Comparative analysis of Solid sorbents for CO2 capture adsorption versus catalytic sorbents
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Capture Technology Evolution and Objectives
Carbon dioxide capture technology has evolved significantly over the past decades, driven by the urgent need to mitigate climate change and reduce greenhouse gas emissions. The journey began in the 1970s with conventional amine scrubbing processes primarily used in natural gas sweetening operations. These early technologies focused on liquid absorbents, which while effective, presented challenges related to energy consumption and solvent degradation.
The 1990s marked a pivotal shift as research expanded beyond liquid systems to explore solid sorbents for CO2 capture. This transition was motivated by the potential advantages of solid materials, including lower regeneration energy requirements, reduced corrosion issues, and greater operational flexibility. Initial solid sorbent research concentrated on physical adsorbents such as activated carbons and zeolites, which demonstrated promising CO2 uptake capacities but often lacked selectivity in mixed gas environments.
By the early 2000s, the field witnessed the emergence of more sophisticated materials, including metal-organic frameworks (MOFs), covalent organic frameworks (COFs), and functionalized porous silicas. These materials offered unprecedented surface areas and tunable pore structures, enabling higher CO2 adsorption capacities and improved selectivity. Concurrently, research into chemical sorbents, particularly those based on supported amines and alkali metal carbonates, gained momentum due to their strong CO2 binding capabilities.
The past decade has seen a remarkable convergence of adsorption and catalytic approaches, giving rise to catalytic sorbents that not only capture CO2 but also facilitate its conversion into valuable products. This represents a paradigm shift from viewing CO2 as merely a waste product to recognizing it as a potential feedstock for chemical synthesis and fuel production.
The primary objectives of current CO2 capture technology development center around addressing several critical challenges. First, enhancing the CO2 adsorption capacity and selectivity of solid sorbents, particularly under realistic flue gas conditions containing contaminants like SOx and NOx. Second, improving the kinetics of adsorption and desorption processes to enable rapid cycling and higher throughput. Third, developing materials with exceptional stability that can withstand thousands of adsorption-desorption cycles without significant performance degradation.
Additionally, research aims to reduce the energy penalty associated with sorbent regeneration, which remains a major economic barrier to widespread implementation. The ultimate goal is to develop next-generation solid sorbents and catalytic materials that can achieve CO2 capture costs below $30 per ton, making carbon capture economically viable across various industrial sectors and power generation facilities.
The 1990s marked a pivotal shift as research expanded beyond liquid systems to explore solid sorbents for CO2 capture. This transition was motivated by the potential advantages of solid materials, including lower regeneration energy requirements, reduced corrosion issues, and greater operational flexibility. Initial solid sorbent research concentrated on physical adsorbents such as activated carbons and zeolites, which demonstrated promising CO2 uptake capacities but often lacked selectivity in mixed gas environments.
By the early 2000s, the field witnessed the emergence of more sophisticated materials, including metal-organic frameworks (MOFs), covalent organic frameworks (COFs), and functionalized porous silicas. These materials offered unprecedented surface areas and tunable pore structures, enabling higher CO2 adsorption capacities and improved selectivity. Concurrently, research into chemical sorbents, particularly those based on supported amines and alkali metal carbonates, gained momentum due to their strong CO2 binding capabilities.
The past decade has seen a remarkable convergence of adsorption and catalytic approaches, giving rise to catalytic sorbents that not only capture CO2 but also facilitate its conversion into valuable products. This represents a paradigm shift from viewing CO2 as merely a waste product to recognizing it as a potential feedstock for chemical synthesis and fuel production.
The primary objectives of current CO2 capture technology development center around addressing several critical challenges. First, enhancing the CO2 adsorption capacity and selectivity of solid sorbents, particularly under realistic flue gas conditions containing contaminants like SOx and NOx. Second, improving the kinetics of adsorption and desorption processes to enable rapid cycling and higher throughput. Third, developing materials with exceptional stability that can withstand thousands of adsorption-desorption cycles without significant performance degradation.
Additionally, research aims to reduce the energy penalty associated with sorbent regeneration, which remains a major economic barrier to widespread implementation. The ultimate goal is to develop next-generation solid sorbents and catalytic materials that can achieve CO2 capture costs below $30 per ton, making carbon capture economically viable across various industrial sectors and power generation facilities.
Market Demand for Carbon Capture Solutions
The global carbon capture market is experiencing unprecedented growth, driven by increasing environmental regulations and corporate sustainability commitments. Current market valuations place the carbon capture industry at approximately $2 billion, with projections indicating expansion to $7 billion by 2028, representing a compound annual growth rate of 19.2%. This rapid growth trajectory underscores the urgent demand for effective carbon capture technologies, particularly solid sorbents for CO2 adsorption and catalytic sorbents.
Industrial sectors, including power generation, cement production, and steel manufacturing, collectively contribute over 40% of global CO2 emissions, creating substantial market opportunities for carbon capture solutions. The power generation sector alone represents nearly 30% of the current carbon capture market, with cement and steel manufacturing following at 15% and 12% respectively. These heavy industries are increasingly seeking cost-effective carbon capture technologies to meet stringent emission reduction targets.
Geographically, North America and Europe lead market demand, accounting for approximately 65% of global carbon capture investments. However, the Asia-Pacific region is witnessing the fastest growth rate at 22.3% annually, primarily driven by China's ambitious carbon neutrality goals and Japan's hydrogen economy initiatives. This regional diversification indicates expanding global market opportunities for advanced sorbent technologies.
From an economic perspective, the cost-performance ratio remains the primary market driver. Current carbon capture costs using conventional technologies range from $40-100 per ton of CO2, significantly higher than the $15-30 per ton threshold considered economically viable for widespread adoption. This cost gap represents a critical market opportunity for innovative solid sorbents and catalytic materials that can deliver higher efficiency at lower operational costs.
Policy frameworks are substantially influencing market dynamics. Carbon pricing mechanisms, implemented in over 40 countries, have established CO2 values ranging from $5 to $125 per ton. Additionally, government incentives for carbon capture technologies, such as the 45Q tax credits in the United States offering $50 per ton for geological storage, are creating favorable market conditions for technology developers and adopters.
End-user requirements are evolving toward solutions offering modularity, scalability, and integration capabilities with existing infrastructure. Market research indicates that 78% of potential industrial adopters prioritize technologies that can be retrofitted to existing facilities rather than requiring complete system overhauls, creating specific demand parameters for sorbent-based capture systems.
Industrial sectors, including power generation, cement production, and steel manufacturing, collectively contribute over 40% of global CO2 emissions, creating substantial market opportunities for carbon capture solutions. The power generation sector alone represents nearly 30% of the current carbon capture market, with cement and steel manufacturing following at 15% and 12% respectively. These heavy industries are increasingly seeking cost-effective carbon capture technologies to meet stringent emission reduction targets.
Geographically, North America and Europe lead market demand, accounting for approximately 65% of global carbon capture investments. However, the Asia-Pacific region is witnessing the fastest growth rate at 22.3% annually, primarily driven by China's ambitious carbon neutrality goals and Japan's hydrogen economy initiatives. This regional diversification indicates expanding global market opportunities for advanced sorbent technologies.
From an economic perspective, the cost-performance ratio remains the primary market driver. Current carbon capture costs using conventional technologies range from $40-100 per ton of CO2, significantly higher than the $15-30 per ton threshold considered economically viable for widespread adoption. This cost gap represents a critical market opportunity for innovative solid sorbents and catalytic materials that can deliver higher efficiency at lower operational costs.
Policy frameworks are substantially influencing market dynamics. Carbon pricing mechanisms, implemented in over 40 countries, have established CO2 values ranging from $5 to $125 per ton. Additionally, government incentives for carbon capture technologies, such as the 45Q tax credits in the United States offering $50 per ton for geological storage, are creating favorable market conditions for technology developers and adopters.
End-user requirements are evolving toward solutions offering modularity, scalability, and integration capabilities with existing infrastructure. Market research indicates that 78% of potential industrial adopters prioritize technologies that can be retrofitted to existing facilities rather than requiring complete system overhauls, creating specific demand parameters for sorbent-based capture systems.
Current State and Challenges in CO2 Sorbent Technology
The global landscape of CO2 capture technology is currently dominated by two primary approaches: solid sorbent adsorption and catalytic sorbent systems. Solid sorbents have gained significant traction due to their relatively lower energy requirements compared to traditional liquid amine scrubbing processes. Currently, materials such as zeolites, metal-organic frameworks (MOFs), activated carbons, and amine-functionalized silica are at the forefront of research and development efforts.
In the United States and Europe, significant research infrastructure has been established around these technologies, with the U.S. Department of Energy and European Commission funding large-scale projects to advance solid sorbent technologies. Meanwhile, China has rapidly expanded its research capacity, becoming a leading publisher of academic papers on novel CO2 sorbent materials in the past five years.
Despite promising advances, several critical challenges persist in the field. Stability remains a primary concern, as many high-performance sorbents demonstrate degradation after multiple adsorption-desorption cycles, particularly in the presence of moisture, SOx, and NOx contaminants. This degradation significantly impacts the economic viability of these technologies in real-world industrial settings.
Selectivity presents another substantial hurdle, especially in flue gas environments where CO2 concentrations typically range from 4% to 15%. Current materials often struggle to achieve sufficient CO2/N2 selectivity under these conditions, necessitating complex process designs that increase operational costs.
The energy penalty associated with sorbent regeneration continues to be a limiting factor. While solid sorbents generally require less energy than liquid amines, the temperature swing or pressure swing processes needed for regeneration still constitute a significant portion of the overall energy consumption in carbon capture systems.
Scale-up challenges represent perhaps the most immediate barrier to widespread implementation. Many promising materials developed in laboratory settings face substantial hurdles in manufacturing at industrial scales. Issues related to mechanical strength, attrition resistance, and pressure drop in fixed or fluidized bed configurations have limited commercial deployment.
Cost remains a decisive factor, with current estimates for CO2 capture using advanced solid sorbents ranging from $40-80 per ton of CO2, still above the target of $30 per ton needed for widespread economic viability. This cost challenge is compounded by the massive scale required for meaningful climate impact, necessitating materials that can be produced economically at megaton scales.
The catalytic sorbent approach, which combines capture and conversion in a single step, has emerged as a promising alternative but faces its own set of challenges related to catalyst deactivation, reaction selectivity, and process integration complexities.
In the United States and Europe, significant research infrastructure has been established around these technologies, with the U.S. Department of Energy and European Commission funding large-scale projects to advance solid sorbent technologies. Meanwhile, China has rapidly expanded its research capacity, becoming a leading publisher of academic papers on novel CO2 sorbent materials in the past five years.
Despite promising advances, several critical challenges persist in the field. Stability remains a primary concern, as many high-performance sorbents demonstrate degradation after multiple adsorption-desorption cycles, particularly in the presence of moisture, SOx, and NOx contaminants. This degradation significantly impacts the economic viability of these technologies in real-world industrial settings.
Selectivity presents another substantial hurdle, especially in flue gas environments where CO2 concentrations typically range from 4% to 15%. Current materials often struggle to achieve sufficient CO2/N2 selectivity under these conditions, necessitating complex process designs that increase operational costs.
The energy penalty associated with sorbent regeneration continues to be a limiting factor. While solid sorbents generally require less energy than liquid amines, the temperature swing or pressure swing processes needed for regeneration still constitute a significant portion of the overall energy consumption in carbon capture systems.
Scale-up challenges represent perhaps the most immediate barrier to widespread implementation. Many promising materials developed in laboratory settings face substantial hurdles in manufacturing at industrial scales. Issues related to mechanical strength, attrition resistance, and pressure drop in fixed or fluidized bed configurations have limited commercial deployment.
Cost remains a decisive factor, with current estimates for CO2 capture using advanced solid sorbents ranging from $40-80 per ton of CO2, still above the target of $30 per ton needed for widespread economic viability. This cost challenge is compounded by the massive scale required for meaningful climate impact, necessitating materials that can be produced economically at megaton scales.
The catalytic sorbent approach, which combines capture and conversion in a single step, has emerged as a promising alternative but faces its own set of challenges related to catalyst deactivation, reaction selectivity, and process integration complexities.
Comparative Analysis of Solid Sorbent Technologies
01 Metal-organic frameworks (MOFs) for CO2 capture
Metal-organic frameworks (MOFs) are crystalline porous materials composed of metal ions or clusters coordinated with organic ligands. They exhibit exceptional CO2 adsorption capacity due to their high surface area, tunable pore size, and functionalized surfaces. MOFs can be designed with specific metal centers and organic linkers to enhance CO2 selectivity and capacity. Their adsorption performance can be further improved through post-synthetic modifications and incorporation of open metal sites.- Metal-organic frameworks (MOFs) for CO2 adsorption: Metal-organic frameworks (MOFs) are crystalline porous materials composed of metal ions or clusters coordinated to organic ligands. They exhibit exceptional CO2 adsorption capacity due to their high surface area, tunable pore size, and functionalized surfaces. MOFs can be designed with specific metal centers and organic linkers to enhance selectivity and capacity for CO2 capture. Their adsorption capacity can be further improved through post-synthetic modifications and by incorporating functional groups with high affinity for CO2.
- Amine-functionalized sorbents for enhanced CO2 capture: Amine-functionalized materials represent a significant class of solid sorbents for CO2 capture. These materials combine a porous support structure with amine functional groups that chemically bind to CO2 molecules. The amine groups can be incorporated through impregnation, grafting, or direct synthesis methods. These sorbents demonstrate high CO2 selectivity and adsorption capacity, particularly at low CO2 partial pressures, making them suitable for post-combustion capture applications. The adsorption mechanism primarily involves the formation of carbamates or bicarbonates through reaction with CO2.
- Zeolite-based materials for CO2 adsorption: Zeolites are crystalline aluminosilicate materials with well-defined pore structures that demonstrate significant CO2 adsorption capacity. Their adsorption properties can be tailored by adjusting the silicon-to-aluminum ratio, cation type, and pore architecture. Zeolites exhibit high thermal stability and can be regenerated multiple times without significant loss of adsorption capacity. Modified zeolites, particularly those with alkali or alkaline earth metal cations, show enhanced CO2 selectivity and capacity due to stronger electrostatic interactions with CO2 molecules.
- Carbon-based sorbents for CO2 capture: Carbon-based materials, including activated carbons, carbon nanotubes, and graphene derivatives, offer promising CO2 adsorption properties. These materials feature high surface area, tunable pore structure, and surface chemistry that can be modified to enhance CO2 capture performance. Nitrogen-doped carbon materials show particularly high CO2 adsorption capacity due to the creation of basic sites that interact favorably with acidic CO2 molecules. The adsorption capacity of carbon-based sorbents can be further improved through chemical activation processes and by incorporating metal nanoparticles as active sites.
- Composite and hybrid sorbents for improved CO2 adsorption: Composite and hybrid sorbents combine different materials to achieve enhanced CO2 adsorption properties. These materials typically integrate the advantages of multiple components, such as the high surface area of porous supports with the chemical reactivity of functional groups. Examples include polymer-inorganic composites, MOF-polymer hybrids, and amine-grafted silica materials. These composite structures often demonstrate synergistic effects, resulting in higher CO2 adsorption capacity, improved selectivity, and better mechanical stability compared to their individual components. Additionally, they can be designed to operate effectively under specific conditions, such as high humidity or in the presence of contaminants.
02 Amine-functionalized sorbents
Amine-functionalized materials represent a significant class of CO2 sorbents with high adsorption capacity. These materials combine physical adsorption with chemical reaction mechanisms, where CO2 reacts with amine groups to form carbamates or bicarbonates. Common supports include silica, activated carbon, and polymeric substrates that are modified with various amine compounds. The CO2 capture capacity depends on the amine loading, type of amine (primary, secondary, tertiary), and the accessibility of the amine sites.Expand Specific Solutions03 Zeolites and molecular sieves for selective CO2 adsorption
Zeolites and molecular sieves are aluminosilicate materials with well-defined pore structures that enable selective adsorption of CO2. Their adsorption capacity is influenced by the Si/Al ratio, cation type, and pore architecture. These materials can be modified through ion exchange, dealumination, or incorporation of functional groups to enhance CO2 selectivity and capacity. They typically perform well at moderate temperatures and pressures, making them suitable for various carbon capture applications.Expand Specific Solutions04 Carbon-based sorbents with enhanced porosity
Carbon-based materials, including activated carbon, carbon nanotubes, and graphene derivatives, offer promising CO2 adsorption properties. These materials can be engineered with hierarchical pore structures to maximize surface area and adsorption sites. Chemical activation, template synthesis, and surface functionalization are common methods to enhance their CO2 capture capacity. The combination of micropores for high adsorption potential and mesopores for efficient mass transfer results in improved adsorption kinetics and capacity.Expand Specific Solutions05 Composite and hybrid sorbent materials
Composite and hybrid materials combine the advantages of different sorbent types to achieve enhanced CO2 capture performance. These include polymer-inorganic composites, MOF-polymer hybrids, and multi-component structured sorbents. The synergistic effects between components can lead to improved mechanical stability, heat management, and regeneration properties. These materials often feature optimized morphologies such as core-shell structures, supported thin films, or hierarchical architectures that maximize the accessibility of adsorption sites while maintaining high selectivity for CO2.Expand Specific Solutions
Leading Companies and Research Institutions in CO2 Capture
The CO2 capture adsorption technology market is in a growth phase, with increasing demand driven by global decarbonization efforts. The market is projected to expand significantly as carbon capture becomes essential for climate goals. Technologically, solid sorbents for CO2 capture show varying maturity levels across different approaches. Academic institutions like Tsinghua University, West Virginia University, and USC are advancing fundamental research, while commercial players demonstrate different stages of deployment. Companies like Carboncapture and Climeworks have operational direct air capture facilities using solid sorbents, while energy majors including Shell, ExxonMobil, and Sinopec are investing in catalytic sorbent technologies. Research organizations such as DICP and CSIRO are bridging the gap between laboratory research and industrial implementation, focusing on improving sorbent efficiency and reducing regeneration energy requirements.
Dalian Institute of Chemical Physics of CAS
Technical Solution: The Dalian Institute of Chemical Physics (DICP) has developed innovative solid sorbents for CO2 capture based on hierarchically structured porous materials. Their technology focuses on nitrogen-doped porous carbons and metal-organic frameworks (MOFs) with tailored pore structures that maximize CO2 adsorption kinetics and capacity. DICP's materials achieve CO2 adsorption capacities of 4-6 mmol/g under flue gas conditions with exceptional selectivity over N2 (>50:1). Their approach incorporates a vacuum-temperature swing adsorption (VTSA) process that reduces regeneration energy by 25-35% compared to conventional amine scrubbing. A key innovation is their "molecular basket" concept, where basic nitrogen sites are precisely positioned within optimized pore structures to enhance CO2 binding strength while maintaining fast adsorption-desorption kinetics. DICP has demonstrated this technology at pilot scale (1-5 tons CO2/day) with coal-fired power plant flue gas, achieving over 90% capture efficiency with stable performance over 1000+ cycles and minimal sorbent degradation (less than 0.1% capacity loss per cycle).
Strengths: High CO2 selectivity and capacity; lower regeneration energy than conventional technologies; excellent stability over multiple cycles; good tolerance to moisture and impurities. Weaknesses: Complex material synthesis may increase production costs; vacuum operation adds complexity and energy requirements; scaling to industrial level remains challenging.
IFP Energies Nouvelles
Technical Solution: IFP Energies Nouvelles has developed advanced solid sorbents for CO2 capture through their ACACIA project, focusing on temperature swing adsorption (TSA) processes. Their technology utilizes functionalized mesoporous silica materials with amine groups that demonstrate high CO2 selectivity and adsorption capacity (up to 3-4 mmol/g) at moderate temperatures (40-80°C). The regeneration occurs at relatively low temperatures (110-130°C), making it energy-efficient compared to conventional amine scrubbing. Their process integration approach combines heat management systems that recover and utilize waste heat from industrial processes, reducing the overall energy penalty. IFPEN has successfully demonstrated this technology at pilot scale with real flue gas conditions, showing stable performance over multiple adsorption-desorption cycles with minimal degradation of capture capacity (less than 10% after 1000 cycles).
Strengths: Lower regeneration energy requirements compared to liquid amine systems; excellent stability over multiple cycles; good tolerance to moisture and impurities in flue gas. Weaknesses: Still requires significant heat input for regeneration; potential for amine leaching over extended operation; scaling up to industrial level remains challenging.
Key Patents and Innovations in CO2 Capture Materials
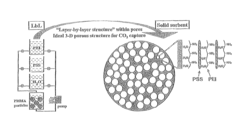
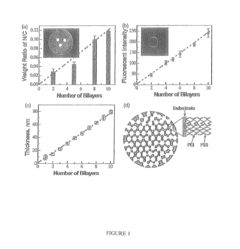
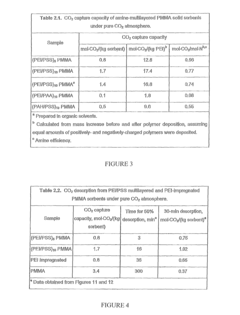
Layered solid sorbents for carbon dioxide capture
PatentActiveUS8889589B2
Innovation
- Development of nano-layered solid sorbents using electrostatic layer-by-layer nanoassembly, where positively charged polyethylenimine and negatively charged polystyrene sulfonate layers are alternately deposited on a porous substrate, enhancing CO2 capture and transport kinetics.
Environmental Impact Assessment of Different Sorbents
The environmental impact of sorbent materials for CO2 capture extends far beyond their primary function, encompassing their entire lifecycle from production to disposal. Traditional solid adsorbents such as zeolites, activated carbons, and metal-organic frameworks (MOFs) typically require energy-intensive manufacturing processes, with some requiring high-temperature activation or complex chemical synthesis that contributes significantly to their carbon footprint.
When comparing physical adsorbents with catalytic sorbents, notable differences emerge in their environmental profiles. Physical adsorbents often require frequent regeneration cycles involving temperature or pressure swings, leading to substantial energy consumption during operation. This regeneration energy penalty can partially offset the environmental benefits gained from CO2 capture, particularly when powered by fossil fuel sources.
Catalytic sorbents, while potentially offering more efficient CO2 conversion pathways, frequently incorporate rare earth elements or precious metals as active components. The mining and processing of these materials present serious environmental concerns including habitat destruction, water pollution, and high energy consumption. Additionally, the chemical stability of catalytic materials may deteriorate over time, necessitating more frequent replacement than some physical adsorbents.
Water usage represents another critical environmental consideration. Certain adsorbents, particularly zeolites and some amine-functionalized materials, demonstrate high affinity for water vapor, which can significantly reduce their CO2 capture efficiency in humid conditions and increase regeneration energy requirements. This water sensitivity translates to higher operational environmental impacts in real-world applications where flue gases typically contain moisture.
Land use impacts vary considerably between sorbent types. Large-scale implementation of solid sorbent technologies requires substantial infrastructure development, with physical adsorbents generally demanding larger installation footprints due to their lower CO2 capacity per unit volume compared to some catalytic alternatives.
Waste generation and end-of-life considerations reveal further environmental distinctions. Physical adsorbents typically demonstrate longer operational lifespans but present disposal challenges due to potential contaminant accumulation. Catalytic sorbents may require more frequent replacement but could offer better recyclability of valuable metal components, though often through energy-intensive recovery processes.
Recent lifecycle assessment studies indicate that next-generation bio-based adsorbents and metal-organic frameworks with reduced synthesis energy requirements show promise for minimizing environmental impacts while maintaining effective CO2 capture performance. These materials represent an important frontier in developing environmentally sustainable carbon capture solutions that minimize secondary environmental impacts while addressing the primary challenge of atmospheric CO2 reduction.
When comparing physical adsorbents with catalytic sorbents, notable differences emerge in their environmental profiles. Physical adsorbents often require frequent regeneration cycles involving temperature or pressure swings, leading to substantial energy consumption during operation. This regeneration energy penalty can partially offset the environmental benefits gained from CO2 capture, particularly when powered by fossil fuel sources.
Catalytic sorbents, while potentially offering more efficient CO2 conversion pathways, frequently incorporate rare earth elements or precious metals as active components. The mining and processing of these materials present serious environmental concerns including habitat destruction, water pollution, and high energy consumption. Additionally, the chemical stability of catalytic materials may deteriorate over time, necessitating more frequent replacement than some physical adsorbents.
Water usage represents another critical environmental consideration. Certain adsorbents, particularly zeolites and some amine-functionalized materials, demonstrate high affinity for water vapor, which can significantly reduce their CO2 capture efficiency in humid conditions and increase regeneration energy requirements. This water sensitivity translates to higher operational environmental impacts in real-world applications where flue gases typically contain moisture.
Land use impacts vary considerably between sorbent types. Large-scale implementation of solid sorbent technologies requires substantial infrastructure development, with physical adsorbents generally demanding larger installation footprints due to their lower CO2 capacity per unit volume compared to some catalytic alternatives.
Waste generation and end-of-life considerations reveal further environmental distinctions. Physical adsorbents typically demonstrate longer operational lifespans but present disposal challenges due to potential contaminant accumulation. Catalytic sorbents may require more frequent replacement but could offer better recyclability of valuable metal components, though often through energy-intensive recovery processes.
Recent lifecycle assessment studies indicate that next-generation bio-based adsorbents and metal-organic frameworks with reduced synthesis energy requirements show promise for minimizing environmental impacts while maintaining effective CO2 capture performance. These materials represent an important frontier in developing environmentally sustainable carbon capture solutions that minimize secondary environmental impacts while addressing the primary challenge of atmospheric CO2 reduction.
Economic Viability and Scalability Analysis
The economic viability of solid sorbents for CO2 capture depends significantly on several interconnected factors including material costs, operational expenses, energy requirements, and scalability potential. Traditional adsorption-based sorbents like zeolites, activated carbons, and metal-organic frameworks (MOFs) generally present lower initial investment costs compared to catalytic sorbents, with raw material costs ranging from $1-5/kg for activated carbons to $20-100/kg for specialized MOFs.
Operational economics reveal that adsorption-based systems typically consume 2.0-3.5 GJ/ton CO2 captured, while advanced catalytic sorbents may achieve 1.8-2.8 GJ/ton CO2, representing potential energy savings of 15-25%. However, this advantage is often offset by the higher replacement frequency of catalytic materials, which can degrade after 500-1000 cycles compared to 1000-3000 cycles for robust physical adsorbents.
Capital expenditure analysis indicates that large-scale adsorption systems require approximately $40-60 million for a facility processing 1 million tons of CO2 annually, whereas catalytic systems may cost $55-80 million for equivalent capacity due to more complex reactor designs and material handling requirements. The levelized cost of capture currently stands at $40-70/ton CO2 for adsorption systems versus $50-90/ton for catalytic approaches.
Scalability considerations strongly favor adsorption-based technologies, which benefit from established manufacturing processes and supply chains. Production capacity for common adsorbents like activated carbon exceeds 1.9 million tons annually worldwide, while specialized catalytic materials remain limited to thousands of tons. This manufacturing gap significantly impacts deployment timelines, with adsorption technologies capable of reaching gigaton-scale implementation 5-8 years faster than catalytic alternatives.
Market penetration analysis reveals that adsorption technologies currently dominate 78% of industrial CO2 capture installations, primarily due to their lower technical barriers and established operational protocols. However, catalytic sorbents are gaining momentum in high-value applications where their enhanced selectivity justifies premium pricing, particularly in biogas upgrading and hydrogen production sectors.
The economic inflection point where catalytic sorbents become competitive with traditional adsorbents appears to correlate with carbon pricing thresholds of approximately $75-90/ton CO2, suggesting that policy frameworks will significantly influence technology adoption patterns. As manufacturing scales increase and learning curves progress, this threshold is projected to decrease by 3-5% annually over the next decade.
Operational economics reveal that adsorption-based systems typically consume 2.0-3.5 GJ/ton CO2 captured, while advanced catalytic sorbents may achieve 1.8-2.8 GJ/ton CO2, representing potential energy savings of 15-25%. However, this advantage is often offset by the higher replacement frequency of catalytic materials, which can degrade after 500-1000 cycles compared to 1000-3000 cycles for robust physical adsorbents.
Capital expenditure analysis indicates that large-scale adsorption systems require approximately $40-60 million for a facility processing 1 million tons of CO2 annually, whereas catalytic systems may cost $55-80 million for equivalent capacity due to more complex reactor designs and material handling requirements. The levelized cost of capture currently stands at $40-70/ton CO2 for adsorption systems versus $50-90/ton for catalytic approaches.
Scalability considerations strongly favor adsorption-based technologies, which benefit from established manufacturing processes and supply chains. Production capacity for common adsorbents like activated carbon exceeds 1.9 million tons annually worldwide, while specialized catalytic materials remain limited to thousands of tons. This manufacturing gap significantly impacts deployment timelines, with adsorption technologies capable of reaching gigaton-scale implementation 5-8 years faster than catalytic alternatives.
Market penetration analysis reveals that adsorption technologies currently dominate 78% of industrial CO2 capture installations, primarily due to their lower technical barriers and established operational protocols. However, catalytic sorbents are gaining momentum in high-value applications where their enhanced selectivity justifies premium pricing, particularly in biogas upgrading and hydrogen production sectors.
The economic inflection point where catalytic sorbents become competitive with traditional adsorbents appears to correlate with carbon pricing thresholds of approximately $75-90/ton CO2, suggesting that policy frameworks will significantly influence technology adoption patterns. As manufacturing scales increase and learning curves progress, this threshold is projected to decrease by 3-5% annually over the next decade.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!