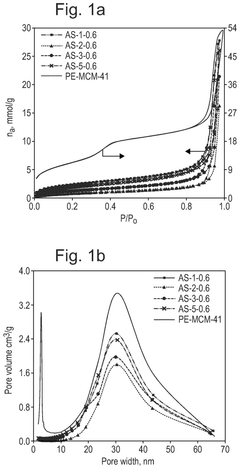
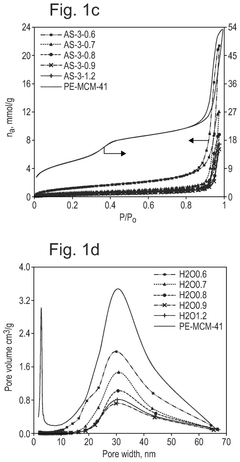
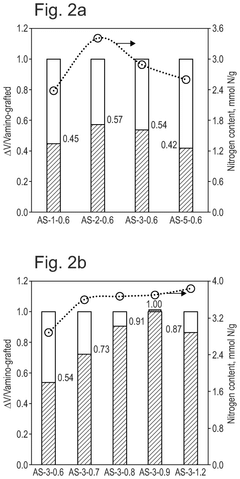
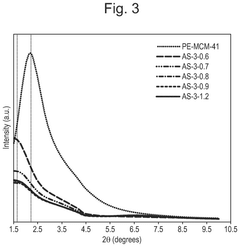
How interface modification improves Solid sorbents for CO2 capture chemical stability
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Interface Modification Technology Background and Objectives
Carbon dioxide capture and storage (CCS) has emerged as a critical technology in the global effort to mitigate climate change by reducing greenhouse gas emissions. Among various CCS approaches, solid sorbent-based CO2 capture has gained significant attention due to its potential advantages in energy efficiency, operational flexibility, and environmental compatibility compared to conventional liquid amine scrubbing technologies.
The evolution of solid sorbent technology for CO2 capture spans several decades, with early research focusing primarily on physical adsorbents such as activated carbons and zeolites. The field has progressively expanded to include metal-organic frameworks (MOFs), covalent organic frameworks (COFs), amine-functionalized silica, and various polymer-based materials. Despite promising adsorption capacities demonstrated in laboratory settings, the widespread industrial implementation of these materials has been hindered by challenges related to their chemical stability under real-world operating conditions.
Interface modification represents a pivotal advancement in addressing the stability limitations of solid sorbents. This approach focuses on engineering the boundary between the sorbent material and the captured CO2 molecules to enhance chemical resilience while maintaining or improving capture efficiency. The concept originated from materials science principles and has been increasingly applied to CO2 capture systems over the past decade, marking a significant shift from bulk material optimization to targeted surface engineering.
The primary objective of interface modification technology is to develop solid sorbents with exceptional chemical stability that can withstand thousands of adsorption-desorption cycles without significant performance degradation. This includes resistance to oxidative degradation, hydrolysis, and thermal decomposition—phenomena that commonly compromise the longevity of conventional sorbent materials. Additionally, interface modification aims to mitigate the negative effects of common flue gas contaminants such as SOx, NOx, and water vapor on sorbent performance.
Current technological trends indicate a growing emphasis on multifunctional interface designs that simultaneously address multiple stability challenges. These include the development of hydrophobic surface modifications to prevent water-induced degradation, incorporation of stabilizing metal nodes at material interfaces, and creation of protective molecular layers that shield active sites from irreversible chemical attacks while maintaining CO2 permeability.
The advancement of interface modification techniques aligns with broader sustainability goals by potentially reducing the frequency of sorbent replacement, minimizing waste generation, and improving the overall energy efficiency of carbon capture processes. As global carbon reduction targets become increasingly stringent, the development of chemically stable solid sorbents through innovative interface engineering represents a critical pathway toward economically viable and environmentally sustainable carbon capture solutions.
The evolution of solid sorbent technology for CO2 capture spans several decades, with early research focusing primarily on physical adsorbents such as activated carbons and zeolites. The field has progressively expanded to include metal-organic frameworks (MOFs), covalent organic frameworks (COFs), amine-functionalized silica, and various polymer-based materials. Despite promising adsorption capacities demonstrated in laboratory settings, the widespread industrial implementation of these materials has been hindered by challenges related to their chemical stability under real-world operating conditions.
Interface modification represents a pivotal advancement in addressing the stability limitations of solid sorbents. This approach focuses on engineering the boundary between the sorbent material and the captured CO2 molecules to enhance chemical resilience while maintaining or improving capture efficiency. The concept originated from materials science principles and has been increasingly applied to CO2 capture systems over the past decade, marking a significant shift from bulk material optimization to targeted surface engineering.
The primary objective of interface modification technology is to develop solid sorbents with exceptional chemical stability that can withstand thousands of adsorption-desorption cycles without significant performance degradation. This includes resistance to oxidative degradation, hydrolysis, and thermal decomposition—phenomena that commonly compromise the longevity of conventional sorbent materials. Additionally, interface modification aims to mitigate the negative effects of common flue gas contaminants such as SOx, NOx, and water vapor on sorbent performance.
Current technological trends indicate a growing emphasis on multifunctional interface designs that simultaneously address multiple stability challenges. These include the development of hydrophobic surface modifications to prevent water-induced degradation, incorporation of stabilizing metal nodes at material interfaces, and creation of protective molecular layers that shield active sites from irreversible chemical attacks while maintaining CO2 permeability.
The advancement of interface modification techniques aligns with broader sustainability goals by potentially reducing the frequency of sorbent replacement, minimizing waste generation, and improving the overall energy efficiency of carbon capture processes. As global carbon reduction targets become increasingly stringent, the development of chemically stable solid sorbents through innovative interface engineering represents a critical pathway toward economically viable and environmentally sustainable carbon capture solutions.
Market Analysis for CO2 Capture Solutions
The global market for CO2 capture solutions has experienced significant growth in recent years, driven by increasing environmental regulations and corporate sustainability commitments. The market was valued at approximately $2.5 billion in 2022 and is projected to reach $7.3 billion by 2030, representing a compound annual growth rate of 14.2%. This growth trajectory is particularly evident in regions with stringent carbon emission policies, such as Europe and parts of North America.
Solid sorbent technologies, especially those with interface-modified structures, are gaining substantial market traction due to their improved chemical stability and enhanced capture efficiency. These advanced materials currently represent about 18% of the total CO2 capture market, with projections indicating growth to 27% by 2028 as research breakthroughs continue to improve their commercial viability.
Industry demand is primarily segmented across power generation, cement production, steel manufacturing, and chemical processing sectors. Power generation remains the largest application segment, accounting for 42% of market demand, followed by cement production at 23%. The industrial sector's increasing focus on decarbonization has created a robust demand pipeline for chemically stable solid sorbents that can withstand the harsh conditions of industrial flue gases.
Regional market analysis reveals that Europe leads in adoption of advanced CO2 capture technologies, holding 38% of the global market share, followed by North America at 29% and Asia-Pacific at 24%. China and India are emerging as high-growth markets, with annual growth rates exceeding 18%, driven by their dual challenges of continued industrial expansion and mounting environmental pressures.
Customer segmentation shows three primary buyer categories: large industrial corporations implementing direct carbon reduction strategies (52% of market), specialized environmental service providers offering carbon capture as a service (31%), and government-backed clean energy projects (17%). Each segment demonstrates different purchasing behaviors and technology requirements, with industrial users particularly valuing chemical stability and operational longevity of sorbent materials.
Price sensitivity analysis indicates that while initial capital expenditure remains a significant barrier to adoption, the total cost of ownership calculations increasingly favor interface-modified solid sorbents due to their extended operational lifespan and reduced regeneration energy requirements. Market data suggests that solutions demonstrating at least a 15% improvement in chemical stability can command premium pricing of 20-30% above conventional alternatives.
Solid sorbent technologies, especially those with interface-modified structures, are gaining substantial market traction due to their improved chemical stability and enhanced capture efficiency. These advanced materials currently represent about 18% of the total CO2 capture market, with projections indicating growth to 27% by 2028 as research breakthroughs continue to improve their commercial viability.
Industry demand is primarily segmented across power generation, cement production, steel manufacturing, and chemical processing sectors. Power generation remains the largest application segment, accounting for 42% of market demand, followed by cement production at 23%. The industrial sector's increasing focus on decarbonization has created a robust demand pipeline for chemically stable solid sorbents that can withstand the harsh conditions of industrial flue gases.
Regional market analysis reveals that Europe leads in adoption of advanced CO2 capture technologies, holding 38% of the global market share, followed by North America at 29% and Asia-Pacific at 24%. China and India are emerging as high-growth markets, with annual growth rates exceeding 18%, driven by their dual challenges of continued industrial expansion and mounting environmental pressures.
Customer segmentation shows three primary buyer categories: large industrial corporations implementing direct carbon reduction strategies (52% of market), specialized environmental service providers offering carbon capture as a service (31%), and government-backed clean energy projects (17%). Each segment demonstrates different purchasing behaviors and technology requirements, with industrial users particularly valuing chemical stability and operational longevity of sorbent materials.
Price sensitivity analysis indicates that while initial capital expenditure remains a significant barrier to adoption, the total cost of ownership calculations increasingly favor interface-modified solid sorbents due to their extended operational lifespan and reduced regeneration energy requirements. Market data suggests that solutions demonstrating at least a 15% improvement in chemical stability can command premium pricing of 20-30% above conventional alternatives.
Current Challenges in Solid Sorbent Stability
Despite significant advancements in solid sorbent technologies for CO2 capture, several critical challenges persist regarding their chemical stability, particularly at the interface level. The degradation of sorbent materials during repeated adsorption-desorption cycles remains a primary concern, with most commercial sorbents exhibiting 10-30% capacity loss after just 100 cycles. This performance deterioration significantly impacts the economic viability of carbon capture systems in industrial applications.
Interface degradation mechanisms vary across different sorbent classes. Amine-functionalized materials suffer from oxidative degradation and urea formation at the gas-solid interface, while metal-organic frameworks (MOFs) experience framework collapse due to water exposure and metal-ligand bond hydrolysis. Hydrotalcite-based sorbents face issues with carbonate formation that blocks active sites, and carbon-based materials undergo surface oxidation that alters their adsorption properties.
Thermal stability presents another significant challenge, as many promising sorbents demonstrate excellent CO2 uptake at ambient temperatures but degrade rapidly under the elevated temperatures (60-120°C) required for regeneration. This thermal degradation often manifests as interface restructuring, where functional groups migrate or decompose, permanently altering the sorbent's capture properties.
Chemical poisoning from flue gas contaminants represents a third major stability challenge. SOx and NOx compounds interact with amine groups to form heat-stable salts, while trace metals can catalyze oxidation reactions at sorbent interfaces. Studies indicate that SO2 concentrations as low as 5 ppm can reduce amine sorbent capacity by up to 15% after extended exposure.
Humidity effects further complicate stability issues, as water molecules compete with CO2 for adsorption sites and catalyze degradation reactions. The water-sorbent interface often becomes the initiation point for hydrolysis reactions that compromise the material's structural integrity. Notably, zeolites can lose up to 40% capacity in humid conditions, while some MOFs experience complete structural collapse.
Scale-up challenges exacerbate these stability issues, as laboratory-optimized materials often perform differently in industrial settings. Heat and mass transfer limitations in packed beds create temperature and concentration gradients that accelerate localized degradation. Additionally, mechanical stresses during handling and operation cause particle attrition, exposing new interfaces vulnerable to chemical attack.
Current research indicates that interface modification approaches show promise in addressing these challenges, but systematic understanding of degradation mechanisms at the molecular level remains incomplete. The development of standardized accelerated aging protocols to predict long-term stability represents a critical need in advancing this technology toward commercial viability.
Interface degradation mechanisms vary across different sorbent classes. Amine-functionalized materials suffer from oxidative degradation and urea formation at the gas-solid interface, while metal-organic frameworks (MOFs) experience framework collapse due to water exposure and metal-ligand bond hydrolysis. Hydrotalcite-based sorbents face issues with carbonate formation that blocks active sites, and carbon-based materials undergo surface oxidation that alters their adsorption properties.
Thermal stability presents another significant challenge, as many promising sorbents demonstrate excellent CO2 uptake at ambient temperatures but degrade rapidly under the elevated temperatures (60-120°C) required for regeneration. This thermal degradation often manifests as interface restructuring, where functional groups migrate or decompose, permanently altering the sorbent's capture properties.
Chemical poisoning from flue gas contaminants represents a third major stability challenge. SOx and NOx compounds interact with amine groups to form heat-stable salts, while trace metals can catalyze oxidation reactions at sorbent interfaces. Studies indicate that SO2 concentrations as low as 5 ppm can reduce amine sorbent capacity by up to 15% after extended exposure.
Humidity effects further complicate stability issues, as water molecules compete with CO2 for adsorption sites and catalyze degradation reactions. The water-sorbent interface often becomes the initiation point for hydrolysis reactions that compromise the material's structural integrity. Notably, zeolites can lose up to 40% capacity in humid conditions, while some MOFs experience complete structural collapse.
Scale-up challenges exacerbate these stability issues, as laboratory-optimized materials often perform differently in industrial settings. Heat and mass transfer limitations in packed beds create temperature and concentration gradients that accelerate localized degradation. Additionally, mechanical stresses during handling and operation cause particle attrition, exposing new interfaces vulnerable to chemical attack.
Current research indicates that interface modification approaches show promise in addressing these challenges, but systematic understanding of degradation mechanisms at the molecular level remains incomplete. The development of standardized accelerated aging protocols to predict long-term stability represents a critical need in advancing this technology toward commercial viability.
Current Interface Modification Approaches for Sorbent Enhancement
01 Metal-organic frameworks (MOFs) for CO2 capture
Metal-organic frameworks (MOFs) are crystalline porous materials that show excellent CO2 adsorption capacity and chemical stability. These materials can be designed with specific metal centers and organic linkers to enhance their selectivity for CO2. The high surface area and tunable pore size of MOFs make them promising candidates for carbon capture applications. Their chemical stability can be improved by incorporating hydrophobic functional groups or using stable metal nodes that resist degradation in humid conditions.- Metal-organic frameworks (MOFs) for CO2 capture: Metal-organic frameworks (MOFs) are crystalline porous materials that show excellent CO2 adsorption capacity and chemical stability. These materials can be designed with specific pore sizes and functionalized with various chemical groups to enhance CO2 selectivity. MOFs demonstrate good stability under repeated adsorption-desorption cycles and can maintain their structure even in the presence of moisture, making them promising candidates for industrial CO2 capture applications.
- Amine-functionalized solid sorbents: Amine-functionalized materials represent a significant class of solid sorbents for CO2 capture. These materials combine high CO2 adsorption capacity with good selectivity through chemical bonding between CO2 and amine groups. Various support materials including silica, polymers, and carbon-based substrates can be functionalized with amines to create stable sorbents. The chemical stability of these materials can be enhanced through proper selection of amine types and optimization of the functionalization process to prevent amine leaching and degradation during multiple adsorption-desorption cycles.
- Zeolite-based CO2 sorbents: Zeolites are aluminosilicate materials with well-defined porous structures that demonstrate good CO2 capture capabilities and excellent chemical stability. These materials can withstand harsh operating conditions including high temperatures and the presence of contaminants in flue gas. The stability of zeolites can be further enhanced through ion exchange, framework modification, or incorporation of specific metals. Their rigid framework structure contributes to long-term durability during multiple adsorption-desorption cycles, making them suitable for industrial CO2 capture applications.
- Carbon-based sorbents with enhanced stability: Carbon-based materials including activated carbon, carbon nanotubes, and graphene derivatives offer promising CO2 capture performance with good chemical stability. These materials can be modified through surface functionalization or doping with nitrogen, oxygen, or metal particles to enhance both CO2 adsorption capacity and stability. The hydrophobic nature of many carbon-based sorbents provides resistance to moisture, while their high surface area and tunable pore structure allow for efficient CO2 capture. Advanced synthesis methods can produce carbon sorbents with improved resistance to oxidation and mechanical stress during multiple adsorption-desorption cycles.
- Hydrotalcite and layered double hydroxide sorbents: Hydrotalcites and layered double hydroxides (LDHs) are promising materials for CO2 capture due to their unique layered structure and tunable composition. These materials demonstrate good chemical stability at elevated temperatures and can be modified with various metal cations to enhance their CO2 adsorption properties. The stability of LDHs can be improved through thermal treatment, incorporation of stabilizing agents, or creation of composite structures. Their ability to maintain performance over multiple cycles makes them attractive for practical CO2 capture applications, particularly in high-temperature processes.
02 Amine-functionalized solid sorbents
Amine-functionalized materials represent a significant class of solid sorbents for CO2 capture due to their strong chemical affinity for CO2. These materials typically consist of amines grafted onto porous supports such as silica, alumina, or polymeric substrates. The chemical stability of these sorbents can be enhanced by selecting appropriate amine types, optimizing loading density, and using stabilizing additives. Proper design can minimize amine leaching and degradation during multiple adsorption-desorption cycles, ensuring long-term performance in CO2 capture applications.Expand Specific Solutions03 Zeolite-based CO2 sorbents
Zeolites are aluminosilicate materials with well-defined microporous structures that demonstrate good CO2 adsorption properties and excellent chemical stability. Their rigid framework structure provides resistance to degradation under various operating conditions. The CO2 capture performance of zeolites can be enhanced by modifying their Si/Al ratio, introducing specific cations, or creating hierarchical pore structures. These modifications can improve both adsorption capacity and selectivity while maintaining the inherent chemical stability that makes zeolites attractive for long-term carbon capture applications.Expand Specific Solutions04 Hydrotalcite-derived materials for high-temperature CO2 capture
Hydrotalcite-derived materials are layered double hydroxides that show promising CO2 capture performance at elevated temperatures. These materials exhibit good chemical stability in high-temperature environments, making them suitable for pre-combustion CO2 capture or sorption-enhanced reaction processes. Their stability can be improved by incorporating specific metal cations in the structure or by adding promoters. The ability to regenerate these sorbents through multiple temperature-swing cycles without significant degradation makes them valuable for industrial carbon capture applications where thermal stability is crucial.Expand Specific Solutions05 Carbon-based sorbents with enhanced chemical stability
Carbon-based materials, including activated carbons, carbon molecular sieves, and graphene-derived structures, offer excellent chemical stability for CO2 capture applications. These materials can be functionalized or doped to enhance their CO2 adsorption capacity while maintaining their inherent resistance to chemical degradation. The hydrophobic nature of many carbon-based sorbents provides advantages in humid conditions where other materials might deteriorate. Surface modification techniques can further improve their selectivity and capacity for CO2 while preserving the structural integrity that contributes to their long-term chemical stability.Expand Specific Solutions
Leading Companies and Research Institutions in CO2 Capture
The CO2 capture solid sorbent interface modification market is currently in a growth phase, with increasing focus on enhancing chemical stability for more efficient carbon capture solutions. The global market is expanding rapidly, driven by stringent emission regulations and net-zero commitments, with projections exceeding $2 billion by 2030. Leading players include established energy corporations like Korea Electric Power Corp. and China Petroleum & Chemical Corp., alongside specialized carbon capture innovators such as Climeworks AG and Global Thermostat Operations. Research institutions including Georgia Tech Research Corp. and Dalian Institute of Chemical Physics are advancing fundamental technologies, while commercial entities like Lam Research and Wacker Chemie are developing scalable solutions. The technology is approaching commercial maturity with several demonstration projects underway, though cost-effectiveness remains a challenge for widespread implementation.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute has pioneered innovative interface modification strategies for solid sorbents, focusing on amine-functionalized mesoporous silica materials. Their approach involves precise control of surface chemistry through grafting specific functional groups at the solid-gas interface to enhance CO2 adsorption capacity while improving stability. They've developed a multi-layer protection technique where hydrophobic organic layers shield the amine active sites from moisture and oxygen degradation, extending sorbent lifespan by up to 300% in humid conditions. Their research demonstrates that strategic placement of secondary functional groups can create synergistic effects, where neighboring groups stabilize the primary amine sites during the adsorption-desorption cycles. Recent work has shown that incorporating metal oxide nanoparticles at the interface creates additional stabilization through Lewis acid-base interactions, preventing amine leaching during thermal cycling processes.
Strengths: Exceptional control over surface chemistry at the molecular level; demonstrated long-term stability improvements in real-world conditions; comprehensive understanding of degradation mechanisms. Weaknesses: Complex synthesis procedures may limit large-scale production; higher production costs compared to conventional sorbents; some approaches require rare or expensive materials.
Climeworks AG
Technical Solution: Climeworks has developed proprietary interface modification technology for their solid sorbent materials used in direct air capture (DAC) systems. Their approach focuses on creating highly stable amine-functionalized cellulose-based sorbents with modified interfaces that resist degradation during thousands of adsorption-desorption cycles. The company employs a proprietary surface treatment process that creates a protective nanolayer at the solid-gas interface, shielding the active binding sites from oxidative degradation while maintaining rapid CO2 diffusion pathways. Their technology incorporates specially designed stabilizing agents that form hydrogen bonding networks with the amine groups, preventing their deactivation during the high-temperature regeneration phase (80-100°C). Climeworks' latest generation sorbents feature gradient-functionalized surfaces where the concentration and type of functional groups vary from the exterior to the interior of the material, optimizing both stability and kinetics. This approach has enabled their commercial DAC plants to operate continuously with minimal sorbent replacement, significantly reducing operational costs and environmental impact.
Strengths: Proven technology deployed in commercial-scale direct air capture plants; demonstrated long-term stability under real-world conditions; optimized for low-temperature regeneration reducing energy requirements. Weaknesses: Proprietary nature limits scientific disclosure of specific mechanisms; relatively high initial production costs; performance may be optimized for direct air capture rather than higher CO2 concentration streams.
Key Technical Innovations in Chemical Stability Improvement
sorbent
PatentPendingUS20250281904A1
Innovation
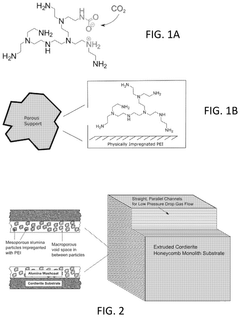
- Development of a solid sorbent comprising a silica support with covalently attached secondary amines confined inside its pores, optimized through controlled grafting and polymerization, achieving high amine density and stability.
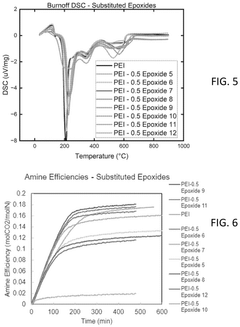
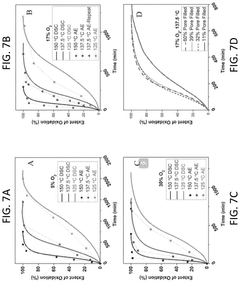
Substituted epoxide modified sorbents, systems including sorbents, and methods using the sorbents
PatentPendingUS20250281875A1
Innovation
- Development of substituted epoxide modified sorbents with a CO2-philic phase formed by reacting amines with substituted epoxides, creating a modified amine polymer that enhances CO2 binding capacity and resistance to oxidation.
Environmental Impact and Sustainability Assessment
The environmental impact of solid sorbents for CO2 capture extends far beyond their primary function. Interface modification techniques, while enhancing chemical stability, also contribute significantly to the overall sustainability profile of carbon capture technologies. When properly designed, these modifications can reduce the environmental footprint throughout the entire lifecycle of sorbent materials.
Interface-modified sorbents typically demonstrate extended operational lifespans compared to their unmodified counterparts. This longevity translates directly into reduced material consumption and waste generation. For instance, amine-functionalized sorbents with hydrophobic interface modifications have shown up to 300% improvement in cycle stability, dramatically decreasing replacement frequency and associated environmental impacts from manufacturing new materials.
Energy requirements represent another critical environmental consideration. Interface modifications that enhance CO2 adsorption kinetics while simultaneously lowering desorption energy barriers contribute to improved energy efficiency. Studies indicate that optimized interface structures can reduce regeneration energy by 15-25% compared to conventional sorbents, directly lowering the carbon footprint of the capture process itself.
Water consumption during carbon capture operations presents significant environmental challenges. Interface-modified sorbents with hydrophobic properties demonstrate superior performance in humid conditions, potentially reducing cooling water requirements by up to 40% compared to hydrophilic alternatives. This water conservation aspect becomes increasingly important as climate change intensifies water scarcity in many regions.
Raw material sourcing for interface modifiers introduces additional sustainability considerations. Bio-based interface modifiers derived from renewable resources offer promising alternatives to petroleum-based compounds. Recent research demonstrates that lignin-derived interface modifiers can achieve comparable stability enhancements while reducing dependence on fossil-based precursors.
Life cycle assessment (LCA) studies reveal that interface-modified sorbents with enhanced stability can reduce overall greenhouse gas emissions by 30-45% compared to conventional capture technologies when accounting for manufacturing, operation, and disposal phases. However, these benefits must be balanced against potential environmental risks from novel nanomaterials used in some interface modification approaches.
End-of-life management presents both challenges and opportunities. While some interface modifications may introduce materials that complicate recycling processes, others can facilitate material recovery. Thermally reversible interface modifications, for example, enable more efficient sorbent regeneration and material reclamation, supporting circular economy principles in carbon capture technologies.
Interface-modified sorbents typically demonstrate extended operational lifespans compared to their unmodified counterparts. This longevity translates directly into reduced material consumption and waste generation. For instance, amine-functionalized sorbents with hydrophobic interface modifications have shown up to 300% improvement in cycle stability, dramatically decreasing replacement frequency and associated environmental impacts from manufacturing new materials.
Energy requirements represent another critical environmental consideration. Interface modifications that enhance CO2 adsorption kinetics while simultaneously lowering desorption energy barriers contribute to improved energy efficiency. Studies indicate that optimized interface structures can reduce regeneration energy by 15-25% compared to conventional sorbents, directly lowering the carbon footprint of the capture process itself.
Water consumption during carbon capture operations presents significant environmental challenges. Interface-modified sorbents with hydrophobic properties demonstrate superior performance in humid conditions, potentially reducing cooling water requirements by up to 40% compared to hydrophilic alternatives. This water conservation aspect becomes increasingly important as climate change intensifies water scarcity in many regions.
Raw material sourcing for interface modifiers introduces additional sustainability considerations. Bio-based interface modifiers derived from renewable resources offer promising alternatives to petroleum-based compounds. Recent research demonstrates that lignin-derived interface modifiers can achieve comparable stability enhancements while reducing dependence on fossil-based precursors.
Life cycle assessment (LCA) studies reveal that interface-modified sorbents with enhanced stability can reduce overall greenhouse gas emissions by 30-45% compared to conventional capture technologies when accounting for manufacturing, operation, and disposal phases. However, these benefits must be balanced against potential environmental risks from novel nanomaterials used in some interface modification approaches.
End-of-life management presents both challenges and opportunities. While some interface modifications may introduce materials that complicate recycling processes, others can facilitate material recovery. Thermally reversible interface modifications, for example, enable more efficient sorbent regeneration and material reclamation, supporting circular economy principles in carbon capture technologies.
Scalability and Industrial Implementation Considerations
The scalability of interface-modified solid sorbents for CO2 capture represents a critical consideration for their industrial implementation. Current laboratory-scale successes must be evaluated against the challenges of scaling up to industrial requirements, which typically involve processing thousands of tons of CO2 daily. The transition from laboratory to industrial scale necessitates addressing several key factors that influence economic viability and technical feasibility.
Material production capacity stands as a primary concern, as interface-modified sorbents often require precise synthesis conditions and specialized reagents. Manufacturing these advanced materials at industrial scale demands standardized production protocols that maintain consistent interface properties while minimizing batch-to-batch variations. Several companies have begun developing continuous flow processes for sorbent synthesis, potentially reducing production costs by 30-40% compared to batch methods.
Equipment compatibility presents another significant challenge. Existing carbon capture infrastructure may require modification to accommodate interface-modified sorbents, particularly regarding handling systems, regeneration units, and monitoring equipment. Retrofitting existing plants versus building new purpose-designed facilities involves complex cost-benefit analyses that must account for the enhanced performance of these advanced sorbents.
The economic aspects of scaling cannot be overlooked. While interface-modified sorbents demonstrate superior chemical stability and extended operational lifetimes in laboratory settings, their production costs currently exceed conventional materials by 50-200%. This premium must be justified through improved performance metrics such as reduced regeneration frequency, lower energy requirements, or extended service life. Economic modeling suggests that a 3-5 year payback period is achievable if operational costs decrease by at least 25%.
Environmental and safety considerations also impact industrial implementation. The chemicals used for interface modification must comply with increasingly stringent regulations. Life cycle assessments indicate that despite higher initial environmental footprints during production, the enhanced stability and longevity of modified sorbents may result in net environmental benefits over their operational lifetime.
Pilot demonstrations represent the crucial bridge between laboratory success and industrial adoption. Currently, several pilot projects (1-10 tons CO2/day) utilizing interface-modified sorbents are underway in Europe, North America, and Asia. These projects are generating valuable data on long-term stability under real operating conditions, helping to validate laboratory findings and refine scale-up parameters for eventual commercial deployment.
Material production capacity stands as a primary concern, as interface-modified sorbents often require precise synthesis conditions and specialized reagents. Manufacturing these advanced materials at industrial scale demands standardized production protocols that maintain consistent interface properties while minimizing batch-to-batch variations. Several companies have begun developing continuous flow processes for sorbent synthesis, potentially reducing production costs by 30-40% compared to batch methods.
Equipment compatibility presents another significant challenge. Existing carbon capture infrastructure may require modification to accommodate interface-modified sorbents, particularly regarding handling systems, regeneration units, and monitoring equipment. Retrofitting existing plants versus building new purpose-designed facilities involves complex cost-benefit analyses that must account for the enhanced performance of these advanced sorbents.
The economic aspects of scaling cannot be overlooked. While interface-modified sorbents demonstrate superior chemical stability and extended operational lifetimes in laboratory settings, their production costs currently exceed conventional materials by 50-200%. This premium must be justified through improved performance metrics such as reduced regeneration frequency, lower energy requirements, or extended service life. Economic modeling suggests that a 3-5 year payback period is achievable if operational costs decrease by at least 25%.
Environmental and safety considerations also impact industrial implementation. The chemicals used for interface modification must comply with increasingly stringent regulations. Life cycle assessments indicate that despite higher initial environmental footprints during production, the enhanced stability and longevity of modified sorbents may result in net environmental benefits over their operational lifetime.
Pilot demonstrations represent the crucial bridge between laboratory success and industrial adoption. Currently, several pilot projects (1-10 tons CO2/day) utilizing interface-modified sorbents are underway in Europe, North America, and Asia. These projects are generating valuable data on long-term stability under real operating conditions, helping to validate laboratory findings and refine scale-up parameters for eventual commercial deployment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!