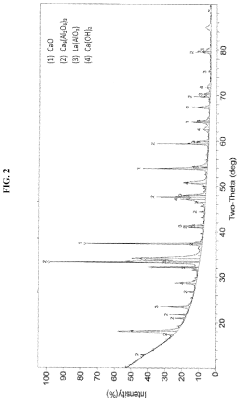
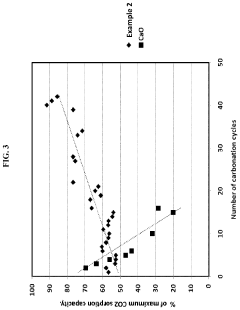
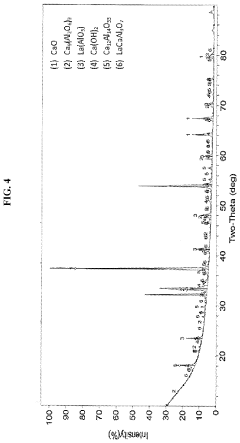
How chemical composition and functionalization improve Solid sorbents for CO2 capture performance
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Capture Sorbent Evolution and Objectives
Carbon dioxide capture technology has evolved significantly over the past decades, driven by the urgent need to mitigate climate change impacts. Initially, CO2 capture focused primarily on liquid amine-based absorption systems, which despite their effectiveness, presented challenges including high energy requirements for regeneration, corrosion issues, and solvent degradation. This prompted researchers to explore solid sorbents as alternative capture media, offering advantages in energy efficiency, stability, and operational flexibility.
The evolution of solid sorbents for CO2 capture can be traced through several developmental phases. Early research concentrated on conventional materials like activated carbons and zeolites, which demonstrated moderate CO2 adsorption capacities but suffered from selectivity limitations in mixed gas environments. The mid-2000s witnessed the emergence of metal-organic frameworks (MOFs) and covalent organic frameworks (COFs), representing a significant leap forward with their exceptional surface areas and tunable pore structures.
Recent advancements have focused on chemically modified solid sorbents, where base materials are functionalized with specific chemical groups to enhance CO2 capture performance. Amine-functionalized silica, for instance, combines the mechanical stability of silica with the chemical reactivity of amines toward CO2. Similarly, the incorporation of alkali metals into carbon-based sorbents has demonstrated remarkable improvements in selectivity and capacity.
The primary objective in solid sorbent development is achieving an optimal balance between adsorption capacity, selectivity, regeneration energy, and long-term stability. Current research aims to develop materials capable of capturing CO2 at concentrations ranging from dilute (400 ppm in direct air capture) to more concentrated streams (12-15% in power plant flue gases), while maintaining performance across multiple adsorption-desorption cycles.
Another critical goal is reducing the energy penalty associated with sorbent regeneration, which currently represents a significant portion of operational costs in carbon capture systems. Researchers are exploring novel regeneration methods, including pressure swing, temperature swing, and hybrid approaches, to minimize energy requirements while maintaining sorbent integrity over thousands of cycles.
Looking forward, the field is moving toward multifunctional sorbents that can simultaneously capture CO2 and convert it into value-added products, potentially transforming carbon capture from a cost center to a revenue-generating process. Additionally, there is growing interest in developing sorbents specifically tailored for challenging environments, such as humid conditions or in the presence of contaminants like SOx and NOx, which often degrade conventional materials.
The evolution of solid sorbents for CO2 capture can be traced through several developmental phases. Early research concentrated on conventional materials like activated carbons and zeolites, which demonstrated moderate CO2 adsorption capacities but suffered from selectivity limitations in mixed gas environments. The mid-2000s witnessed the emergence of metal-organic frameworks (MOFs) and covalent organic frameworks (COFs), representing a significant leap forward with their exceptional surface areas and tunable pore structures.
Recent advancements have focused on chemically modified solid sorbents, where base materials are functionalized with specific chemical groups to enhance CO2 capture performance. Amine-functionalized silica, for instance, combines the mechanical stability of silica with the chemical reactivity of amines toward CO2. Similarly, the incorporation of alkali metals into carbon-based sorbents has demonstrated remarkable improvements in selectivity and capacity.
The primary objective in solid sorbent development is achieving an optimal balance between adsorption capacity, selectivity, regeneration energy, and long-term stability. Current research aims to develop materials capable of capturing CO2 at concentrations ranging from dilute (400 ppm in direct air capture) to more concentrated streams (12-15% in power plant flue gases), while maintaining performance across multiple adsorption-desorption cycles.
Another critical goal is reducing the energy penalty associated with sorbent regeneration, which currently represents a significant portion of operational costs in carbon capture systems. Researchers are exploring novel regeneration methods, including pressure swing, temperature swing, and hybrid approaches, to minimize energy requirements while maintaining sorbent integrity over thousands of cycles.
Looking forward, the field is moving toward multifunctional sorbents that can simultaneously capture CO2 and convert it into value-added products, potentially transforming carbon capture from a cost center to a revenue-generating process. Additionally, there is growing interest in developing sorbents specifically tailored for challenging environments, such as humid conditions or in the presence of contaminants like SOx and NOx, which often degrade conventional materials.
Market Analysis for Carbon Capture Technologies
The global carbon capture and storage (CCS) market is experiencing significant growth, driven by increasing environmental concerns and stringent regulations aimed at reducing greenhouse gas emissions. As of 2023, the market was valued at approximately $7.3 billion and is projected to reach $15.2 billion by 2030, representing a compound annual growth rate (CAGR) of 11.5% during the forecast period.
Solid sorbents for CO2 capture represent a rapidly expanding segment within this market, with particular interest in chemically functionalized materials. This segment is expected to grow at a faster rate than the overall CCS market, with projections indicating a CAGR of 14.8% through 2030, driven by their superior performance characteristics and lower energy requirements compared to traditional liquid amine scrubbing technologies.
Regionally, North America currently dominates the solid sorbent market with approximately 38% market share, followed by Europe at 32% and Asia-Pacific at 24%. The Asia-Pacific region, particularly China and India, is expected to witness the highest growth rate due to increasing industrial activities and government initiatives to reduce carbon emissions from power plants and industrial facilities.
Key market drivers include increasingly stringent carbon emission regulations across major economies, growing corporate commitments to carbon neutrality, and substantial government funding for carbon capture research and deployment. The European Union's Carbon Border Adjustment Mechanism and the enhanced 45Q tax credits in the United States are creating strong financial incentives for CCS technology adoption.
Market challenges include high initial capital costs for implementation, energy penalties associated with the capture process, and competition from alternative carbon reduction strategies. The average cost of CO2 capture using current solid sorbent technologies ranges from $40-80 per ton, which remains a significant barrier to widespread adoption despite being lower than many alternative capture methods.
End-use sectors showing the strongest demand include power generation (36% of market share), cement production (22%), steel manufacturing (18%), and chemical processing (15%). These industries are under particular pressure to decarbonize their operations while maintaining production capacity.
Investment in solid sorbent technologies has seen remarkable growth, with venture capital funding increasing by 215% between 2020 and 2023. Strategic partnerships between technology developers and industrial end-users are becoming increasingly common, accelerating the commercialization pathway for novel sorbent materials with enhanced chemical compositions and functionalization.
Solid sorbents for CO2 capture represent a rapidly expanding segment within this market, with particular interest in chemically functionalized materials. This segment is expected to grow at a faster rate than the overall CCS market, with projections indicating a CAGR of 14.8% through 2030, driven by their superior performance characteristics and lower energy requirements compared to traditional liquid amine scrubbing technologies.
Regionally, North America currently dominates the solid sorbent market with approximately 38% market share, followed by Europe at 32% and Asia-Pacific at 24%. The Asia-Pacific region, particularly China and India, is expected to witness the highest growth rate due to increasing industrial activities and government initiatives to reduce carbon emissions from power plants and industrial facilities.
Key market drivers include increasingly stringent carbon emission regulations across major economies, growing corporate commitments to carbon neutrality, and substantial government funding for carbon capture research and deployment. The European Union's Carbon Border Adjustment Mechanism and the enhanced 45Q tax credits in the United States are creating strong financial incentives for CCS technology adoption.
Market challenges include high initial capital costs for implementation, energy penalties associated with the capture process, and competition from alternative carbon reduction strategies. The average cost of CO2 capture using current solid sorbent technologies ranges from $40-80 per ton, which remains a significant barrier to widespread adoption despite being lower than many alternative capture methods.
End-use sectors showing the strongest demand include power generation (36% of market share), cement production (22%), steel manufacturing (18%), and chemical processing (15%). These industries are under particular pressure to decarbonize their operations while maintaining production capacity.
Investment in solid sorbent technologies has seen remarkable growth, with venture capital funding increasing by 215% between 2020 and 2023. Strategic partnerships between technology developers and industrial end-users are becoming increasingly common, accelerating the commercialization pathway for novel sorbent materials with enhanced chemical compositions and functionalization.
Current Solid Sorbents Landscape and Technical Barriers
The current landscape of solid sorbents for CO2 capture is characterized by diverse material categories with varying performance characteristics. Physical adsorbents such as activated carbons and zeolites offer high surface areas and established manufacturing processes, but generally exhibit limited CO2 selectivity and capacity under practical conditions. Chemical adsorbents including amine-functionalized silicas and metal-organic frameworks (MOFs) demonstrate superior CO2 affinity but face stability challenges during multiple adsorption-desorption cycles.
Recent advancements in hybrid materials combining physical and chemical adsorption mechanisms have shown promising results, particularly in addressing the selectivity-capacity trade-off that has historically limited sorbent performance. However, these materials often require complex synthesis procedures that present significant barriers to large-scale production and commercial deployment.
The technical landscape is further complicated by the diversity of operating conditions across potential applications. Power plant flue gas typically contains 12-15% CO2 at atmospheric pressure, while direct air capture must address ultra-dilute CO2 concentrations of approximately 400 ppm. This variation necessitates application-specific sorbent optimization, creating additional complexity in material design and evaluation protocols.
Despite significant research progress, several critical technical barriers persist in solid sorbent development. The energy penalty associated with sorbent regeneration remains a primary challenge, with most current materials requiring substantial energy input for CO2 release, negatively impacting overall process efficiency. Material stability represents another major hurdle, as many high-performance sorbents exhibit degradation under realistic operating conditions involving moisture, SOx, NOx, and repeated thermal cycling.
Scalability concerns present additional obstacles, with many laboratory-developed materials utilizing expensive precursors or complex synthesis procedures that prove challenging to implement at industrial scale. The gap between laboratory performance metrics and real-world operation remains substantial, with many materials showing dramatically reduced performance when tested with actual flue gas or under dynamic flow conditions.
Cost considerations further constrain implementation, as economic viability requires materials with not only high technical performance but also reasonable production costs and operational lifetimes. Current estimates suggest that material costs must decrease significantly to achieve economically viable carbon capture processes, particularly for applications beyond high-concentration point sources.
The competitive landscape features increasing activity from both academic institutions and industrial players, with notable contributions from specialized materials companies and energy sector incumbents. Recent patent activity indicates growing interest in functionalized porous materials, particularly those incorporating novel amine configurations or metal sites designed for optimal CO2 interaction.
Recent advancements in hybrid materials combining physical and chemical adsorption mechanisms have shown promising results, particularly in addressing the selectivity-capacity trade-off that has historically limited sorbent performance. However, these materials often require complex synthesis procedures that present significant barriers to large-scale production and commercial deployment.
The technical landscape is further complicated by the diversity of operating conditions across potential applications. Power plant flue gas typically contains 12-15% CO2 at atmospheric pressure, while direct air capture must address ultra-dilute CO2 concentrations of approximately 400 ppm. This variation necessitates application-specific sorbent optimization, creating additional complexity in material design and evaluation protocols.
Despite significant research progress, several critical technical barriers persist in solid sorbent development. The energy penalty associated with sorbent regeneration remains a primary challenge, with most current materials requiring substantial energy input for CO2 release, negatively impacting overall process efficiency. Material stability represents another major hurdle, as many high-performance sorbents exhibit degradation under realistic operating conditions involving moisture, SOx, NOx, and repeated thermal cycling.
Scalability concerns present additional obstacles, with many laboratory-developed materials utilizing expensive precursors or complex synthesis procedures that prove challenging to implement at industrial scale. The gap between laboratory performance metrics and real-world operation remains substantial, with many materials showing dramatically reduced performance when tested with actual flue gas or under dynamic flow conditions.
Cost considerations further constrain implementation, as economic viability requires materials with not only high technical performance but also reasonable production costs and operational lifetimes. Current estimates suggest that material costs must decrease significantly to achieve economically viable carbon capture processes, particularly for applications beyond high-concentration point sources.
The competitive landscape features increasing activity from both academic institutions and industrial players, with notable contributions from specialized materials companies and energy sector incumbents. Recent patent activity indicates growing interest in functionalized porous materials, particularly those incorporating novel amine configurations or metal sites designed for optimal CO2 interaction.
Chemical Functionalization Approaches for Enhanced CO2 Adsorption
01 Metal-organic frameworks (MOFs) for CO2 capture
Metal-organic frameworks represent a promising class of solid sorbents for CO2 capture due to their high surface area, tunable pore size, and chemical functionality. These crystalline materials consist of metal ions or clusters coordinated to organic ligands, forming porous structures that can selectively adsorb CO2. MOFs can be designed with specific binding sites for enhanced CO2 selectivity and capacity, making them effective for post-combustion carbon capture applications.- Metal-organic frameworks (MOFs) for CO2 capture: Metal-organic frameworks (MOFs) are crystalline porous materials that have shown exceptional performance as solid sorbents for CO2 capture. These materials offer high surface area, tunable pore size, and chemical functionality that can be optimized for selective CO2 adsorption. MOFs can be designed with specific metal centers and organic linkers to enhance CO2 binding affinity and capacity, making them promising candidates for carbon capture applications in various industrial settings.
- Amine-functionalized sorbents for enhanced CO2 adsorption: Amine-functionalized solid sorbents demonstrate superior CO2 capture performance through chemical adsorption mechanisms. These materials incorporate various amine groups onto porous supports such as silica, activated carbon, or polymeric substrates, creating strong binding sites for CO2 molecules. The amine functionalization significantly increases CO2 selectivity and capacity under both dry and humid conditions, while also providing good regeneration properties at moderate temperatures, making them suitable for practical carbon capture applications.
- Zeolite-based materials for selective CO2 adsorption: Zeolites are crystalline aluminosilicate materials with well-defined pore structures that exhibit excellent performance as CO2 sorbents. Their molecular sieving properties allow for selective adsorption of CO2 over other gases based on molecular size and polarity. Zeolites can be modified through ion exchange, framework substitution, or surface functionalization to enhance their CO2 capture capacity and selectivity. These materials demonstrate good thermal stability and can be regenerated multiple times without significant loss of performance.
- Carbon-based sorbents for CO2 capture: Carbon-based materials, including activated carbon, carbon nanotubes, and graphene derivatives, serve as effective solid sorbents for CO2 capture. These materials offer advantages such as high surface area, tunable porosity, and excellent thermal stability. Surface modification techniques can enhance their CO2 adsorption capacity and selectivity. Carbon-based sorbents are particularly attractive due to their relatively low cost, abundance, and environmental compatibility, making them suitable for large-scale carbon capture applications.
- Regeneration and cyclic performance of CO2 sorbents: The regeneration capability and cyclic stability of solid sorbents are critical factors for practical CO2 capture applications. Various regeneration methods, including temperature swing adsorption (TSA), pressure swing adsorption (PSA), and vacuum swing adsorption (VSA), can be employed depending on the sorbent properties. Advanced sorbent materials are designed to maintain high CO2 capture capacity over multiple adsorption-desorption cycles while minimizing energy requirements for regeneration. Innovations in regeneration processes and sorbent stability significantly impact the overall efficiency and economic viability of carbon capture technologies.
02 Amine-functionalized solid sorbents
Amine-functionalized materials are widely used as solid sorbents for CO2 capture due to their strong chemical affinity for CO2 molecules. These sorbents typically consist of amines grafted onto high-surface-area supports such as silica, activated carbon, or polymeric substrates. The amine groups form carbamates or bicarbonates upon reaction with CO2, enabling efficient capture even at low CO2 concentrations. These materials offer advantages including high selectivity, good capacity, and the ability to operate at moderate temperatures.Expand Specific Solutions03 Zeolite-based CO2 capture systems
Zeolites are crystalline aluminosilicate materials with well-defined pore structures that make them effective for CO2 adsorption. Their molecular sieving properties allow for selective capture of CO2 from gas mixtures. The performance of zeolites for CO2 capture depends on factors such as the Si/Al ratio, cation type, and pore architecture. These materials offer benefits including high thermal stability, mechanical strength, and resistance to contaminants, making them suitable for industrial-scale carbon capture applications.Expand Specific Solutions04 Carbon-based sorbents for CO2 capture
Carbon-based materials, including activated carbon, carbon nanotubes, and graphene derivatives, serve as effective solid sorbents for CO2 capture. These materials can be engineered with high surface areas and tailored pore structures to enhance CO2 adsorption capacity. Carbon-based sorbents offer advantages such as low cost, high thermal stability, and ease of regeneration. Their performance can be further improved through surface functionalization or incorporation of metal nanoparticles to increase CO2 binding affinity.Expand Specific Solutions05 Regeneration methods for solid CO2 sorbents
Effective regeneration of solid sorbents is crucial for sustainable CO2 capture processes. Various methods are employed, including temperature swing adsorption (TSA), pressure swing adsorption (PSA), and vacuum swing adsorption (VSA). The choice of regeneration technique affects the energy requirements, cycle time, and overall efficiency of the capture system. Advanced regeneration approaches, such as microwave-assisted desorption or steam stripping, can significantly reduce the energy penalty associated with sorbent regeneration while maintaining sorbent performance over multiple capture-release cycles.Expand Specific Solutions
Leading Organizations in Solid Sorbent Research
The solid sorbent CO2 capture technology landscape is evolving rapidly, currently positioned in the early commercialization phase with an estimated market size of $2-3 billion, projected to grow significantly as carbon capture becomes essential for climate goals. Technical maturity varies across players, with research institutions like Arizona State University, Zhejiang University, and Dalian Institute focusing on fundamental chemical composition improvements. Commercial entities demonstrate different specialization levels: Climeworks AG has deployed operational direct air capture plants, while energy companies like Korea Electric Power Corp and its subsidiaries are integrating carbon capture into power generation. Industrial players including BASF, Wacker Chemie, and Corning are advancing material functionalization, while specialized firms like Global Thermostat and Susteon are developing proprietary sorbent technologies with enhanced CO2 affinity and regeneration capabilities.
Dalian Institute of Chemical Physics of CAS
Technical Solution: The Dalian Institute has pioneered innovative solid sorbents for CO2 capture through precise molecular engineering of porous materials. Their research focuses on developing polyethyleneimine (PEI)-impregnated mesoporous silica with controlled pore structures that achieve CO2 adsorption capacities up to 5.6 mmol/g under simulated flue gas conditions. Their scientists have systematically investigated the relationship between amine loading density, pore size distribution, and capture performance, establishing optimal parameters for maximizing CO2 uptake while maintaining rapid kinetics. The institute has also developed novel composite materials combining alkali metal carbonates with transition metal oxides that demonstrate exceptional stability through hundreds of adsorption-desorption cycles. Their recent work explores the incorporation of ionic liquids into porous frameworks, creating hybrid materials that combine the selectivity of chemical absorption with the structural advantages of solid supports.
Strengths: Cutting-edge fundamental research capabilities; systematic approach to structure-property relationships; strong focus on understanding molecular-level interactions for rational design. Weaknesses: Some materials still face challenges in scaling to industrial production; potential cost barriers for complex synthesized materials; regeneration energy requirements remain a challenge for certain sorbent classes.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed proprietary solid sorbent technologies for CO2 capture focusing on practical industrial applications. Their approach centers on amine-modified activated carbon materials and zeolite-based molecular sieves with tailored pore structures. Sinopec's research has yielded composite sorbents that combine high surface area carbon substrates with carefully selected amine functional groups, achieving CO2 capacities of 3-4 mmol/g under typical flue gas conditions. Their materials are engineered for mechanical stability in pressure swing adsorption systems, withstanding thousands of pressurization cycles without significant degradation. Sinopec has also pioneered the development of alkali-metal-based double salt sorbents that operate effectively at elevated temperatures (300-500°C), making them suitable for integration with high-temperature industrial processes. These materials demonstrate rapid sorption kinetics and can be regenerated at relatively low energy penalties compared to conventional liquid amine systems.
Strengths: Materials designed specifically for industrial-scale implementation; focus on practical aspects like mechanical stability and regeneration efficiency; extensive testing under realistic conditions. Weaknesses: Some materials show performance degradation in the presence of SOx and NOx contaminants; higher temperature sorbents may face challenges with thermal management; regeneration energy requirements still present economic barriers.
Key Innovations in Sorbent Composition and Structure
Solid sorbent materials functionalized with polyamines having oxygen-containing units selected from carbonyl units, hydroxyl units, and combinations thereof
PatentPendingUS20250288970A1
Innovation
- Development of solid sorbent materials functionalized with polyamines having oxygen-containing units such as carbonyl and hydroxyl units, which exhibit high adsorption capacity for carbon dioxide and low adsorption for water, with low desorption residuals under mild conditions.
Composition and Process for Capturing Carbon Dioxide
PatentInactiveUS20200009527A1
Innovation
- A novel solid sorbent composition comprising calcium oxide, calcium aluminate, and a mixed metal oxide with a perovskite crystalline structure, which is synthesized through a specific process involving milling, drying, and calcination, enabling efficient carbon dioxide capture and regeneration with improved sorbent capacity and attrition resistance across multiple cycles.
Environmental Impact Assessment of Sorbent Materials
The environmental impact of solid sorbents for CO2 capture extends beyond their primary function of carbon sequestration. A comprehensive assessment reveals that while these materials contribute positively to climate change mitigation, their production, use, and disposal present complex environmental considerations that must be carefully evaluated.
The life cycle analysis of solid sorbents demonstrates varying ecological footprints depending on their chemical composition. Amine-functionalized materials, while highly effective for CO2 capture, often require energy-intensive synthesis processes and potentially toxic precursors. Studies indicate that the environmental burden of manufacturing these sorbents can partially offset their carbon reduction benefits, particularly when production involves high-temperature calcination or extensive chemical processing.
Water consumption represents another significant environmental factor, especially for zeolites and metal-organic frameworks (MOFs) that may require substantial water volumes during synthesis and regeneration cycles. This becomes particularly problematic in water-stressed regions where industrial water usage competes with agricultural and municipal needs.
The degradation of sorbent materials over multiple capture-regeneration cycles introduces additional environmental concerns. Chemical modifications that enhance CO2 capture performance often involve compounds that may leach into the environment during use or disposal. Research indicates that functionalized silica materials typically demonstrate lower toxicity profiles compared to certain metal-based sorbents that may release heavy metals under specific conditions.
End-of-life management of spent sorbents presents both challenges and opportunities. While some materials can be regenerated and reused multiple times, others become hazardous waste requiring specialized disposal. Recent innovations in green chemistry approaches have yielded promising bio-based sorbents with enhanced biodegradability, potentially reducing long-term environmental impacts.
Energy requirements for sorbent regeneration constitute a significant portion of the overall environmental footprint. Advanced functionalization techniques that lower regeneration temperatures can substantially reduce operational energy demands, improving the net environmental benefit of capture systems. Studies demonstrate that optimized chemical compositions can reduce regeneration energy by up to 30% compared to first-generation materials.
The geographical context of sorbent deployment also influences environmental impact assessments. Proximity to renewable energy sources can significantly improve the sustainability profile of energy-intensive sorbent systems, while local regulations on chemical use and waste management shape implementation strategies across different regions.
The life cycle analysis of solid sorbents demonstrates varying ecological footprints depending on their chemical composition. Amine-functionalized materials, while highly effective for CO2 capture, often require energy-intensive synthesis processes and potentially toxic precursors. Studies indicate that the environmental burden of manufacturing these sorbents can partially offset their carbon reduction benefits, particularly when production involves high-temperature calcination or extensive chemical processing.
Water consumption represents another significant environmental factor, especially for zeolites and metal-organic frameworks (MOFs) that may require substantial water volumes during synthesis and regeneration cycles. This becomes particularly problematic in water-stressed regions where industrial water usage competes with agricultural and municipal needs.
The degradation of sorbent materials over multiple capture-regeneration cycles introduces additional environmental concerns. Chemical modifications that enhance CO2 capture performance often involve compounds that may leach into the environment during use or disposal. Research indicates that functionalized silica materials typically demonstrate lower toxicity profiles compared to certain metal-based sorbents that may release heavy metals under specific conditions.
End-of-life management of spent sorbents presents both challenges and opportunities. While some materials can be regenerated and reused multiple times, others become hazardous waste requiring specialized disposal. Recent innovations in green chemistry approaches have yielded promising bio-based sorbents with enhanced biodegradability, potentially reducing long-term environmental impacts.
Energy requirements for sorbent regeneration constitute a significant portion of the overall environmental footprint. Advanced functionalization techniques that lower regeneration temperatures can substantially reduce operational energy demands, improving the net environmental benefit of capture systems. Studies demonstrate that optimized chemical compositions can reduce regeneration energy by up to 30% compared to first-generation materials.
The geographical context of sorbent deployment also influences environmental impact assessments. Proximity to renewable energy sources can significantly improve the sustainability profile of energy-intensive sorbent systems, while local regulations on chemical use and waste management shape implementation strategies across different regions.
Scalability and Industrial Implementation Challenges
The scaling of solid sorbent technologies for CO2 capture from laboratory to industrial scale presents significant challenges that must be addressed for successful implementation. Current pilot-scale demonstrations have shown promising results, but the transition to full commercial scale requires overcoming several critical barriers. Material production represents a primary challenge, as manufacturing high-performance functionalized sorbents at industrial quantities demands consistent quality control and cost-effective synthesis methods that may not directly translate from laboratory procedures.
Equipment design and engineering considerations present another major hurdle. Industrial implementation requires specialized contactors, regeneration systems, and handling equipment that can maintain optimal performance while processing large gas volumes. The physical properties of solid sorbents, including particle size distribution, mechanical strength, and attrition resistance, become increasingly critical at larger scales where material losses can significantly impact operational economics.
Process integration challenges emerge when incorporating CO2 capture systems into existing industrial facilities. Heat management during adsorption-desorption cycles requires careful engineering to minimize energy penalties, while pressure drop considerations across packed beds or fluidized systems must be optimized to reduce parasitic energy consumption. Additionally, the integration of capture systems with downstream CO2 compression, transportation, and storage infrastructure necessitates comprehensive system engineering.
Economic viability remains a decisive factor in industrial implementation. The capital expenditure for large-scale solid sorbent systems must compete with established technologies, while operational costs—including sorbent replacement due to degradation, energy requirements for regeneration, and maintenance—significantly influence the overall cost per ton of CO2 captured. Current estimates suggest costs ranging from $50-120 per ton of CO2, which must be further reduced to enable widespread adoption.
Regulatory frameworks and environmental considerations also impact scalability. Permitting requirements, safety protocols for handling large quantities of potentially hazardous materials, and environmental impact assessments for waste streams all influence implementation timelines and costs. Furthermore, the environmental footprint of sorbent production itself must be evaluated to ensure net carbon reduction benefits.
Technology readiness represents the culmination of these challenges. While laboratory studies demonstrate impressive CO2 capture performance through chemical composition optimization and functionalization, the technology readiness level (TRL) for most advanced solid sorbents remains between 4-6, indicating significant development is still required before full commercial deployment. Bridging this gap requires coordinated efforts between material scientists, chemical engineers, equipment manufacturers, and end-users to develop integrated solutions that maintain the performance advantages of advanced sorbents while addressing practical implementation challenges.
Equipment design and engineering considerations present another major hurdle. Industrial implementation requires specialized contactors, regeneration systems, and handling equipment that can maintain optimal performance while processing large gas volumes. The physical properties of solid sorbents, including particle size distribution, mechanical strength, and attrition resistance, become increasingly critical at larger scales where material losses can significantly impact operational economics.
Process integration challenges emerge when incorporating CO2 capture systems into existing industrial facilities. Heat management during adsorption-desorption cycles requires careful engineering to minimize energy penalties, while pressure drop considerations across packed beds or fluidized systems must be optimized to reduce parasitic energy consumption. Additionally, the integration of capture systems with downstream CO2 compression, transportation, and storage infrastructure necessitates comprehensive system engineering.
Economic viability remains a decisive factor in industrial implementation. The capital expenditure for large-scale solid sorbent systems must compete with established technologies, while operational costs—including sorbent replacement due to degradation, energy requirements for regeneration, and maintenance—significantly influence the overall cost per ton of CO2 captured. Current estimates suggest costs ranging from $50-120 per ton of CO2, which must be further reduced to enable widespread adoption.
Regulatory frameworks and environmental considerations also impact scalability. Permitting requirements, safety protocols for handling large quantities of potentially hazardous materials, and environmental impact assessments for waste streams all influence implementation timelines and costs. Furthermore, the environmental footprint of sorbent production itself must be evaluated to ensure net carbon reduction benefits.
Technology readiness represents the culmination of these challenges. While laboratory studies demonstrate impressive CO2 capture performance through chemical composition optimization and functionalization, the technology readiness level (TRL) for most advanced solid sorbents remains between 4-6, indicating significant development is still required before full commercial deployment. Bridging this gap requires coordinated efforts between material scientists, chemical engineers, equipment manufacturers, and end-users to develop integrated solutions that maintain the performance advantages of advanced sorbents while addressing practical implementation challenges.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!