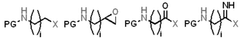How surface area and porosity influence Solid sorbents for CO2 capture thermal and mechanical stability
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Capture Sorbent Evolution and Objectives
Carbon dioxide capture technology has evolved significantly over the past decades, driven by the urgent need to mitigate climate change impacts. Initially, CO2 capture focused primarily on liquid sorbents, particularly amine-based solutions, which dominated industrial applications since the 1930s. However, these systems faced substantial challenges including high regeneration energy requirements, equipment corrosion, and degradation issues during thermal cycling.
The emergence of solid sorbents represents a pivotal shift in CO2 capture technology, offering potentially lower energy penalties and improved stability. Early solid sorbents included basic metal oxides and hydroxides, which gradually gave way to more sophisticated materials including zeolites, activated carbons, and metal-organic frameworks (MOFs). Each generation of materials has demonstrated incremental improvements in capture capacity, selectivity, and operational stability.
Surface area and porosity have emerged as critical parameters governing solid sorbent performance. Historical data indicates that materials with higher specific surface areas generally exhibit enhanced CO2 adsorption capacities due to increased available binding sites. The evolution of synthesis techniques has enabled precise control over these properties, with modern materials routinely achieving surface areas exceeding 1000 m²/g compared to early materials with only 200-300 m²/g.
Porosity characteristics have similarly undergone significant refinement. Early research focused primarily on total pore volume, while contemporary approaches emphasize pore size distribution and architecture. The recognition that micropores (< 2 nm) contribute significantly to CO2 capture at low partial pressures, while mesopores (2-50 nm) facilitate mass transport kinetics, has guided material design strategies toward hierarchical pore structures.
The primary technical objective in this field is developing solid sorbents that maintain structural integrity and adsorption performance over thousands of thermal and pressure swing cycles. Current targets include achieving CO2 working capacities exceeding 3 mmol/g under practical conditions, with regeneration temperatures below 100°C to minimize energy penalties. Additionally, materials must demonstrate mechanical stability under process conditions including rapid temperature changes, pressure fluctuations, and exposure to contaminants.
Future development aims to establish quantitative structure-property relationships between surface area, pore characteristics, and thermal/mechanical stability. This includes understanding how pore collapse mechanisms relate to specific surface area distributions and identifying optimal pore architectures that balance high adsorption capacity with structural resilience. The ultimate goal remains creating economically viable solid sorbents that can reduce CO2 capture costs below $40 per ton while maintaining performance over thousands of operating cycles.
The emergence of solid sorbents represents a pivotal shift in CO2 capture technology, offering potentially lower energy penalties and improved stability. Early solid sorbents included basic metal oxides and hydroxides, which gradually gave way to more sophisticated materials including zeolites, activated carbons, and metal-organic frameworks (MOFs). Each generation of materials has demonstrated incremental improvements in capture capacity, selectivity, and operational stability.
Surface area and porosity have emerged as critical parameters governing solid sorbent performance. Historical data indicates that materials with higher specific surface areas generally exhibit enhanced CO2 adsorption capacities due to increased available binding sites. The evolution of synthesis techniques has enabled precise control over these properties, with modern materials routinely achieving surface areas exceeding 1000 m²/g compared to early materials with only 200-300 m²/g.
Porosity characteristics have similarly undergone significant refinement. Early research focused primarily on total pore volume, while contemporary approaches emphasize pore size distribution and architecture. The recognition that micropores (< 2 nm) contribute significantly to CO2 capture at low partial pressures, while mesopores (2-50 nm) facilitate mass transport kinetics, has guided material design strategies toward hierarchical pore structures.
The primary technical objective in this field is developing solid sorbents that maintain structural integrity and adsorption performance over thousands of thermal and pressure swing cycles. Current targets include achieving CO2 working capacities exceeding 3 mmol/g under practical conditions, with regeneration temperatures below 100°C to minimize energy penalties. Additionally, materials must demonstrate mechanical stability under process conditions including rapid temperature changes, pressure fluctuations, and exposure to contaminants.
Future development aims to establish quantitative structure-property relationships between surface area, pore characteristics, and thermal/mechanical stability. This includes understanding how pore collapse mechanisms relate to specific surface area distributions and identifying optimal pore architectures that balance high adsorption capacity with structural resilience. The ultimate goal remains creating economically viable solid sorbents that can reduce CO2 capture costs below $40 per ton while maintaining performance over thousands of operating cycles.
Market Analysis for Carbon Capture Technologies
The global carbon capture and storage (CCS) market is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. As of 2023, the market was valued at approximately $7.5 billion and is projected to reach $15.3 billion by 2030, representing a compound annual growth rate of 10.7%. This growth trajectory is supported by substantial government investments, with the US Inflation Reduction Act allocating $369 billion toward climate change initiatives, including carbon capture technologies.
Solid sorbents represent a rapidly expanding segment within the carbon capture technology landscape, currently accounting for about 18% of the total market share. This segment is expected to grow at a faster rate than traditional solvent-based approaches due to the inherent advantages of solid sorbents in terms of energy efficiency and operational costs. Industries with high CO2 emissions, such as power generation, cement production, and steel manufacturing, are the primary adopters of these technologies.
Regional analysis indicates that North America currently leads the carbon capture market with approximately 40% market share, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is expected to demonstrate the highest growth rate over the next decade due to rapid industrialization coupled with stringent emission regulations being implemented in countries like China and India.
The competitive landscape features both established industrial gas companies and specialized technology providers. Major players include Honeywell UOP, Air Liquide, Climeworks, Carbon Engineering, and Global Thermostat. These companies are increasingly focusing on developing advanced solid sorbents with optimized surface area and porosity characteristics to enhance thermal and mechanical stability – critical factors for commercial viability.
Customer demand is shifting toward solutions that offer lower regeneration energy requirements and longer operational lifetimes. Market research indicates that end-users are willing to pay a premium of up to 15% for sorbent technologies that demonstrate superior thermal cycling stability, which directly translates to reduced operational costs over the system lifetime.
The market is also witnessing a trend toward modular and scalable carbon capture systems, particularly for distributed emission sources. This trend favors solid sorbent technologies that can be more easily scaled down compared to traditional liquid solvent systems. Venture capital investment in startups developing novel solid sorbent materials has reached $850 million in 2023, a 35% increase from the previous year, indicating strong market confidence in this technological approach.
Solid sorbents represent a rapidly expanding segment within the carbon capture technology landscape, currently accounting for about 18% of the total market share. This segment is expected to grow at a faster rate than traditional solvent-based approaches due to the inherent advantages of solid sorbents in terms of energy efficiency and operational costs. Industries with high CO2 emissions, such as power generation, cement production, and steel manufacturing, are the primary adopters of these technologies.
Regional analysis indicates that North America currently leads the carbon capture market with approximately 40% market share, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is expected to demonstrate the highest growth rate over the next decade due to rapid industrialization coupled with stringent emission regulations being implemented in countries like China and India.
The competitive landscape features both established industrial gas companies and specialized technology providers. Major players include Honeywell UOP, Air Liquide, Climeworks, Carbon Engineering, and Global Thermostat. These companies are increasingly focusing on developing advanced solid sorbents with optimized surface area and porosity characteristics to enhance thermal and mechanical stability – critical factors for commercial viability.
Customer demand is shifting toward solutions that offer lower regeneration energy requirements and longer operational lifetimes. Market research indicates that end-users are willing to pay a premium of up to 15% for sorbent technologies that demonstrate superior thermal cycling stability, which directly translates to reduced operational costs over the system lifetime.
The market is also witnessing a trend toward modular and scalable carbon capture systems, particularly for distributed emission sources. This trend favors solid sorbent technologies that can be more easily scaled down compared to traditional liquid solvent systems. Venture capital investment in startups developing novel solid sorbent materials has reached $850 million in 2023, a 35% increase from the previous year, indicating strong market confidence in this technological approach.
Surface-Porosity Challenges in CO2 Sorbents
The surface area and porosity of solid sorbents represent critical parameters that fundamentally determine their CO2 capture performance. These structural characteristics directly influence the number of active sites available for CO2 adsorption, thereby affecting capture capacity. However, the relationship between these parameters and the thermal and mechanical stability of sorbents presents significant challenges that require comprehensive investigation.
High surface area materials, while offering superior adsorption capacity, often exhibit compromised structural integrity under thermal cycling conditions. This trade-off manifests particularly in microporous materials where the extensive internal surface area creates numerous potential failure points during temperature fluctuations. The thermal expansion coefficient mismatch between different structural components in high-surface-area materials can induce internal stresses, leading to microcrack formation and eventual structural collapse after multiple adsorption-desorption cycles.
Porosity configuration presents another dimension of complexity. Materials with predominantly micropores (<2 nm) typically demonstrate higher CO2 selectivity but may suffer from diffusion limitations and pore blockage during extended operation. Conversely, materials with larger mesopores (2-50 nm) offer improved mass transfer kinetics but potentially reduced adsorption capacity per unit volume. The hierarchical arrangement of pores significantly impacts thermal stability, as heat transfer pathways through the material structure determine temperature gradient distribution during cycling.
The mechanical stability challenges are equally significant. Highly porous structures often exhibit reduced mechanical strength due to decreased material density and increased void fraction. During pressure swing operations, these materials experience substantial mechanical stress from gas pressure fluctuations. The pore wall thickness represents a critical parameter - thinner walls maximize surface area but compromise mechanical resilience. This creates an engineering dilemma where optimizing for adsorption capacity may inherently reduce operational durability.
Surface chemistry further complicates this relationship. Surface functionalization methods that enhance CO2 affinity often modify pore structures and can introduce thermal instability. Amine-grafted silicas, for example, demonstrate excellent CO2 capture performance but may suffer from amine leaching and degradation at elevated temperatures, compromising both surface area and mechanical integrity over time.
Recent research indicates that controlled hierarchical porosity - strategically combining micro, meso, and macropores - may offer a pathway to balance these competing requirements. Materials with "scaffold" structures that provide mechanical support while maintaining high internal surface area show promising stability characteristics. Additionally, composite approaches that integrate mechanically robust components with high-surface-area materials represent an emerging direction for addressing these fundamental challenges.
High surface area materials, while offering superior adsorption capacity, often exhibit compromised structural integrity under thermal cycling conditions. This trade-off manifests particularly in microporous materials where the extensive internal surface area creates numerous potential failure points during temperature fluctuations. The thermal expansion coefficient mismatch between different structural components in high-surface-area materials can induce internal stresses, leading to microcrack formation and eventual structural collapse after multiple adsorption-desorption cycles.
Porosity configuration presents another dimension of complexity. Materials with predominantly micropores (<2 nm) typically demonstrate higher CO2 selectivity but may suffer from diffusion limitations and pore blockage during extended operation. Conversely, materials with larger mesopores (2-50 nm) offer improved mass transfer kinetics but potentially reduced adsorption capacity per unit volume. The hierarchical arrangement of pores significantly impacts thermal stability, as heat transfer pathways through the material structure determine temperature gradient distribution during cycling.
The mechanical stability challenges are equally significant. Highly porous structures often exhibit reduced mechanical strength due to decreased material density and increased void fraction. During pressure swing operations, these materials experience substantial mechanical stress from gas pressure fluctuations. The pore wall thickness represents a critical parameter - thinner walls maximize surface area but compromise mechanical resilience. This creates an engineering dilemma where optimizing for adsorption capacity may inherently reduce operational durability.
Surface chemistry further complicates this relationship. Surface functionalization methods that enhance CO2 affinity often modify pore structures and can introduce thermal instability. Amine-grafted silicas, for example, demonstrate excellent CO2 capture performance but may suffer from amine leaching and degradation at elevated temperatures, compromising both surface area and mechanical integrity over time.
Recent research indicates that controlled hierarchical porosity - strategically combining micro, meso, and macropores - may offer a pathway to balance these competing requirements. Materials with "scaffold" structures that provide mechanical support while maintaining high internal surface area show promising stability characteristics. Additionally, composite approaches that integrate mechanically robust components with high-surface-area materials represent an emerging direction for addressing these fundamental challenges.
Current Surface Engineering Approaches
01 Metal-organic frameworks (MOFs) for CO2 capture
Metal-organic frameworks (MOFs) are crystalline porous materials that show excellent CO2 adsorption capacity and selectivity. These materials can be engineered to have high thermal stability by incorporating strong metal-ligand bonds and rigid frameworks. Their mechanical stability can be enhanced through structural modifications such as interpenetration or mixed-metal approaches. MOFs can maintain their structural integrity during multiple adsorption-desorption cycles, making them promising candidates for practical CO2 capture applications.- Metal-organic frameworks (MOFs) for CO2 capture: Metal-organic frameworks (MOFs) are crystalline porous materials that have shown excellent potential for CO2 capture due to their high surface area and tunable pore structures. These materials can be engineered to have enhanced thermal stability through the incorporation of strong metal-ligand bonds and rigid organic linkers. Their mechanical stability can be improved by using interpenetrated structures or incorporating flexible components that can absorb mechanical stress without structural collapse. MOFs can be designed with specific functional groups that selectively bind CO2 while maintaining structural integrity under repeated adsorption-desorption cycles.
- Amine-functionalized silica-based sorbents: Amine-functionalized silica materials represent a significant class of solid sorbents for CO2 capture. These materials combine the mechanical strength of silica supports with the CO2-philic nature of amine groups. The thermal stability of these sorbents can be enhanced by using sterically hindered amines or by grafting rather than impregnation methods. Mechanical stability is achieved through the rigid silica framework, which can withstand pressure fluctuations in industrial settings. Various approaches to anchor amine groups to silica surfaces have been developed to prevent amine leaching during temperature swing operations, thereby improving the long-term stability of these materials.
- Hydrotalcite-derived mixed metal oxides: Hydrotalcite-derived mixed metal oxides are promising CO2 sorbents that exhibit excellent thermal stability at high temperatures. These layered double hydroxide materials can maintain their CO2 capture capacity even after multiple high-temperature regeneration cycles. Their mechanical stability is enhanced by the strong ionic bonds between the metal cations and the hydroxide/carbonate anions in their structure. The composition can be tailored by varying the ratio of divalent to trivalent metals to optimize both CO2 adsorption capacity and stability. These materials are particularly suitable for pre-combustion CO2 capture applications where high temperatures are encountered.
- Carbon-based sorbents with enhanced stability: Carbon-based materials, including activated carbons, carbon molecular sieves, and graphene-derived structures, offer excellent platforms for CO2 capture due to their high surface area and pore volume. Their thermal stability can be enhanced through high-temperature treatment processes that remove unstable functional groups. Mechanical stability is improved by controlling the synthesis conditions to create robust carbon frameworks. Surface modification with nitrogen-containing groups or metal nanoparticles can increase both CO2 selectivity and the material's resistance to degradation. These carbon-based sorbents are advantageous due to their low cost, light weight, and resistance to moisture, making them suitable for various industrial applications.
- Zeolites and zeolite-like materials with improved stability: Zeolites and zeolite-like materials are crystalline aluminosilicates with well-defined pore structures that can effectively capture CO2. Their inherent thermal stability can be further enhanced by ion exchange with specific cations or by creating hierarchical pore structures. The mechanical stability of these materials is improved through careful control of the synthesis conditions to minimize defects and optimize crystal size. Post-synthesis treatments, such as steam treatment or acid washing, can also strengthen the framework structure. These materials maintain their adsorption properties over numerous temperature and pressure swing cycles, making them suitable for long-term industrial CO2 capture applications.
02 Amine-functionalized solid sorbents
Amine-functionalized materials represent a significant class of solid sorbents for CO2 capture. These materials combine high CO2 selectivity with good adsorption capacity through chemical bonding. The thermal stability of these sorbents can be improved by selecting appropriate amine types and optimizing the loading amount. Mechanical stability is enhanced by using robust support materials such as silica, alumina, or polymeric substrates. Various techniques including grafting, impregnation, and in-situ polymerization are employed to incorporate amine groups onto the support materials.Expand Specific Solutions03 Zeolite-based CO2 sorbents
Zeolites are aluminosilicate materials with well-defined pore structures that demonstrate good CO2 capture performance. Their inherent crystalline structure provides excellent thermal stability, often withstanding temperatures above 500°C. The mechanical stability of zeolites can be enhanced through various synthesis methods and post-treatment processes. Ion exchange with specific cations can improve both CO2 adsorption capacity and the material's resistance to degradation under cyclic operation conditions. These materials are particularly suitable for pressure swing adsorption systems due to their robust nature.Expand Specific Solutions04 Carbon-based sorbents with enhanced stability
Carbon-based materials, including activated carbons, carbon molecular sieves, and graphene-derived sorbents, offer excellent thermal and mechanical stability for CO2 capture applications. These materials can be modified through various activation processes, chemical treatments, and doping with heteroatoms to enhance their CO2 adsorption properties. The inherent thermal stability of carbon materials allows them to operate effectively at elevated temperatures. Their mechanical robustness makes them suitable for fixed-bed adsorption systems where pressure fluctuations occur. Surface functionalization can be tailored to improve selectivity while maintaining the structural integrity of the carbon framework.Expand Specific Solutions05 Composite and hybrid sorbents for improved stability
Composite and hybrid sorbents combine different materials to achieve enhanced thermal and mechanical stability for CO2 capture. These materials often integrate the advantages of various components, such as the high adsorption capacity of amines with the structural stability of inorganic supports. Polymer-inorganic composites, mixed matrix materials, and core-shell structures are common approaches to developing these advanced sorbents. The synergistic effects between components can lead to improved resistance to thermal degradation and mechanical stress during cycling operations. Various synthesis methods including sol-gel processing, hydrothermal treatment, and template-assisted growth are employed to create these complex structures with optimized properties.Expand Specific Solutions
Leading Organizations in CO2 Capture Materials
The solid sorbent CO2 capture market is currently in a growth phase, with increasing global focus on carbon reduction technologies. Market size is expanding rapidly, projected to reach significant scale as carbon capture becomes essential for climate goals. Technologically, the field shows varying maturity levels across different sorbent types. Leading players like ExxonMobil and Climeworks are advancing commercial-scale direct air capture solutions, while research institutions such as Dalian Institute of Chemical Physics and Georgia Tech are developing fundamental understanding of surface area and porosity effects on thermal and mechanical stability. Academic-industrial partnerships between universities (Ohio State, Rice, USC) and corporations (Corning, Halliburton) are accelerating innovation in sorbent design, focusing on optimizing pore structure for enhanced CO2 capture performance while maintaining structural integrity under operational conditions.
ExxonMobil Technology & Engineering Co.
Technical Solution: ExxonMobil has developed advanced metal-organic frameworks (MOFs) with optimized surface area (>2000 m²/g) and controlled porosity for CO2 capture. Their proprietary technology focuses on hierarchical pore structures that combine micropores (<2 nm) for selective CO2 adsorption with mesopores (2-50 nm) for efficient gas transport. This design minimizes diffusion limitations while maximizing adsorption capacity. ExxonMobil's approach includes surface functionalization with amine groups to enhance CO2 selectivity and binding strength. Their materials undergo extensive thermal cycling tests (up to 1000 cycles at temperatures ranging from 40-120°C) to ensure stability in industrial conditions. The company has also developed proprietary binder systems that maintain mechanical integrity while minimizing pore blockage, resulting in pelletized sorbents with crush strengths exceeding 2 N/mm while retaining >90% of powder-form CO2 capacity.
Strengths: Superior thermal stability during multiple adsorption-desorption cycles; excellent mechanical properties suitable for industrial-scale fixed bed operations; high CO2 selectivity even in humid conditions. Weaknesses: Higher production costs compared to conventional sorbents; potential for performance degradation in the presence of SOx and NOx contaminants; energy requirements for regeneration remain relatively high.
Climeworks AG
Technical Solution: Climeworks has pioneered a direct air capture (DAC) technology utilizing specialized solid sorbents with optimized surface area and porosity characteristics. Their proprietary sorbent materials feature a cellulose-based support structure with amine-functionalized surfaces, providing high CO2 selectivity from ambient air. The sorbent design incorporates a hierarchical pore network with macropores (>50 nm) for air flow and micropores (<2 nm) for CO2 binding sites, achieving surface areas of approximately 300-500 m²/g. Climeworks' innovation lies in their modular "collector" units that house these sorbents in a configuration that maximizes air contact while protecting the material from mechanical stress. Their regeneration process operates at relatively low temperatures (80-100°C) compared to other DAC technologies, which helps preserve the thermal stability of the sorbent over thousands of cycles. The company has demonstrated sorbent durability exceeding 3 years of continuous operation in field conditions, with minimal degradation in capture capacity (less than 10% reduction annually).
Strengths: Modular design allows for scalable deployment; relatively low regeneration temperature reduces energy requirements; demonstrated long-term stability in real-world conditions. Weaknesses: Lower CO2 capacity per unit mass compared to some competing materials; requires significant air flow which increases fan energy consumption; performance is humidity-dependent, requiring moisture management strategies.
Critical Patents in Sorbent Stability Enhancement
Sorbent material for co2 capture, uses thereof and methods for making same
PatentWO2025124872A1
Innovation
- A sorbent material composed of a mixture of 75-98 wt.% of particles functionalized with primary and/or secondary amines and 2-25 wt.% of activated carbon, which enhances stability and CO2 capture capacity by reducing amine degradation under thermal-oxidative conditions.
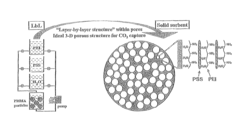
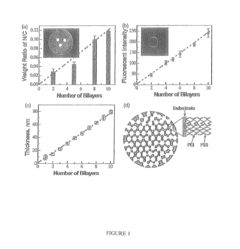
Layered solid sorbents for carbon dioxide capture
PatentActiveUS8889589B2
Innovation
- Development of nano-layered solid sorbents using electrostatic layer-by-layer nanoassembly, where positively charged polyethylenimine and negatively charged polystyrene sulfonate layers are alternately deposited on a porous substrate, enhancing CO2 capture and transport kinetics.
Environmental Policy Impact on Carbon Capture
Environmental policies worldwide have increasingly recognized carbon capture technologies as critical tools in the fight against climate change. The regulatory landscape has evolved significantly, with many nations implementing carbon pricing mechanisms, emissions trading systems, and direct subsidies to incentivize the development and deployment of carbon capture solutions. These policy frameworks directly influence research priorities and investment in solid sorbent technologies, particularly those focused on optimizing surface area and porosity for enhanced thermal and mechanical stability.
The Paris Agreement has been instrumental in accelerating policy support for carbon capture technologies. Countries' Nationally Determined Contributions (NDCs) frequently reference carbon capture as a pathway to meeting emissions reduction targets, creating a favorable environment for research funding. In the United States, the 45Q tax credit provides up to $50 per metric ton of CO2 permanently sequestered, significantly improving the economic viability of projects utilizing advanced sorbent materials.
European Union policies have similarly evolved, with the Innovation Fund allocating substantial resources to demonstration projects featuring novel solid sorbents. These policies specifically encourage materials with optimized surface characteristics that can withstand the thermal cycling and mechanical stresses inherent in industrial carbon capture applications. The EU Emissions Trading System (ETS) further enhances the economic case for deploying such technologies by putting a price on carbon emissions.
In Asia, China's 14th Five-Year Plan explicitly mentions carbon capture technologies, with research institutes receiving directed funding for developing thermally stable sorbent materials. Japan's Green Innovation Fund similarly prioritizes materials science approaches to carbon capture, with specific emphasis on durability under industrial conditions.
Policy frameworks increasingly incorporate technical specifications that directly influence sorbent development. Regulatory requirements for operational longevity, energy efficiency, and capture rates drive research toward materials with optimized surface area and porosity distributions that can maintain structural integrity under thermal and mechanical stress.
International standards bodies are beginning to develop benchmarks for sorbent performance, creating a regulatory environment that rewards innovations in material stability. These standards typically emphasize cyclic capacity retention under thermal stress and resistance to mechanical degradation—properties directly linked to surface area and pore structure engineering.
The interplay between environmental policy and technical research creates feedback loops that accelerate innovation. As policies become more stringent regarding emissions, the demand for more efficient and durable sorbents increases, directing research funding toward fundamental studies of how surface characteristics influence long-term stability under industrial conditions.
The Paris Agreement has been instrumental in accelerating policy support for carbon capture technologies. Countries' Nationally Determined Contributions (NDCs) frequently reference carbon capture as a pathway to meeting emissions reduction targets, creating a favorable environment for research funding. In the United States, the 45Q tax credit provides up to $50 per metric ton of CO2 permanently sequestered, significantly improving the economic viability of projects utilizing advanced sorbent materials.
European Union policies have similarly evolved, with the Innovation Fund allocating substantial resources to demonstration projects featuring novel solid sorbents. These policies specifically encourage materials with optimized surface characteristics that can withstand the thermal cycling and mechanical stresses inherent in industrial carbon capture applications. The EU Emissions Trading System (ETS) further enhances the economic case for deploying such technologies by putting a price on carbon emissions.
In Asia, China's 14th Five-Year Plan explicitly mentions carbon capture technologies, with research institutes receiving directed funding for developing thermally stable sorbent materials. Japan's Green Innovation Fund similarly prioritizes materials science approaches to carbon capture, with specific emphasis on durability under industrial conditions.
Policy frameworks increasingly incorporate technical specifications that directly influence sorbent development. Regulatory requirements for operational longevity, energy efficiency, and capture rates drive research toward materials with optimized surface area and porosity distributions that can maintain structural integrity under thermal and mechanical stress.
International standards bodies are beginning to develop benchmarks for sorbent performance, creating a regulatory environment that rewards innovations in material stability. These standards typically emphasize cyclic capacity retention under thermal stress and resistance to mechanical degradation—properties directly linked to surface area and pore structure engineering.
The interplay between environmental policy and technical research creates feedback loops that accelerate innovation. As policies become more stringent regarding emissions, the demand for more efficient and durable sorbents increases, directing research funding toward fundamental studies of how surface characteristics influence long-term stability under industrial conditions.
Scalability and Industrial Implementation
The scalability of solid sorbent technologies for CO2 capture represents a critical bridge between laboratory success and industrial implementation. Current pilot-scale demonstrations have shown promising results for materials with optimized surface area and porosity characteristics, but significant engineering challenges remain for full industrial deployment. The transition from gram-scale laboratory testing to ton-scale industrial operations requires careful consideration of how surface properties affect long-term stability under real-world conditions.
Manufacturing processes for high-surface-area sorbents must be adapted for industrial scale while maintaining the precise porosity characteristics that enable effective CO2 capture. Techniques such as spray drying, hydrothermal synthesis, and template-assisted fabrication have demonstrated potential for scaling, though each presents unique challenges in maintaining uniform pore distribution and surface area consistency across large production volumes. Materials with hierarchical pore structures often show superior performance but present greater manufacturing complexity.
Economic viability remains closely tied to the thermal and mechanical stability of sorbents during repeated cycling. Materials requiring frequent replacement due to degradation of surface properties significantly impact operational costs. Analysis indicates that sorbents maintaining at least 80% of their initial capacity after 1,000 cycles could achieve economically viable implementation in power plants and industrial facilities. Surface area degradation rates below 0.5% per cycle appear to be the threshold for commercial feasibility.
Integration with existing industrial infrastructure presents another dimension of scalability challenges. Retrofitting existing facilities requires sorbent systems with flexible form factors that can accommodate space constraints while maintaining sufficient surface area for effective capture. Modular designs utilizing structured sorbents with engineered porosity have shown promise in pilot implementations, allowing for phased deployment and capacity expansion.
Regulatory frameworks and carbon pricing mechanisms increasingly influence implementation timelines. Regions with established carbon markets show accelerated adoption of advanced sorbent technologies, with particular emphasis on materials demonstrating superior thermal stability for energy-efficient regeneration. The correlation between surface area preservation during cycling and regeneration energy requirements has emerged as a key performance indicator for industrial implementation potential.
Manufacturing processes for high-surface-area sorbents must be adapted for industrial scale while maintaining the precise porosity characteristics that enable effective CO2 capture. Techniques such as spray drying, hydrothermal synthesis, and template-assisted fabrication have demonstrated potential for scaling, though each presents unique challenges in maintaining uniform pore distribution and surface area consistency across large production volumes. Materials with hierarchical pore structures often show superior performance but present greater manufacturing complexity.
Economic viability remains closely tied to the thermal and mechanical stability of sorbents during repeated cycling. Materials requiring frequent replacement due to degradation of surface properties significantly impact operational costs. Analysis indicates that sorbents maintaining at least 80% of their initial capacity after 1,000 cycles could achieve economically viable implementation in power plants and industrial facilities. Surface area degradation rates below 0.5% per cycle appear to be the threshold for commercial feasibility.
Integration with existing industrial infrastructure presents another dimension of scalability challenges. Retrofitting existing facilities requires sorbent systems with flexible form factors that can accommodate space constraints while maintaining sufficient surface area for effective capture. Modular designs utilizing structured sorbents with engineered porosity have shown promise in pilot implementations, allowing for phased deployment and capacity expansion.
Regulatory frameworks and carbon pricing mechanisms increasingly influence implementation timelines. Regions with established carbon markets show accelerated adoption of advanced sorbent technologies, with particular emphasis on materials demonstrating superior thermal stability for energy-efficient regeneration. The correlation between surface area preservation during cycling and regeneration energy requirements has emerged as a key performance indicator for industrial implementation potential.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!