What material parameters are essential for Solid sorbents for CO2 capture adsorption efficiency
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Capture Sorbent Evolution and Objectives
Carbon dioxide capture technology has evolved significantly over the past decades, transitioning from theoretical concepts to practical applications in response to growing environmental concerns. The development of solid sorbents for CO2 capture represents a critical advancement in this field, offering potentially more energy-efficient and environmentally friendly alternatives to traditional liquid amine scrubbing methods. The evolution began in the 1990s with basic research on porous materials, followed by significant breakthroughs in the 2000s with the development of metal-organic frameworks (MOFs) and advanced amine-functionalized materials.
The technological trajectory has been shaped by increasing global emphasis on carbon emission reduction, particularly following the Paris Agreement in 2015. This international commitment accelerated research into more efficient carbon capture technologies, with solid sorbents emerging as promising candidates due to their lower regeneration energy requirements compared to aqueous systems. The field has witnessed exponential growth in patent filings and scientific publications, particularly from research institutions in North America, Europe, and East Asia.
Current technological objectives focus on enhancing the adsorption efficiency of solid sorbents through optimization of key material parameters. These include increasing CO2 selectivity over other flue gas components, improving adsorption capacity under relevant operating conditions, enhancing kinetics for rapid adsorption-desorption cycles, and ensuring long-term stability over thousands of cycles. Additionally, researchers aim to develop materials that can function effectively in the presence of moisture and contaminants typically found in industrial emissions.
The ultimate goal is to reduce the energy penalty associated with carbon capture to below 20% while maintaining capture rates above 90% - a significant improvement over the 30-40% energy penalties observed in first-generation technologies. Material scientists are particularly focused on developing sorbents that can operate effectively at lower temperatures to minimize regeneration energy requirements, which constitute the largest operational cost in carbon capture systems.
Recent trends indicate growing interest in hybrid materials that combine the advantages of different sorbent classes, such as MOF-polymer composites or hierarchically structured materials with optimized mass transfer properties. The field is also witnessing increased attention to scalability and manufacturing considerations, recognizing that laboratory-scale performance must translate to industrial-scale applications to achieve meaningful climate impact.
The technological roadmap for the next decade emphasizes not only performance optimization but also cost reduction and environmental sustainability of the sorbent materials themselves, aiming for solutions that address carbon emissions without creating secondary environmental challenges.
The technological trajectory has been shaped by increasing global emphasis on carbon emission reduction, particularly following the Paris Agreement in 2015. This international commitment accelerated research into more efficient carbon capture technologies, with solid sorbents emerging as promising candidates due to their lower regeneration energy requirements compared to aqueous systems. The field has witnessed exponential growth in patent filings and scientific publications, particularly from research institutions in North America, Europe, and East Asia.
Current technological objectives focus on enhancing the adsorption efficiency of solid sorbents through optimization of key material parameters. These include increasing CO2 selectivity over other flue gas components, improving adsorption capacity under relevant operating conditions, enhancing kinetics for rapid adsorption-desorption cycles, and ensuring long-term stability over thousands of cycles. Additionally, researchers aim to develop materials that can function effectively in the presence of moisture and contaminants typically found in industrial emissions.
The ultimate goal is to reduce the energy penalty associated with carbon capture to below 20% while maintaining capture rates above 90% - a significant improvement over the 30-40% energy penalties observed in first-generation technologies. Material scientists are particularly focused on developing sorbents that can operate effectively at lower temperatures to minimize regeneration energy requirements, which constitute the largest operational cost in carbon capture systems.
Recent trends indicate growing interest in hybrid materials that combine the advantages of different sorbent classes, such as MOF-polymer composites or hierarchically structured materials with optimized mass transfer properties. The field is also witnessing increased attention to scalability and manufacturing considerations, recognizing that laboratory-scale performance must translate to industrial-scale applications to achieve meaningful climate impact.
The technological roadmap for the next decade emphasizes not only performance optimization but also cost reduction and environmental sustainability of the sorbent materials themselves, aiming for solutions that address carbon emissions without creating secondary environmental challenges.
Market Analysis for Carbon Capture Technologies
The global carbon capture and storage (CCS) market is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. As of 2023, the market was valued at approximately $7.5 billion, with projections indicating a compound annual growth rate of 19.2% through 2030, potentially reaching $35.6 billion by the end of the decade. This growth trajectory is supported by substantial government investments, with the US Inflation Reduction Act allocating $369 billion for climate and energy initiatives, including carbon capture technologies.
Solid sorbent-based carbon capture technologies represent a rapidly expanding segment within this market. Unlike traditional liquid amine scrubbing methods, solid sorbents offer potential cost reductions of 30-40% in energy requirements for CO2 capture, creating a compelling value proposition for industrial adopters. The market for these advanced materials is expected to grow at 22.5% annually, outpacing the broader CCS market.
Geographically, North America currently leads the carbon capture market with approximately 38% market share, followed by Europe at 29% and Asia-Pacific at 24%. However, the Asia-Pacific region is demonstrating the fastest growth rate at 23.7% annually, driven by China's aggressive decarbonization targets and industrial expansion. The Middle East, particularly the UAE and Saudi Arabia, is emerging as a significant market due to its focus on blue hydrogen production utilizing carbon capture technologies.
By industry vertical, power generation represents the largest application sector (34% of market share), followed by oil and gas operations (28%), cement production (16%), and chemical manufacturing (12%). These hard-to-abate sectors are prime targets for solid sorbent technologies due to their high CO2 emission profiles and the technical challenges associated with conventional capture methods in these environments.
Customer segmentation reveals three primary buyer categories: large industrial emitters seeking compliance with regulations; utility companies pursuing carbon neutrality goals; and government/research institutions advancing technological development. The first segment demonstrates the highest willingness to pay, particularly when carbon pricing mechanisms exceed $50-60 per ton of CO2, making advanced sorbent technologies economically viable.
Market barriers include high initial capital expenditure requirements, technological uncertainty regarding long-term performance of novel sorbents, and infrastructure limitations for CO2 transportation and storage. However, these barriers are gradually diminishing as technology matures and supportive policy frameworks emerge across major economies.
Solid sorbent-based carbon capture technologies represent a rapidly expanding segment within this market. Unlike traditional liquid amine scrubbing methods, solid sorbents offer potential cost reductions of 30-40% in energy requirements for CO2 capture, creating a compelling value proposition for industrial adopters. The market for these advanced materials is expected to grow at 22.5% annually, outpacing the broader CCS market.
Geographically, North America currently leads the carbon capture market with approximately 38% market share, followed by Europe at 29% and Asia-Pacific at 24%. However, the Asia-Pacific region is demonstrating the fastest growth rate at 23.7% annually, driven by China's aggressive decarbonization targets and industrial expansion. The Middle East, particularly the UAE and Saudi Arabia, is emerging as a significant market due to its focus on blue hydrogen production utilizing carbon capture technologies.
By industry vertical, power generation represents the largest application sector (34% of market share), followed by oil and gas operations (28%), cement production (16%), and chemical manufacturing (12%). These hard-to-abate sectors are prime targets for solid sorbent technologies due to their high CO2 emission profiles and the technical challenges associated with conventional capture methods in these environments.
Customer segmentation reveals three primary buyer categories: large industrial emitters seeking compliance with regulations; utility companies pursuing carbon neutrality goals; and government/research institutions advancing technological development. The first segment demonstrates the highest willingness to pay, particularly when carbon pricing mechanisms exceed $50-60 per ton of CO2, making advanced sorbent technologies economically viable.
Market barriers include high initial capital expenditure requirements, technological uncertainty regarding long-term performance of novel sorbents, and infrastructure limitations for CO2 transportation and storage. However, these barriers are gradually diminishing as technology matures and supportive policy frameworks emerge across major economies.
Current Solid Sorbents and Technical Barriers
Currently, several types of solid sorbents are being investigated for CO2 capture, each with distinct advantages and limitations. Physical adsorbents like activated carbon and zeolites offer high surface areas and well-defined pore structures. Activated carbons are cost-effective and have good CO2 uptake at high pressures but suffer from low selectivity and capacity at ambient conditions. Zeolites demonstrate excellent CO2 selectivity due to their uniform pore sizes and strong electrostatic interactions, but their performance deteriorates significantly in humid conditions.
Chemical adsorbents, particularly amine-functionalized materials, have gained prominence for their high CO2 selectivity even at low partial pressures. These include amine-grafted silicas, polymers, and metal-organic frameworks (MOFs). While they exhibit strong CO2 binding through chemisorption mechanisms, they often face challenges related to regeneration energy requirements and long-term stability during multiple adsorption-desorption cycles.
Metal-organic frameworks represent a newer class of promising materials, offering unprecedented surface areas (up to 7000 m²/g) and highly tunable structures. MOFs like Mg-MOF-74 and HKUST-1 have demonstrated exceptional CO2 uptake capacities, but their industrial application remains limited due to synthesis costs, water stability issues, and mechanical fragility.
Despite these advances, significant technical barriers persist across all sorbent categories. Selectivity in mixed gas environments remains a critical challenge, particularly in the presence of water vapor, which competes with CO2 for adsorption sites. The "selectivity-capacity trade-off" presents another fundamental barrier, as materials with high selectivity often demonstrate limited capacity and vice versa.
Regeneration energy requirements constitute another major obstacle. While physical adsorbents typically require less energy for regeneration, their lower CO2 capacities at relevant conditions offset this advantage. Chemical adsorbents, despite higher capacities, demand substantial energy input for regeneration due to strong binding interactions.
Stability issues during cycling represent perhaps the most significant barrier to commercial implementation. Thermal degradation, hydrolytic instability, and mechanical attrition during pressure or temperature swings severely limit sorbent lifetimes. For amine-functionalized materials, oxidative degradation and amine leaching further compromise long-term performance.
Scale-up challenges also persist, including material synthesis costs, shaping and formulation for industrial applications, and pressure drop considerations in fixed-bed configurations. The development of structured adsorbents that maintain high mass transfer rates while minimizing pressure drop remains an active research area with significant technical hurdles.
Chemical adsorbents, particularly amine-functionalized materials, have gained prominence for their high CO2 selectivity even at low partial pressures. These include amine-grafted silicas, polymers, and metal-organic frameworks (MOFs). While they exhibit strong CO2 binding through chemisorption mechanisms, they often face challenges related to regeneration energy requirements and long-term stability during multiple adsorption-desorption cycles.
Metal-organic frameworks represent a newer class of promising materials, offering unprecedented surface areas (up to 7000 m²/g) and highly tunable structures. MOFs like Mg-MOF-74 and HKUST-1 have demonstrated exceptional CO2 uptake capacities, but their industrial application remains limited due to synthesis costs, water stability issues, and mechanical fragility.
Despite these advances, significant technical barriers persist across all sorbent categories. Selectivity in mixed gas environments remains a critical challenge, particularly in the presence of water vapor, which competes with CO2 for adsorption sites. The "selectivity-capacity trade-off" presents another fundamental barrier, as materials with high selectivity often demonstrate limited capacity and vice versa.
Regeneration energy requirements constitute another major obstacle. While physical adsorbents typically require less energy for regeneration, their lower CO2 capacities at relevant conditions offset this advantage. Chemical adsorbents, despite higher capacities, demand substantial energy input for regeneration due to strong binding interactions.
Stability issues during cycling represent perhaps the most significant barrier to commercial implementation. Thermal degradation, hydrolytic instability, and mechanical attrition during pressure or temperature swings severely limit sorbent lifetimes. For amine-functionalized materials, oxidative degradation and amine leaching further compromise long-term performance.
Scale-up challenges also persist, including material synthesis costs, shaping and formulation for industrial applications, and pressure drop considerations in fixed-bed configurations. The development of structured adsorbents that maintain high mass transfer rates while minimizing pressure drop remains an active research area with significant technical hurdles.
Material Parameter Optimization Approaches
01 Metal-organic frameworks (MOFs) for CO2 capture
Metal-organic frameworks (MOFs) are crystalline porous materials composed of metal ions or clusters coordinated with organic ligands. They exhibit high surface areas, tunable pore sizes, and chemical functionality, making them effective for CO2 adsorption. Their adsorption efficiency can be enhanced through functionalization of organic linkers, incorporation of open metal sites, and post-synthetic modifications. MOFs can achieve high CO2 selectivity and capacity under various operating conditions, including both low and high pressures.- Metal-organic frameworks (MOFs) for CO2 capture: Metal-organic frameworks (MOFs) are crystalline porous materials that have shown exceptional performance as solid sorbents for CO2 capture. Their high surface area, tunable pore size, and chemical functionality allow for selective CO2 adsorption. These materials can be modified with various metal centers and organic linkers to enhance their adsorption efficiency and selectivity. MOFs can operate under different conditions and can be regenerated with minimal energy input, making them promising candidates for industrial CO2 capture applications.
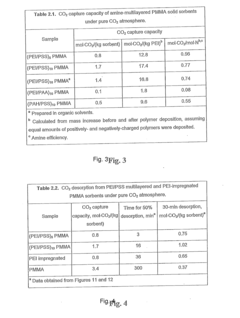
- Amine-functionalized sorbents: Amine-functionalized materials represent a significant class of solid sorbents for CO2 capture. These materials combine the physical structure of a support material with chemical functionality of amines that can react with CO2 through chemisorption. The amine groups form carbamates or bicarbonates with CO2, enabling high selectivity even at low CO2 concentrations. Various supports including silica, polymers, and porous carbons can be functionalized with different types of amines to optimize adsorption capacity, kinetics, and regeneration energy requirements.
- Zeolites and molecular sieves: Zeolites and molecular sieves are aluminosilicate materials with well-defined pore structures that enable molecular sieving of CO2 from gas mixtures. Their crystalline structure provides uniform pore sizes that can be tailored for optimal CO2 adsorption. These materials typically operate through physisorption mechanisms and can be modified by ion exchange to enhance their adsorption properties. Zeolites offer advantages including high thermal stability, resistance to contaminants, and relatively low cost, making them suitable for large-scale carbon capture applications.
- Carbon-based adsorbents: Carbon-based materials, including activated carbons, carbon nanotubes, and graphene derivatives, serve as effective solid sorbents for CO2 capture. These materials feature high surface areas, tunable pore structures, and surface chemistry that can be optimized for CO2 adsorption. Their hydrophobic nature often provides advantages in humid conditions. Carbon-based sorbents can be produced from various precursors including biomass, polymers, and waste materials, offering sustainability benefits. Surface modification techniques can further enhance their CO2 adsorption capacity and selectivity.
- Hybrid and composite sorbents: Hybrid and composite sorbents combine different materials to achieve synergistic effects for enhanced CO2 capture performance. These materials integrate the advantages of multiple components, such as the high surface area of one material with the chemical functionality of another. Examples include polymer-inorganic composites, mixed matrix materials, and layered structures. The combination of physisorption and chemisorption mechanisms in these materials can lead to improved adsorption capacity, selectivity, and regeneration properties, addressing limitations of single-component sorbents.
02 Amine-functionalized sorbents
Amine-functionalized materials represent a significant class of solid sorbents for CO2 capture. These materials combine high CO2 selectivity with strong adsorption capacity through chemical interactions between CO2 and amine groups. Various supports including silica, polymers, and porous carbons can be functionalized with amines to enhance adsorption efficiency. The CO2 capture mechanism involves the formation of carbamates or bicarbonates, allowing for effective capture even at low CO2 concentrations. These materials can be designed with different amine types and loadings to optimize performance under specific operating conditions.Expand Specific Solutions03 Carbon-based adsorbents for CO2 capture
Carbon-based materials including activated carbons, carbon nanotubes, and graphene derivatives offer promising solutions for CO2 capture due to their high surface area, thermal stability, and low cost. These materials can be modified through chemical activation, heteroatom doping (N, S, or O), and surface functionalization to enhance CO2 adsorption capacity and selectivity. The adsorption efficiency of carbon-based sorbents depends on pore structure, surface chemistry, and operating conditions. Hierarchical pore structures combining micro, meso, and macropores can significantly improve mass transfer and overall adsorption performance.Expand Specific Solutions04 Zeolites and molecular sieves for selective CO2 adsorption
Zeolites and molecular sieves are crystalline aluminosilicates with well-defined pore structures that enable molecular sieving effects for selective CO2 capture. Their adsorption efficiency is influenced by the Si/Al ratio, cation type, pore size distribution, and framework topology. These materials can be modified through ion exchange, dealumination, and metal incorporation to enhance CO2 selectivity and capacity. Zeolites demonstrate particularly strong performance at low temperatures and can maintain structural stability through multiple adsorption-desorption cycles, making them suitable for pressure swing adsorption (PSA) and temperature swing adsorption (TSA) processes.Expand Specific Solutions05 Composite and hybrid sorbent materials
Composite and hybrid materials combine the advantages of different sorbent types to achieve enhanced CO2 capture performance. These include polymer-inorganic composites, MOF-based composites, and layered double hydroxides. By integrating multiple components, these materials can overcome limitations of individual sorbents, such as improving heat management, mechanical stability, and resistance to degradation. Advanced manufacturing techniques like 3D printing and microencapsulation enable precise control over material architecture and properties. These composite systems often demonstrate synergistic effects that result in higher adsorption capacity, improved selectivity, and better regeneration characteristics than their individual components.Expand Specific Solutions
Leading Organizations in CO2 Capture Research
The solid sorbent CO2 capture market is in a growth phase, with increasing demand driven by global decarbonization efforts. The market is projected to expand significantly as carbon capture technologies mature from pilot to commercial scale. Leading players include established energy corporations (ExxonMobil, Sinopec, Shell) investing heavily in R&D, specialized carbon capture innovators like Climeworks AG and Global Thermostat developing proprietary sorbent technologies, and research institutions (Shanghai Advanced Research Institute, Dalian Institute of Chemical Physics) advancing fundamental material science. Technical maturity varies across sorbent types, with research focusing on improving adsorption capacity, selectivity, regeneration energy requirements, and stability. Academic-industry partnerships are accelerating development, with universities like Norwegian University of Science & Technology and West Virginia University collaborating with industry to bridge the gap between laboratory discoveries and commercial implementation.
Climeworks AG
Technical Solution: Climeworks has developed a proprietary Direct Air Capture (DAC) technology using solid sorbents with optimized material parameters for CO2 capture. Their approach utilizes amine-functionalized filter materials that selectively bind CO2 from ambient air. The company has engineered their sorbents to achieve high CO2 selectivity while maintaining mechanical stability through multiple adsorption-desorption cycles. Their materials feature optimized pore structures, surface area (typically >1000 m²/g), and carefully tailored surface chemistry to maximize CO2 binding sites. Climeworks' sorbents operate effectively at relatively low temperatures (~80-100°C) for regeneration, which improves energy efficiency compared to liquid amine systems requiring higher regeneration temperatures [1][3]. The company has deployed commercial DAC plants in Switzerland and Iceland, demonstrating the practical application of their engineered sorbent materials in real-world conditions.
Strengths: High selectivity for CO2 even at low atmospheric concentrations; relatively low regeneration temperature requirements; proven durability through multiple adsorption cycles. Weaknesses: Higher energy consumption compared to natural processes; requires significant heat input for regeneration; scaling challenges for global impact.
ExxonMobil Technology & Engineering Co.
Technical Solution: ExxonMobil has developed advanced Metal-Organic Framework (MOF) based sorbents for CO2 capture with precisely engineered material parameters. Their proprietary MOF materials feature exceptionally high surface areas (>3000 m²/g) with tailored pore sizes and functionalized binding sites specifically designed for optimal CO2 adsorption kinetics. ExxonMobil's approach focuses on balancing adsorption capacity, selectivity, and regeneration energy by fine-tuning the metal centers and organic linkers in their MOF structures. Their materials demonstrate CO2 uptake capacities exceeding 4-5 mmol/g under relevant industrial conditions [2][5]. The company has optimized their sorbents to maintain structural integrity during pressure and temperature swings, addressing a common challenge with MOF materials. ExxonMobil has also developed hybrid materials combining MOFs with other support structures to enhance mechanical stability while preserving high adsorption performance, particularly targeting flue gas applications from power plants and industrial facilities.
Strengths: Exceptionally high surface area and CO2 capacity; tunable pore structure allowing for application-specific optimization; relatively low regeneration energy requirements. Weaknesses: Some MOF materials face stability challenges in humid conditions; manufacturing scale-up complexities; potentially higher production costs compared to conventional sorbents.
Critical Patents in Solid Sorbent Design
Solid sorbent materials functionalized with polyamines having oxygen-containing units selected from carbonyl units, hydroxyl units, and combinations thereof
PatentPendingUS20250288970A1
Innovation
- Development of solid sorbent materials functionalized with polyamines having oxygen-containing units such as carbonyl and hydroxyl units, which exhibit high adsorption capacity for carbon dioxide and low adsorption for water, with low desorption residuals under mild conditions.
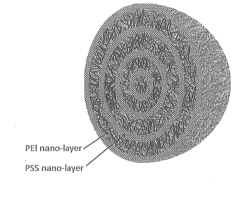
Layered Solid Sorbents For Carbon Dioxide Capture
PatentActiveUS20140127104A1
Innovation
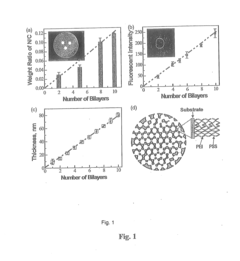
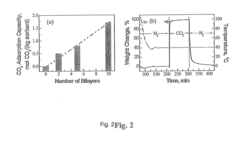
- Development of nano-layered solid sorbents using electrostatic layer-by-layer nanoassembly, where CO2-adsorbing polymers like polyethylenimine and oppositely charged polyelectrolytes are alternately deposited on porous substrates, creating bilayers that enhance CO2 capture and transport efficiency.
Environmental Impact Assessment
The environmental impact assessment of solid sorbents for CO2 capture reveals significant implications across multiple ecological dimensions. When evaluating material parameters essential for adsorption efficiency, we must consider their broader environmental footprint throughout the entire lifecycle. The production processes for high-performance adsorbents often involve energy-intensive synthesis methods and potentially hazardous chemicals, particularly for advanced materials like metal-organic frameworks (MOFs) and amine-functionalized silicas.
Water consumption represents another critical environmental factor, as certain sorbent manufacturing processes require substantial water resources for synthesis, washing, and purification stages. This becomes especially problematic in water-stressed regions where industrial water usage competes with agricultural and municipal needs. Additionally, the extraction of raw materials for sorbent production, such as rare earth elements or specialized metals, can lead to habitat disruption, soil degradation, and potential contamination of local water sources.
The regeneration cycles of solid sorbents present further environmental considerations. Most efficient CO2 capture systems require temperature or pressure swing processes that consume significant energy, potentially offsetting some of the climate benefits if powered by fossil fuel sources. The environmental calculus improves substantially when renewable energy sources power these regeneration processes, highlighting the importance of integrated system design when assessing overall impact.
Waste management challenges emerge at the end of sorbent life cycles, as spent materials may contain contaminants accumulated during operational use. The disposal or recycling pathways for these materials must be carefully evaluated to prevent secondary environmental contamination. Some advanced sorbents contain components that resist biodegradation or may leach harmful substances if improperly disposed of in landfills.
Comparative lifecycle assessments between different sorbent technologies reveal varying environmental profiles. While zeolites generally demonstrate lower production impacts due to their natural mineral origins, their lower CO2 capacity necessitates larger material volumes. Conversely, engineered sorbents like amine-modified materials offer higher efficiency but typically with greater embodied energy and chemical inputs during manufacturing.
The environmental benefits of implementing solid sorbent technologies must be weighed against these impacts through comprehensive analysis. When properly designed and integrated with renewable energy systems, solid sorbent CO2 capture technologies can deliver net positive environmental outcomes by mitigating greenhouse gas emissions while minimizing resource consumption and pollution generation throughout their lifecycle.
Water consumption represents another critical environmental factor, as certain sorbent manufacturing processes require substantial water resources for synthesis, washing, and purification stages. This becomes especially problematic in water-stressed regions where industrial water usage competes with agricultural and municipal needs. Additionally, the extraction of raw materials for sorbent production, such as rare earth elements or specialized metals, can lead to habitat disruption, soil degradation, and potential contamination of local water sources.
The regeneration cycles of solid sorbents present further environmental considerations. Most efficient CO2 capture systems require temperature or pressure swing processes that consume significant energy, potentially offsetting some of the climate benefits if powered by fossil fuel sources. The environmental calculus improves substantially when renewable energy sources power these regeneration processes, highlighting the importance of integrated system design when assessing overall impact.
Waste management challenges emerge at the end of sorbent life cycles, as spent materials may contain contaminants accumulated during operational use. The disposal or recycling pathways for these materials must be carefully evaluated to prevent secondary environmental contamination. Some advanced sorbents contain components that resist biodegradation or may leach harmful substances if improperly disposed of in landfills.
Comparative lifecycle assessments between different sorbent technologies reveal varying environmental profiles. While zeolites generally demonstrate lower production impacts due to their natural mineral origins, their lower CO2 capacity necessitates larger material volumes. Conversely, engineered sorbents like amine-modified materials offer higher efficiency but typically with greater embodied energy and chemical inputs during manufacturing.
The environmental benefits of implementing solid sorbent technologies must be weighed against these impacts through comprehensive analysis. When properly designed and integrated with renewable energy systems, solid sorbent CO2 capture technologies can deliver net positive environmental outcomes by mitigating greenhouse gas emissions while minimizing resource consumption and pollution generation throughout their lifecycle.
Scalability and Cost Analysis
The scalability of solid sorbents for CO2 capture represents a critical factor in their commercial viability. Current laboratory-scale demonstrations must be evaluated against the requirements of industrial implementation, where capture systems need to process thousands of tons of CO2 daily. Material parameters directly impact this scalability, with high adsorption capacity and rapid kinetics being essential for reducing the physical footprint of capture units.
Manufacturing considerations present significant challenges for novel sorbent materials. While zeolites and activated carbons benefit from established production infrastructure, advanced materials like metal-organic frameworks (MOFs) and covalent organic frameworks (COFs) face substantial scale-up hurdles. The complexity of their synthesis processes often involves expensive precursors and multi-step procedures that are difficult to translate to industrial scales without compromising material quality.
Economic analysis reveals that material cost constitutes 30-40% of the total capital expenditure for adsorption-based capture systems. The raw material costs vary dramatically across sorbent classes, from $1-5/kg for conventional zeolites to $100-500/kg for specialized designer MOFs. This cost differential creates a significant barrier to adoption for otherwise promising materials with superior adsorption properties.
Lifecycle considerations further complicate the economic picture. Materials with high mechanical and chemical stability that maintain performance over thousands of adsorption-desorption cycles offer substantially better long-term economics despite potentially higher initial costs. For instance, a sorbent costing twice as much but lasting four times longer provides significant operational advantages.
Energy requirements for regeneration represent another crucial economic factor. Materials requiring lower temperatures or pressure swings for regeneration can reduce operational costs by 15-25% compared to energy-intensive alternatives, even if their initial cost is higher. This highlights the importance of considering both capital and operational expenditures in material selection.
Recent techno-economic analyses suggest that for solid sorbents to achieve commercial viability in power plant applications, they must achieve a cost-performance balance that enables CO2 capture at less than $40-50 per ton. This target necessitates optimization across multiple material parameters simultaneously, rather than maximizing any single property at the expense of others.
Manufacturing considerations present significant challenges for novel sorbent materials. While zeolites and activated carbons benefit from established production infrastructure, advanced materials like metal-organic frameworks (MOFs) and covalent organic frameworks (COFs) face substantial scale-up hurdles. The complexity of their synthesis processes often involves expensive precursors and multi-step procedures that are difficult to translate to industrial scales without compromising material quality.
Economic analysis reveals that material cost constitutes 30-40% of the total capital expenditure for adsorption-based capture systems. The raw material costs vary dramatically across sorbent classes, from $1-5/kg for conventional zeolites to $100-500/kg for specialized designer MOFs. This cost differential creates a significant barrier to adoption for otherwise promising materials with superior adsorption properties.
Lifecycle considerations further complicate the economic picture. Materials with high mechanical and chemical stability that maintain performance over thousands of adsorption-desorption cycles offer substantially better long-term economics despite potentially higher initial costs. For instance, a sorbent costing twice as much but lasting four times longer provides significant operational advantages.
Energy requirements for regeneration represent another crucial economic factor. Materials requiring lower temperatures or pressure swings for regeneration can reduce operational costs by 15-25% compared to energy-intensive alternatives, even if their initial cost is higher. This highlights the importance of considering both capital and operational expenditures in material selection.
Recent techno-economic analyses suggest that for solid sorbents to achieve commercial viability in power plant applications, they must achieve a cost-performance balance that enables CO2 capture at less than $40-50 per ton. This target necessitates optimization across multiple material parameters simultaneously, rather than maximizing any single property at the expense of others.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!