What technical mechanisms govern Solid sorbents for CO2 capture kinetics and thermodynamics
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Capture Sorbent Evolution and Objectives
Carbon dioxide capture technology has evolved significantly over the past decades, driven by the urgent need to mitigate climate change impacts. The journey began with conventional absorption methods using liquid amines in the 1930s, which, while effective, presented challenges including high energy requirements for regeneration and equipment corrosion. The 1990s marked a pivotal shift toward solid sorbents as researchers recognized their potential advantages in energy efficiency and operational stability.
Solid sorbents for CO2 capture have progressed through several generations. First-generation materials included activated carbons and zeolites, which offered moderate CO2 selectivity but suffered from moisture sensitivity. The early 2000s witnessed the emergence of second-generation sorbents, notably metal-organic frameworks (MOFs) and amine-functionalized silica, which demonstrated significantly improved CO2 capacity and selectivity under various conditions.
The technological evolution has been guided by fundamental thermodynamic and kinetic principles. Thermodynamically, the ideal sorbent must balance strong CO2 affinity during adsorption with reasonable energy requirements for desorption. This delicate equilibrium has driven research toward materials with tunable binding energies and innovative regeneration methods. Kinetically, researchers have focused on enhancing mass transfer rates through optimized pore structures and surface chemistry modifications.
Current research objectives center on developing third-generation sorbents that address multiple performance metrics simultaneously. These include achieving CO2 capture capacities exceeding 3 mmol/g under realistic conditions, maintaining stability over thousands of adsorption-desorption cycles, and reducing regeneration energy to below 2 GJ/ton CO2. Additionally, researchers aim to design materials that maintain performance under the challenging conditions of real flue gases, including moisture, SOx, and NOx contaminants.
The field is now moving toward rational design approaches, leveraging computational modeling and high-throughput screening to identify promising material candidates before synthesis. This represents a significant shift from earlier trial-and-error methodologies. Machine learning algorithms are increasingly employed to predict material properties and optimize formulations, accelerating the discovery process.
Looking forward, the ultimate objective is to develop economically viable solid sorbents that can be deployed at industrial scale, reducing the cost of CO2 capture to below $30/ton while maintaining environmental sustainability throughout their lifecycle. This includes considerations of raw material availability, manufacturing scalability, and end-of-life management.
Solid sorbents for CO2 capture have progressed through several generations. First-generation materials included activated carbons and zeolites, which offered moderate CO2 selectivity but suffered from moisture sensitivity. The early 2000s witnessed the emergence of second-generation sorbents, notably metal-organic frameworks (MOFs) and amine-functionalized silica, which demonstrated significantly improved CO2 capacity and selectivity under various conditions.
The technological evolution has been guided by fundamental thermodynamic and kinetic principles. Thermodynamically, the ideal sorbent must balance strong CO2 affinity during adsorption with reasonable energy requirements for desorption. This delicate equilibrium has driven research toward materials with tunable binding energies and innovative regeneration methods. Kinetically, researchers have focused on enhancing mass transfer rates through optimized pore structures and surface chemistry modifications.
Current research objectives center on developing third-generation sorbents that address multiple performance metrics simultaneously. These include achieving CO2 capture capacities exceeding 3 mmol/g under realistic conditions, maintaining stability over thousands of adsorption-desorption cycles, and reducing regeneration energy to below 2 GJ/ton CO2. Additionally, researchers aim to design materials that maintain performance under the challenging conditions of real flue gases, including moisture, SOx, and NOx contaminants.
The field is now moving toward rational design approaches, leveraging computational modeling and high-throughput screening to identify promising material candidates before synthesis. This represents a significant shift from earlier trial-and-error methodologies. Machine learning algorithms are increasingly employed to predict material properties and optimize formulations, accelerating the discovery process.
Looking forward, the ultimate objective is to develop economically viable solid sorbents that can be deployed at industrial scale, reducing the cost of CO2 capture to below $30/ton while maintaining environmental sustainability throughout their lifecycle. This includes considerations of raw material availability, manufacturing scalability, and end-of-life management.
Market Analysis for Carbon Capture Technologies
The global carbon capture and storage (CCS) market is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. As of 2023, the market was valued at approximately $7.5 billion, with projections indicating growth to reach $15-20 billion by 2030, representing a compound annual growth rate of 12-15%. This growth trajectory is supported by substantial government investments, with the US Inflation Reduction Act allocating $369 billion toward climate change initiatives, including carbon capture technologies.
Solid sorbent-based carbon capture technologies are gaining particular traction within this expanding market. These technologies currently represent about 25% of the carbon capture technology market share, competing with solvent-based approaches (55%) and membrane technologies (15%). The solid sorbent segment is expected to grow at a faster rate than the overall market, potentially reaching 35% market share by 2028 due to advantages in energy efficiency and operational costs.
Key market drivers for solid sorbent technologies include their lower regeneration energy requirements compared to traditional amine solvents, reduced corrosion issues, and greater stability under various operating conditions. These advantages translate to potential cost reductions of 30-40% in the carbon capture process, a critical factor for widespread commercial adoption.
Regional analysis shows North America leading the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). China and India are emerging as particularly fast-growing markets, with annual growth rates exceeding 18%, driven by their dual needs for continued industrial development and emissions reduction.
Industry segmentation reveals that power generation represents the largest application sector (45%), followed by cement production (20%), steel manufacturing (15%), and chemical processing (12%). The remaining market share is distributed across various industrial applications. This distribution reflects the carbon-intensive nature of these industries and regulatory pressures they face.
Market challenges include high initial capital costs, technological scalability issues, and uncertain regulatory frameworks in some regions. Despite these challenges, the solid sorbent carbon capture market benefits from increasing carbon pricing mechanisms, with carbon prices in the EU Emissions Trading System reaching record levels of €80-90 per tonne, creating strong economic incentives for adoption.
Solid sorbent-based carbon capture technologies are gaining particular traction within this expanding market. These technologies currently represent about 25% of the carbon capture technology market share, competing with solvent-based approaches (55%) and membrane technologies (15%). The solid sorbent segment is expected to grow at a faster rate than the overall market, potentially reaching 35% market share by 2028 due to advantages in energy efficiency and operational costs.
Key market drivers for solid sorbent technologies include their lower regeneration energy requirements compared to traditional amine solvents, reduced corrosion issues, and greater stability under various operating conditions. These advantages translate to potential cost reductions of 30-40% in the carbon capture process, a critical factor for widespread commercial adoption.
Regional analysis shows North America leading the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). China and India are emerging as particularly fast-growing markets, with annual growth rates exceeding 18%, driven by their dual needs for continued industrial development and emissions reduction.
Industry segmentation reveals that power generation represents the largest application sector (45%), followed by cement production (20%), steel manufacturing (15%), and chemical processing (12%). The remaining market share is distributed across various industrial applications. This distribution reflects the carbon-intensive nature of these industries and regulatory pressures they face.
Market challenges include high initial capital costs, technological scalability issues, and uncertain regulatory frameworks in some regions. Despite these challenges, the solid sorbent carbon capture market benefits from increasing carbon pricing mechanisms, with carbon prices in the EU Emissions Trading System reaching record levels of €80-90 per tonne, creating strong economic incentives for adoption.
Solid Sorbents: Current Status and Technical Barriers
Solid sorbents have emerged as promising alternatives to conventional liquid amine-based systems for CO2 capture, offering potential advantages in energy efficiency, environmental impact, and operational flexibility. Currently, a diverse range of solid sorbents is being investigated globally, including metal-organic frameworks (MOFs), zeolites, activated carbons, amine-functionalized silicas, and hydrotalcites. Each category exhibits distinct adsorption mechanisms, with physisorption dominating in zeolites and MOFs, while chemisorption characterizes amine-functionalized materials.
Despite significant research progress, several technical barriers impede the widespread commercial deployment of solid sorbents. The primary challenge remains the trade-off between adsorption capacity and selectivity versus regeneration energy requirements. Materials demonstrating high CO2 uptake often require substantial energy input for regeneration, compromising the overall energy efficiency of the capture process. This fundamental challenge stems from the thermodynamic relationship between binding strength and regeneration energy.
Stability issues present another significant barrier. Many promising sorbents exhibit performance degradation under realistic operating conditions, including exposure to moisture, SOx, NOx, and other flue gas components. Hydrothermal stability is particularly critical, as water vapor is ubiquitous in most industrial gas streams. The molecular mechanisms of degradation often involve irreversible chemical reactions or structural collapse that permanently diminish capture capacity.
Heat management during adsorption-desorption cycles represents a substantial engineering challenge. The exothermic nature of CO2 adsorption generates heat that must be effectively managed to maintain optimal operating conditions. Conversely, the endothermic desorption process requires efficient heat transfer to minimize energy consumption. Current heat transfer limitations in packed bed configurations restrict the kinetics of both processes.
Mass transfer limitations constitute another significant barrier, particularly for structured sorbents like MOFs and zeolites. Diffusion constraints within microporous structures can severely limit the practical adsorption rates, reducing the effective capacity under realistic contact times. This kinetic limitation becomes especially pronounced at the industrial scale, where rapid cycling is essential for economic viability.
Scale-up challenges further complicate commercial implementation. Laboratory-scale synthesis methods often prove difficult to translate to industrial production volumes while maintaining consistent material properties and performance. Additionally, the mechanical properties of many advanced sorbents remain inadequate for industrial applications, with attrition and crushing during cycling leading to particle breakdown and pressure drop increases.
The economic viability of solid sorbent systems is further challenged by the current high production costs of advanced materials like MOFs and functionalized adsorbents, which must decrease substantially to compete with established technologies.
Despite significant research progress, several technical barriers impede the widespread commercial deployment of solid sorbents. The primary challenge remains the trade-off between adsorption capacity and selectivity versus regeneration energy requirements. Materials demonstrating high CO2 uptake often require substantial energy input for regeneration, compromising the overall energy efficiency of the capture process. This fundamental challenge stems from the thermodynamic relationship between binding strength and regeneration energy.
Stability issues present another significant barrier. Many promising sorbents exhibit performance degradation under realistic operating conditions, including exposure to moisture, SOx, NOx, and other flue gas components. Hydrothermal stability is particularly critical, as water vapor is ubiquitous in most industrial gas streams. The molecular mechanisms of degradation often involve irreversible chemical reactions or structural collapse that permanently diminish capture capacity.
Heat management during adsorption-desorption cycles represents a substantial engineering challenge. The exothermic nature of CO2 adsorption generates heat that must be effectively managed to maintain optimal operating conditions. Conversely, the endothermic desorption process requires efficient heat transfer to minimize energy consumption. Current heat transfer limitations in packed bed configurations restrict the kinetics of both processes.
Mass transfer limitations constitute another significant barrier, particularly for structured sorbents like MOFs and zeolites. Diffusion constraints within microporous structures can severely limit the practical adsorption rates, reducing the effective capacity under realistic contact times. This kinetic limitation becomes especially pronounced at the industrial scale, where rapid cycling is essential for economic viability.
Scale-up challenges further complicate commercial implementation. Laboratory-scale synthesis methods often prove difficult to translate to industrial production volumes while maintaining consistent material properties and performance. Additionally, the mechanical properties of many advanced sorbents remain inadequate for industrial applications, with attrition and crushing during cycling leading to particle breakdown and pressure drop increases.
The economic viability of solid sorbent systems is further challenged by the current high production costs of advanced materials like MOFs and functionalized adsorbents, which must decrease substantially to compete with established technologies.
Contemporary Solid Sorbent Mechanisms and Solutions
01 Metal-organic frameworks (MOFs) for CO2 capture
Metal-organic frameworks represent a promising class of solid sorbents for CO2 capture due to their high surface area, tunable pore size, and adjustable chemical functionality. These crystalline materials consist of metal ions or clusters coordinated to organic ligands, forming porous structures that can selectively adsorb CO2. The kinetics of CO2 adsorption in MOFs is generally rapid due to their open framework structure, while the thermodynamics can be tuned by modifying the metal centers or organic linkers to achieve optimal binding energies for efficient capture and release cycles.- Metal-organic frameworks (MOFs) for CO2 capture: Metal-organic frameworks represent a promising class of solid sorbents for CO2 capture due to their high surface area, tunable pore size, and customizable chemistry. These crystalline materials consist of metal ions or clusters coordinated to organic ligands, forming porous structures that can selectively adsorb CO2. The kinetics of CO2 adsorption in MOFs is typically rapid due to their open framework structure, while the thermodynamics can be tuned by modifying the metal centers or organic linkers to achieve optimal binding energies for efficient capture and release cycles.
- Amine-functionalized sorbents for enhanced CO2 capture: Amine-functionalized materials represent a significant advancement in solid sorbent technology for CO2 capture. These materials combine the physical structure of a support material (such as silica, alumina, or porous polymers) with chemically reactive amine groups that form carbamates with CO2. The thermodynamics of these materials show favorable enthalpy of adsorption for CO2, while the kinetics can vary based on the accessibility of the amine sites and the diffusion limitations within the porous structure. The regeneration energy requirements are typically lower than traditional liquid amine systems, making them attractive for practical applications.
- Zeolites and molecular sieves for selective CO2 adsorption: Zeolites and molecular sieves are crystalline aluminosilicates with well-defined pore structures that enable selective adsorption of CO2 based on molecular size and polarity. These materials exhibit favorable thermodynamics for CO2 capture at ambient conditions but may require careful management of water co-adsorption. The kinetics of adsorption in zeolites is governed by diffusion through the microporous channels, which can be optimized by controlling crystal size and introducing hierarchical pore structures. Their thermal and chemical stability makes them suitable for multiple adsorption-desorption cycles in practical carbon capture applications.
- Carbon-based sorbents for CO2 capture: Carbon-based materials, including activated carbons, carbon nanotubes, and graphene derivatives, offer excellent properties for CO2 capture applications. These materials provide high surface area, tunable pore structure, and surface chemistry that can be modified to enhance CO2 selectivity. The thermodynamics of CO2 adsorption on carbon materials typically shows moderate binding energies, facilitating both efficient capture and regeneration. The kinetics of adsorption is generally favorable due to the accessible pore structure and low diffusion barriers. Additionally, these materials benefit from low cost, high thermal stability, and resistance to degradation in the presence of contaminants.
- Temperature and pressure swing adsorption processes for CO2 capture: Temperature and pressure swing adsorption processes represent critical operational approaches for utilizing solid sorbents in CO2 capture systems. These processes leverage the thermodynamic relationships between temperature, pressure, and adsorption capacity to efficiently capture and release CO2. The kinetics of adsorption and desorption during these cycles significantly impacts overall system performance, determining cycle times and energy requirements. Process optimization involves balancing thermodynamic efficiency with kinetic limitations to maximize working capacity while minimizing energy consumption. Advanced process configurations, including vacuum swing adsorption and combined temperature-pressure swing approaches, can further enhance performance for specific applications.
02 Amine-functionalized sorbents for enhanced CO2 capture
Amine-functionalized materials represent a significant advancement in solid sorbent technology for CO2 capture. These materials combine the physical structure of a support material (such as silica, alumina, or porous polymers) with chemically reactive amine groups that form carbamates or carbonates with CO2. The incorporation of amine groups significantly enhances CO2 selectivity and adsorption capacity under various conditions. The kinetics of these materials is governed by the accessibility of the amine sites, while the thermodynamics is influenced by the type and density of amine groups, which determine binding strength and regeneration energy requirements.Expand Specific Solutions03 Zeolites and molecular sieves for selective CO2 adsorption
Zeolites and molecular sieves are crystalline aluminosilicate materials with well-defined pore structures that enable molecular sieving effects for selective CO2 capture. These materials offer advantages including high thermal stability, resistance to contaminants, and tunable adsorption properties through ion exchange or framework modification. The kinetics of CO2 adsorption in zeolites is influenced by diffusion limitations within the microporous structure, while the thermodynamics is determined by the framework composition, cation type, and Si/Al ratio, which affect the strength of interaction with CO2 molecules.Expand Specific Solutions04 Carbon-based sorbents for CO2 capture applications
Carbon-based materials, including activated carbons, carbon nanotubes, and graphene derivatives, offer versatile platforms for CO2 capture due to their high surface area, tunable porosity, and surface chemistry. These materials can be modified through physical or chemical activation processes to enhance their CO2 adsorption capacity and selectivity. The kinetics of adsorption on carbon materials is generally favorable due to their accessible pore structure, while the thermodynamics can be optimized through surface functionalization to introduce specific binding sites that increase the enthalpy of adsorption for CO2.Expand Specific Solutions05 Regeneration strategies and cyclic performance of CO2 sorbents
The practical application of solid sorbents for CO2 capture depends critically on their regeneration capabilities and performance stability over multiple adsorption-desorption cycles. Various regeneration strategies, including temperature swing adsorption (TSA), pressure swing adsorption (PSA), and vacuum swing adsorption (VSA), are employed based on the thermodynamic properties of the sorbent-CO2 interaction. The kinetics of desorption and the energy requirements for regeneration are key factors affecting the overall efficiency of the capture process. Advanced sorbent designs focus on optimizing the balance between strong CO2 binding for efficient capture and moderate binding energies that facilitate economical regeneration.Expand Specific Solutions
Leading Organizations in Solid Sorbent Development
The solid sorbents for CO2 capture market is in a growth phase, with increasing global focus on carbon reduction technologies. The market size is expanding rapidly, projected to reach significant scale as carbon capture becomes essential for climate goals. Technologically, the field is advancing from early-stage development toward commercial maturity. Leading players demonstrate varying levels of technical sophistication: academic institutions like Norwegian University of Science & Technology and Zhejiang University focus on fundamental kinetics research; energy corporations including China Petroleum & Chemical Corp., ExxonMobil, and Shell pursue integration with existing infrastructure; while specialized firms such as Carboncapture and TDA Research develop innovative sorbent technologies. Korean power companies (KEPCO and subsidiaries) are actively advancing practical implementation, indicating the technology's growing commercial viability across global markets.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has pioneered composite solid sorbents combining amine-functionalized mesoporous silica with hydrophobic components to enhance CO2 capture in humid conditions. Their technology achieves CO2 adsorption capacities of 3-4 mmol/g with fast kinetics (reaching 80% of equilibrium capacity within 5 minutes)[2]. Sinopec's approach focuses on the thermodynamic optimization through controlled distribution of basic sites, achieving adsorption enthalpies around 65-75 kJ/mol that balance capture efficiency with reasonable regeneration energy[4]. The company has developed a temperature-swing adsorption process operating between 40-120°C that maintains sorbent performance over 1000+ cycles with minimal degradation. Their industrial-scale demonstration units incorporate innovative fluidized bed reactors with integrated heat exchangers to optimize heat transfer during adsorption and regeneration phases, significantly improving energy efficiency compared to conventional amine scrubbing technologies[5]. Sinopec has also developed specialized manufacturing techniques to produce these sorbents at scale with consistent quality and performance metrics.
Strengths: High tolerance to moisture in flue gas streams; good mechanical stability in fluidized bed operations; scalable manufacturing process for commercial deployment. Weaknesses: Higher regeneration temperatures compared to some competing technologies; potential for amine leaching during extended operation; requires careful handling of spent sorbents for environmental compliance.
Carboncapture, Inc.
Technical Solution: Carboncapture has developed a modular direct air capture (DAC) system utilizing proprietary solid sorbents with tailored kinetic and thermodynamic properties specifically for low CO2 concentration environments. Their technology employs zeolite-based materials with specialized surface modifications that enhance CO2 adsorption rates even at atmospheric concentrations (400 ppm), achieving uptake rates 3-5 times faster than conventional materials[2]. The company's approach focuses on optimizing the thermodynamics through careful control of adsorption sites, achieving binding energies in the range of 40-50 kJ/mol that enable efficient capture while maintaining reasonable regeneration requirements[4]. Carboncapture's system operates on a temperature-vacuum swing adsorption cycle that minimizes energy consumption through innovative heat recovery mechanisms and renewable energy integration. Their modular design allows for distributed deployment with capacity ranging from tons to kilotons of CO2 per year, with demonstrated stability over thousands of cycles with minimal performance degradation[9]. The company has also developed advanced process control algorithms that continuously optimize operating parameters based on ambient conditions and energy availability.
Strengths: Highly effective for direct air capture applications with low CO2 concentrations; modular and scalable system architecture; ability to operate using renewable energy sources. Weaknesses: Higher energy requirements per ton of CO2 compared to point-source capture; more complex regeneration process involving both temperature and pressure swings; higher capital costs compared to traditional carbon capture technologies.
Key Adsorption Kinetics and Thermodynamic Principles
Solid sorbents for capturing co 2
PatentWO2023232666A1
Innovation
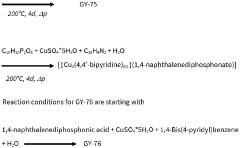
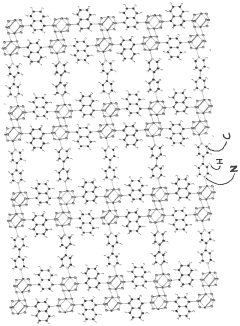
- Development of phosphonate and organoarsonate MOFs with specific molecular formulas, such as [{M2(4,4’-bipyridine)0.5}(l,4-naphthalenediphosphonate)] and [{M2(4,4’-bipyridine)0.5}(l,4-naphthalenediarsonate)], which maintain selectivity and stability under harsh conditions, including high humidity and temperatures up to 360°C, by creating a hydrophobic environment that favors CO2 physisorption over H2O.
Layered solid sorbents for carbon dioxide capture
PatentActiveUS8889589B2
Innovation
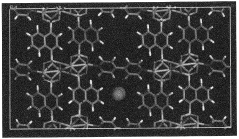
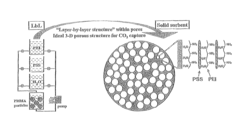
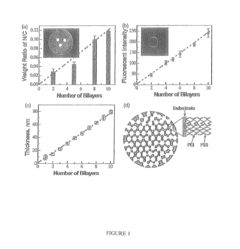
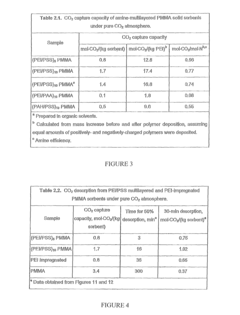
- Development of nano-layered solid sorbents using electrostatic layer-by-layer nanoassembly, where positively charged polyethylenimine and negatively charged polystyrene sulfonate layers are alternately deposited on a porous substrate, enhancing CO2 capture and transport kinetics.
Environmental Impact Assessment of Sorbent Technologies
The environmental impact of solid sorbent technologies for CO2 capture extends beyond their primary function of reducing greenhouse gas emissions. These technologies must be evaluated through a comprehensive life cycle assessment to understand their true environmental footprint.
When examining solid sorbents such as zeolites, metal-organic frameworks (MOFs), and amine-functionalized materials, their production processes involve significant energy consumption and resource extraction. The synthesis of advanced materials like MOFs typically requires organic solvents and metal precursors, which can generate hazardous waste streams if not properly managed.
During operational phases, solid sorbents demonstrate varying environmental profiles depending on their regeneration requirements. Temperature swing adsorption (TSA) systems may demand substantial thermal energy, while pressure swing adsorption (PSA) systems consume electricity for compression. The source of this energy significantly influences the net environmental benefit of the capture process. Renewable energy integration can substantially improve the environmental performance of these systems.
Water consumption represents another critical environmental consideration. Some sorbents, particularly those with hydrophilic properties, may compete with water molecules for adsorption sites, potentially requiring additional dehumidification processes that increase the system's complexity and environmental burden.
The durability and lifetime of sorbents directly impact their sustainability profile. Materials requiring frequent replacement generate additional waste and manufacturing impacts. Research indicates that most advanced sorbents maintain stability for hundreds to thousands of cycles, though performance degradation rates vary significantly based on operating conditions and contaminant exposure.
End-of-life management presents both challenges and opportunities. While some spent sorbents may contain heavy metals or other hazardous components requiring special disposal protocols, others offer recycling potential. Innovative approaches to sorbent regeneration and material recovery are emerging as important research directions to minimize waste generation.
Land use impacts must also be considered, particularly for large-scale deployment scenarios. The physical footprint of capture facilities using solid sorbents is generally smaller than alternative technologies like solvent-based systems, potentially reducing habitat disruption and land transformation impacts.
Comparative analyses with alternative carbon capture technologies reveal that solid sorbents often present lower toxicity profiles than amine solvents, which can degrade into potentially harmful compounds. However, the environmental advantages must be weighed against performance metrics like capture efficiency and energy requirements to determine the most sustainable approach for specific applications.
When examining solid sorbents such as zeolites, metal-organic frameworks (MOFs), and amine-functionalized materials, their production processes involve significant energy consumption and resource extraction. The synthesis of advanced materials like MOFs typically requires organic solvents and metal precursors, which can generate hazardous waste streams if not properly managed.
During operational phases, solid sorbents demonstrate varying environmental profiles depending on their regeneration requirements. Temperature swing adsorption (TSA) systems may demand substantial thermal energy, while pressure swing adsorption (PSA) systems consume electricity for compression. The source of this energy significantly influences the net environmental benefit of the capture process. Renewable energy integration can substantially improve the environmental performance of these systems.
Water consumption represents another critical environmental consideration. Some sorbents, particularly those with hydrophilic properties, may compete with water molecules for adsorption sites, potentially requiring additional dehumidification processes that increase the system's complexity and environmental burden.
The durability and lifetime of sorbents directly impact their sustainability profile. Materials requiring frequent replacement generate additional waste and manufacturing impacts. Research indicates that most advanced sorbents maintain stability for hundreds to thousands of cycles, though performance degradation rates vary significantly based on operating conditions and contaminant exposure.
End-of-life management presents both challenges and opportunities. While some spent sorbents may contain heavy metals or other hazardous components requiring special disposal protocols, others offer recycling potential. Innovative approaches to sorbent regeneration and material recovery are emerging as important research directions to minimize waste generation.
Land use impacts must also be considered, particularly for large-scale deployment scenarios. The physical footprint of capture facilities using solid sorbents is generally smaller than alternative technologies like solvent-based systems, potentially reducing habitat disruption and land transformation impacts.
Comparative analyses with alternative carbon capture technologies reveal that solid sorbents often present lower toxicity profiles than amine solvents, which can degrade into potentially harmful compounds. However, the environmental advantages must be weighed against performance metrics like capture efficiency and energy requirements to determine the most sustainable approach for specific applications.
Scalability and Industrial Implementation Challenges
The scaling of solid sorbent technologies for CO2 capture from laboratory to industrial scale presents significant engineering and economic challenges. Current pilot-scale implementations have demonstrated promising results, but the transition to full commercial deployment requires addressing several critical barriers. Material production represents a primary challenge, as manufacturing high-performance sorbents at industrial quantities demands consistent quality control and cost-effective synthesis methods that may not directly translate from laboratory procedures.
Equipment design and process integration pose additional complexities. Traditional equipment used in liquid absorption processes requires substantial modification to accommodate solid sorbents with different flow properties, heat transfer characteristics, and mechanical behaviors. Novel reactor designs such as fluidized beds, moving beds, and rotary systems are being developed specifically for solid sorbent applications, but these require extensive validation under realistic operating conditions.
Mechanical stability of sorbents during repeated cycling represents another significant hurdle. Industrial implementation requires materials that can withstand thousands of adsorption-desorption cycles without substantial degradation in capacity or kinetics. Attrition, sintering, and chemical poisoning from trace impurities in industrial gas streams can dramatically reduce operational lifetimes, necessitating robust material formulations and potentially protective engineering strategies.
Heat management systems present particular engineering challenges. The exothermic nature of CO2 adsorption and endothermic desorption processes requires efficient heat transfer mechanisms to maintain optimal operating conditions. This becomes increasingly difficult at industrial scales where heat transfer limitations can significantly impact system performance and energy efficiency.
Economic viability remains perhaps the most significant barrier to widespread implementation. Capital expenditures for first-generation solid sorbent systems are typically higher than conventional technologies, requiring clear operational advantages to justify investment. Additionally, the operational expenditures, including energy requirements for regeneration, sorbent replacement costs, and maintenance, must be competitive with established technologies to achieve market penetration.
Regulatory frameworks and carbon pricing mechanisms will significantly influence implementation timelines. Current uncertainty in many regions regarding long-term carbon policies complicates investment decisions for large-scale carbon capture infrastructure. Industries are hesitant to commit substantial resources without clear regulatory signals and economic incentives that ensure return on investment for decarbonization technologies.
Equipment design and process integration pose additional complexities. Traditional equipment used in liquid absorption processes requires substantial modification to accommodate solid sorbents with different flow properties, heat transfer characteristics, and mechanical behaviors. Novel reactor designs such as fluidized beds, moving beds, and rotary systems are being developed specifically for solid sorbent applications, but these require extensive validation under realistic operating conditions.
Mechanical stability of sorbents during repeated cycling represents another significant hurdle. Industrial implementation requires materials that can withstand thousands of adsorption-desorption cycles without substantial degradation in capacity or kinetics. Attrition, sintering, and chemical poisoning from trace impurities in industrial gas streams can dramatically reduce operational lifetimes, necessitating robust material formulations and potentially protective engineering strategies.
Heat management systems present particular engineering challenges. The exothermic nature of CO2 adsorption and endothermic desorption processes requires efficient heat transfer mechanisms to maintain optimal operating conditions. This becomes increasingly difficult at industrial scales where heat transfer limitations can significantly impact system performance and energy efficiency.
Economic viability remains perhaps the most significant barrier to widespread implementation. Capital expenditures for first-generation solid sorbent systems are typically higher than conventional technologies, requiring clear operational advantages to justify investment. Additionally, the operational expenditures, including energy requirements for regeneration, sorbent replacement costs, and maintenance, must be competitive with established technologies to achieve market penetration.
Regulatory frameworks and carbon pricing mechanisms will significantly influence implementation timelines. Current uncertainty in many regions regarding long-term carbon policies complicates investment decisions for large-scale carbon capture infrastructure. Industries are hesitant to commit substantial resources without clear regulatory signals and economic incentives that ensure return on investment for decarbonization technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!