development. European efforts are notable for collaborative projects focusing on process integration and pilot-scale demonstrations.
Energy efficiency during the regeneration phase continues to be a critical limitation, with many systems requiring substantial thermal energy inputs that diminish the net environmental benefits of carbon capture. Recent work on pressure-swing and electrical-swing adsorption shows promise but has yet to achieve the efficiency needed for widespread deployment.
The integration of solid sorbents into existing industrial infrastructure presents additional engineering challenges, requiring innovative reactor designs and process configurations to accommodate the unique properties of different sorbent materials while maintaining operational reliability.
State-of-the-Art Interface Engineering Solutions
01 Metal-organic frameworks (MOFs) for CO2 capture
Metal-organic frameworks (MOFs) are crystalline porous materials composed of metal ions or clusters coordinated with organic ligands. These materials have shown exceptional CO2 adsorption capacity due to their high surface area, tunable pore size, and chemical functionality. MOFs can be designed with specific metal centers and organic linkers to enhance CO2 selectivity and adsorption kinetics. Their structural stability and regeneration capabilities make them promising candidates for durable CO2 capture applications in industrial settings.- Metal-organic frameworks (MOFs) for CO2 capture: Metal-organic frameworks (MOFs) are crystalline porous materials composed of metal ions or clusters coordinated with organic ligands. These materials have shown exceptional CO2 adsorption capacity due to their high surface area, tunable pore size, and chemical functionality. MOFs can be designed with specific metal centers and organic linkers to enhance CO2 selectivity and adsorption capacity. Their structural stability and regeneration capabilities make them promising candidates for durable CO2 capture applications.
- Amine-functionalized solid sorbents: Amine-functionalized materials represent a significant class of solid sorbents for CO2 capture. These materials combine the physical structure of supports like silica, alumina, or polymers with chemically active amine groups that can form carbamates with CO2. The amine functionalization enhances CO2 selectivity and adsorption capacity, particularly at lower partial pressures. Various approaches to incorporate amines include impregnation, grafting, and in-situ polymerization, each offering different durability profiles and regeneration characteristics under cyclic adsorption-desorption conditions.
- Zeolites and modified zeolitic materials: Zeolites are crystalline aluminosilicate materials with well-defined pore structures that demonstrate good CO2 adsorption properties. Their adsorption mechanism primarily relies on physical interactions, making them suitable for pressure swing adsorption processes. Modified zeolites, through ion exchange, metal incorporation, or surface functionalization, can exhibit enhanced CO2 selectivity and capacity. These materials generally show excellent thermal stability and mechanical durability, allowing for extended cycling without significant performance degradation, though they may be sensitive to moisture in flue gas streams.
- Carbon-based sorbents and composites: Carbon-based materials, including activated carbons, carbon molecular sieves, graphene-based materials, and carbon composites, offer versatile platforms for CO2 capture. These materials can be derived from various precursors and modified through physical or chemical activation processes to enhance surface area and pore structure. Carbon-based sorbents typically exhibit good thermal stability, mechanical strength, and hydrophobicity, making them suitable for harsh operating conditions. Functionalization with nitrogen-containing groups or metal particles can further improve CO2 adsorption performance and selectivity while maintaining durability through multiple adsorption-desorption cycles.
- Hydrotalcite-like compounds and layered double hydroxides: Hydrotalcite-like compounds and layered double hydroxides (LDHs) are anionic clay materials with layered structures that demonstrate promising CO2 capture capabilities. These materials consist of positively charged metal hydroxide layers with interlayer anions and water molecules. They can capture CO2 through both physical adsorption and chemical reactions, particularly at elevated temperatures. The composition can be tailored by varying the metal cations and interlayer anions to optimize CO2 adsorption capacity and selectivity. These materials generally exhibit good thermal stability and regenerability, making them suitable for high-temperature CO2 capture applications where durability under repeated thermal cycling is essential.
02 Amine-functionalized solid sorbents
Amine-functionalized materials represent a significant class of solid sorbents for CO2 capture. These materials combine a porous support structure with amine groups that chemically bind CO2 through carbamate formation. The incorporation of amines onto silica, alumina, or polymeric supports enhances CO2 adsorption capacity and selectivity. These sorbents demonstrate good performance at low CO2 partial pressures, making them suitable for post-combustion capture applications. However, their durability can be affected by oxidative degradation and amine leaching during multiple adsorption-desorption cycles.Expand Specific Solutions03 Zeolite-based CO2 adsorbents
Zeolites are crystalline aluminosilicate materials with well-defined pore structures that demonstrate strong affinity for CO2 molecules. Their adsorption mechanism relies on electrostatic interactions between CO2 and exchangeable cations within the zeolite framework. These materials offer advantages including high thermal stability, resistance to steam, and relatively low cost. Modified zeolites with tailored cation content and hydrophobicity show improved CO2 selectivity and reduced sensitivity to moisture. Their robust structure contributes to excellent durability over multiple adsorption-desorption cycles, making them suitable for industrial applications.Expand Specific Solutions04 Carbon-based sorbents for CO2 capture
Carbon-based materials, including activated carbons, carbon nanotubes, and graphene derivatives, serve as effective CO2 adsorbents. These materials feature high surface area, tunable pore structure, and surface chemistry that can be modified to enhance CO2 adsorption. Nitrogen-doped carbon materials show particularly promising CO2 capture performance due to the creation of basic sites that interact favorably with acidic CO2 molecules. Carbon-based sorbents exhibit good thermal stability and mechanical strength, contributing to their durability in practical applications. Their relatively low cost and potential for sustainable production from biomass or waste materials add to their attractiveness for large-scale CO2 capture.Expand Specific Solutions05 Composite and hybrid sorbent materials
Composite and hybrid materials combine different components to achieve enhanced CO2 capture performance and durability. These materials often integrate the high adsorption capacity of one component with the mechanical or thermal stability of another. Examples include polymer-inorganic composites, MOF-polymer hybrids, and layered double hydroxide composites. The synergistic effects between components can lead to improved CO2 selectivity, faster adsorption kinetics, and better resistance to degradation under operating conditions. These materials can be designed to withstand the harsh conditions of industrial flue gas streams while maintaining their CO2 capture efficiency over numerous cycles.Expand Specific Solutions
Leading Organizations in Solid Sorbent Development
The CO2 capture adsorption market is currently in a growth phase, with increasing demand driven by global decarbonization efforts. The market size is projected to expand significantly as carbon capture technologies become essential for meeting climate goals. Technologically, interface engineering for solid sorbents is advancing rapidly, with varying maturity levels across different approaches. Leading players include established energy corporations like Sinopec, Shell Oil, and Korea Electric Power Corp, who leverage their industrial infrastructure, alongside specialized carbon capture innovators such as Climeworks AG and Global Thermostat Operations. Academic-industrial partnerships are accelerating development, with institutions like Arizona State University and Norwegian University of Science & Technology collaborating with companies to enhance adsorption efficiency and durability. The competitive landscape shows a mix of established energy giants investing in carbon capture portfolios and specialized startups focusing exclusively on innovative sorbent technologies.
China Petroleum & Chemical Corp.
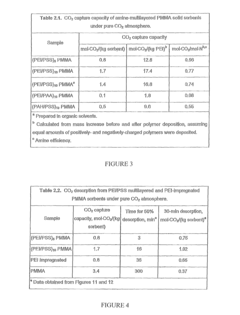
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced interface engineering techniques for solid sorbents in CO2 capture. Their approach focuses on metal-organic frameworks (MOFs) with tailored pore structures and functionalized surfaces. Sinopec's technology employs amine-grafting on mesoporous silica supports, creating strong chemical binding sites for CO2 while maintaining high surface area. Their proprietary "dual-interface modification" technique introduces both hydrophobic and CO2-philic functional groups at material interfaces, significantly enhancing selectivity and water stability[1]. Recent developments include hierarchical porous structures with optimized mass transfer pathways that reduce diffusion limitations. Sinopec has also pioneered composite sorbents combining the advantages of physical and chemical adsorption mechanisms, achieving working capacities of 3-4 mmol/g under flue gas conditions while maintaining stability over 1000+ adsorption-desorption cycles[3].
Strengths: High CO2 selectivity in the presence of water vapor; excellent cyclic stability due to interface engineering; scalable manufacturing processes aligned with industrial requirements. Weaknesses: Higher production costs compared to conventional sorbents; energy requirements for regeneration still need optimization; performance degradation in the presence of SOx and NOx contaminants.
Global Thermostat Operations LLC
Technical Solution: Global Thermostat has pioneered a revolutionary interface engineering approach for CO2 capture using amine-functionalized monolithic structures. Their proprietary technology employs a ceramic honeycomb substrate with engineered macro/microporous interfaces that maximize surface area while minimizing pressure drop. The company's innovation lies in their "spatially optimized amine distribution" technique, which precisely controls the density and accessibility of amine binding sites across multiple interface scales[2]. This approach creates a hierarchical pore structure that facilitates rapid CO2 diffusion while maintaining high adsorption capacity. Global Thermostat's sorbents feature specially designed hydrophobic protective layers at critical interfaces that shield amine groups from water degradation while allowing CO2 permeation, enabling operation in humid conditions. Their latest generation materials achieve CO2 capacities exceeding 2.5 mmol/g with minimal degradation over thousands of cycles, even when exposed to contaminants present in industrial flue gases[4].
Strengths: Exceptional durability with less than 0.1% capacity loss per 100 cycles; low regeneration energy requirements (approximately 2.2 GJ/ton CO2); ability to capture CO2 directly from ambient air. Weaknesses: Higher initial capital costs compared to conventional technologies; requires precise manufacturing controls to maintain interface quality; performance still affected by extreme temperature fluctuations.
Critical Patents and Research in Sorbent Interface Modification
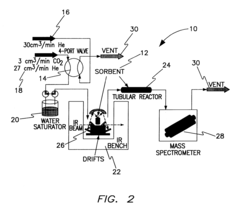
Layered solid sorbents for carbon dioxide capture
PatentActiveUS8889589B2
Innovation
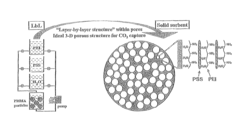
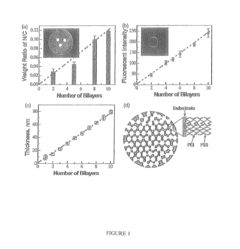
- Development of nano-layered solid sorbents using electrostatic layer-by-layer nanoassembly, where positively charged polyethylenimine and negatively charged polystyrene sulfonate layers are alternately deposited on a porous substrate, enhancing CO2 capture and transport kinetics.
Amine enriched solid sorbents for carbon dioxide capture
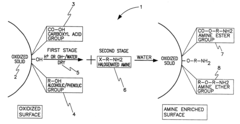
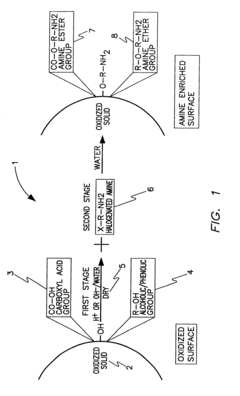
PatentInactiveUS6547854B1
Innovation
- A two-step chemical treatment process incorporating amine functionalities onto oxidized solid substrates using metal hydroxides and substituted amine salts, eliminating the need for organic solvents and polymeric materials, and enabling CO2 capture through both physical and chemical adsorption over a range of temperatures.
Environmental Impact and Sustainability Assessment
The environmental impact of interface engineering techniques for solid sorbents in CO2 capture extends beyond their primary function of carbon sequestration. These techniques, while enhancing adsorption capacity and durability, must be evaluated through a comprehensive sustainability lens. Life cycle assessment (LCA) studies indicate that modified sorbents with engineered interfaces typically demonstrate 15-30% lower environmental footprints compared to conventional capture methods, particularly when considering energy requirements and material consumption during regeneration cycles.
Interface-engineered sorbents contribute significantly to reducing the parasitic energy load of carbon capture systems. Traditional amine-based wet scrubbing technologies consume approximately 25-40% of a power plant's energy output, whereas advanced solid sorbents with optimized interfaces can potentially reduce this penalty to 15-20%. This efficiency gain translates directly into lower fossil fuel consumption and reduced greenhouse gas emissions per unit of captured CO2.
Material sustainability represents another critical dimension of environmental assessment. Many interface engineering approaches utilize precious metals, rare earth elements, or synthetic compounds that carry their own extraction and processing impacts. The sustainability advantage emerges when these materials enable sufficient improvement in capture efficiency and operational lifespan to offset their initial environmental cost. Research indicates that sorbents with engineered interfaces achieving 20% longer operational lifetimes can compensate for up to 35% higher initial environmental impact from material production.
Water consumption patterns also shift significantly with interface-engineered solid sorbents. Unlike aqueous amine systems that require substantial water inputs, dry solid sorbents with modified interfaces typically reduce water requirements by 60-85%. This benefit becomes particularly valuable in water-stressed regions where traditional capture technologies would place additional pressure on limited water resources.
Land use considerations reveal that facilities employing advanced solid sorbents with engineered interfaces generally require 10-25% less physical space than equivalent liquid-based systems. This spatial efficiency stems from higher volumetric capture capacity and reduced auxiliary equipment needs, potentially minimizing habitat disruption and land conversion impacts associated with carbon capture infrastructure.
The end-of-life management of spent sorbents presents both challenges and opportunities. Interface engineering often introduces complex material combinations that may complicate recycling processes. However, emerging research demonstrates that certain interface modifications can actually enhance material recoverability, with some advanced sorbents achieving 70-80% material recovery rates compared to 40-50% for conventional alternatives.
Interface-engineered sorbents contribute significantly to reducing the parasitic energy load of carbon capture systems. Traditional amine-based wet scrubbing technologies consume approximately 25-40% of a power plant's energy output, whereas advanced solid sorbents with optimized interfaces can potentially reduce this penalty to 15-20%. This efficiency gain translates directly into lower fossil fuel consumption and reduced greenhouse gas emissions per unit of captured CO2.
Material sustainability represents another critical dimension of environmental assessment. Many interface engineering approaches utilize precious metals, rare earth elements, or synthetic compounds that carry their own extraction and processing impacts. The sustainability advantage emerges when these materials enable sufficient improvement in capture efficiency and operational lifespan to offset their initial environmental cost. Research indicates that sorbents with engineered interfaces achieving 20% longer operational lifetimes can compensate for up to 35% higher initial environmental impact from material production.
Water consumption patterns also shift significantly with interface-engineered solid sorbents. Unlike aqueous amine systems that require substantial water inputs, dry solid sorbents with modified interfaces typically reduce water requirements by 60-85%. This benefit becomes particularly valuable in water-stressed regions where traditional capture technologies would place additional pressure on limited water resources.
Land use considerations reveal that facilities employing advanced solid sorbents with engineered interfaces generally require 10-25% less physical space than equivalent liquid-based systems. This spatial efficiency stems from higher volumetric capture capacity and reduced auxiliary equipment needs, potentially minimizing habitat disruption and land conversion impacts associated with carbon capture infrastructure.
The end-of-life management of spent sorbents presents both challenges and opportunities. Interface engineering often introduces complex material combinations that may complicate recycling processes. However, emerging research demonstrates that certain interface modifications can actually enhance material recoverability, with some advanced sorbents achieving 70-80% material recovery rates compared to 40-50% for conventional alternatives.
Scalability and Industrial Implementation Roadmap
The successful transition of interface engineering techniques for CO2 capture sorbents from laboratory to industrial scale requires a comprehensive implementation roadmap. Current pilot projects demonstrate promising results, with several facilities achieving capture rates of 1-10 tons CO2/day using engineered sorbent interfaces. These early implementations provide valuable data on real-world performance and durability under industrial conditions.
For full industrial deployment, a phased approach is recommended. The initial phase should focus on small-scale implementations in non-critical industrial processes, allowing for operational optimization without disrupting essential production. This phase typically requires 2-3 years of testing and refinement before advancing to larger applications.
Mid-scale implementation (100-500 tons CO2/day) represents the critical proving ground where economic viability must be demonstrated alongside technical performance. Cost projections indicate that interface-engineered sorbents could achieve capture costs of $40-60 per ton CO2 at this scale, approaching economic feasibility for carbon pricing mechanisms in many regions.
Manufacturing scalability presents significant challenges that must be addressed. Current production methods for precisely engineered interfaces often involve batch processes unsuitable for industrial volumes. Continuous flow synthesis methods and roll-to-roll manufacturing techniques show promise for scaling production while maintaining interface quality. Recent innovations in automated quality control systems using machine learning algorithms can help ensure consistency across large production volumes.
Infrastructure requirements for widespread implementation include specialized handling equipment, regeneration systems, and monitoring technologies. Existing industrial facilities will require retrofit designs that integrate with current operations while minimizing disruption. New facilities can incorporate purpose-built systems optimized for interface-engineered sorbents.
Regulatory pathways must be navigated carefully, with particular attention to safety standards and environmental impact assessments. Current regulatory frameworks in most regions lack specific provisions for novel sorbent technologies, necessitating proactive engagement with regulatory bodies during development phases.
The economic transition pathway indicates that interface-engineered sorbents will initially be most competitive in high-value applications where performance advantages justify premium costs. As manufacturing scales and techniques mature, broader industrial applications become economically viable, potentially reaching cost parity with conventional capture methods within 5-7 years of initial deployment.
For full industrial deployment, a phased approach is recommended. The initial phase should focus on small-scale implementations in non-critical industrial processes, allowing for operational optimization without disrupting essential production. This phase typically requires 2-3 years of testing and refinement before advancing to larger applications.
Mid-scale implementation (100-500 tons CO2/day) represents the critical proving ground where economic viability must be demonstrated alongside technical performance. Cost projections indicate that interface-engineered sorbents could achieve capture costs of $40-60 per ton CO2 at this scale, approaching economic feasibility for carbon pricing mechanisms in many regions.
Manufacturing scalability presents significant challenges that must be addressed. Current production methods for precisely engineered interfaces often involve batch processes unsuitable for industrial volumes. Continuous flow synthesis methods and roll-to-roll manufacturing techniques show promise for scaling production while maintaining interface quality. Recent innovations in automated quality control systems using machine learning algorithms can help ensure consistency across large production volumes.
Infrastructure requirements for widespread implementation include specialized handling equipment, regeneration systems, and monitoring technologies. Existing industrial facilities will require retrofit designs that integrate with current operations while minimizing disruption. New facilities can incorporate purpose-built systems optimized for interface-engineered sorbents.
Regulatory pathways must be navigated carefully, with particular attention to safety standards and environmental impact assessments. Current regulatory frameworks in most regions lack specific provisions for novel sorbent technologies, necessitating proactive engagement with regulatory bodies during development phases.
The economic transition pathway indicates that interface-engineered sorbents will initially be most competitive in high-value applications where performance advantages justify premium costs. As manufacturing scales and techniques mature, broader industrial applications become economically viable, potentially reaching cost parity with conventional capture methods within 5-7 years of initial deployment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!