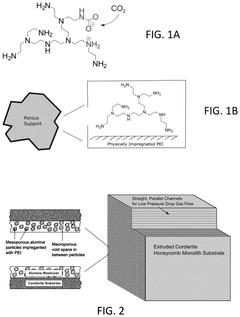
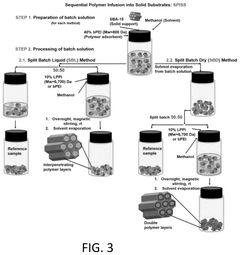
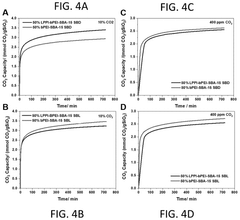
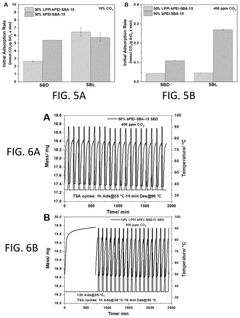
How surface chemistry and functionalization enhance Solid sorbents for CO2 capture performance
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Capture Sorbent Evolution and Objectives
Carbon dioxide capture technology has evolved significantly over the past decades, driven by the urgent need to mitigate climate change impacts. Initially, CO2 capture focused primarily on liquid sorbents, particularly amine-based solutions, which dominated industrial applications since the 1930s. However, these systems faced persistent challenges including high energy requirements for regeneration, equipment corrosion, and solvent degradation, prompting researchers to explore alternative approaches.
Solid sorbents emerged as promising alternatives in the 1990s, offering advantages such as reduced energy consumption, minimal corrosion issues, and greater operational flexibility. The evolution of solid sorbents has progressed through several generations, beginning with basic porous materials like activated carbon and zeolites, advancing to functionalized materials with enhanced selectivity, and most recently developing into highly engineered composite materials with unprecedented capture capacities.
Surface chemistry has played a pivotal role in this evolution. Early research established that the physical properties of sorbents—surface area, pore structure, and particle size—while important, were insufficient alone to achieve high CO2 capture performance. The breakthrough came with the recognition that chemical interactions between CO2 molecules and sorbent surfaces could dramatically enhance capture efficiency, leading to intensive research into surface functionalization techniques.
The primary objective in solid sorbent development is to achieve optimal balance between adsorption capacity, selectivity, and regeneration energy. This requires precise engineering of surface chemistry to create binding sites with ideal interaction strengths—strong enough to capture CO2 efficiently from dilute streams but weak enough to enable energy-efficient regeneration. Secondary objectives include enhancing mechanical stability, resistance to contaminants, and maintaining performance over multiple adsorption-desorption cycles.
Current research aims to develop next-generation sorbents with capture capacities exceeding 3 mmol CO2/g sorbent under realistic conditions, selectivity factors above 100 for CO2 over N2, and regeneration energy requirements below 2 GJ/ton CO2. These ambitious targets necessitate fundamental advances in surface chemistry understanding and functionalization techniques.
The technological trajectory points toward increasingly sophisticated control over surface properties at the molecular level, with particular emphasis on tailoring binding site distribution, strength, and accessibility. Emerging objectives include developing sorbents capable of operating across wider temperature ranges, tolerating higher moisture levels, and integrating seamlessly with renewable energy sources for regeneration processes.
Solid sorbents emerged as promising alternatives in the 1990s, offering advantages such as reduced energy consumption, minimal corrosion issues, and greater operational flexibility. The evolution of solid sorbents has progressed through several generations, beginning with basic porous materials like activated carbon and zeolites, advancing to functionalized materials with enhanced selectivity, and most recently developing into highly engineered composite materials with unprecedented capture capacities.
Surface chemistry has played a pivotal role in this evolution. Early research established that the physical properties of sorbents—surface area, pore structure, and particle size—while important, were insufficient alone to achieve high CO2 capture performance. The breakthrough came with the recognition that chemical interactions between CO2 molecules and sorbent surfaces could dramatically enhance capture efficiency, leading to intensive research into surface functionalization techniques.
The primary objective in solid sorbent development is to achieve optimal balance between adsorption capacity, selectivity, and regeneration energy. This requires precise engineering of surface chemistry to create binding sites with ideal interaction strengths—strong enough to capture CO2 efficiently from dilute streams but weak enough to enable energy-efficient regeneration. Secondary objectives include enhancing mechanical stability, resistance to contaminants, and maintaining performance over multiple adsorption-desorption cycles.
Current research aims to develop next-generation sorbents with capture capacities exceeding 3 mmol CO2/g sorbent under realistic conditions, selectivity factors above 100 for CO2 over N2, and regeneration energy requirements below 2 GJ/ton CO2. These ambitious targets necessitate fundamental advances in surface chemistry understanding and functionalization techniques.
The technological trajectory points toward increasingly sophisticated control over surface properties at the molecular level, with particular emphasis on tailoring binding site distribution, strength, and accessibility. Emerging objectives include developing sorbents capable of operating across wider temperature ranges, tolerating higher moisture levels, and integrating seamlessly with renewable energy sources for regeneration processes.
Market Analysis for Carbon Capture Technologies
The global carbon capture and storage (CCS) market is experiencing significant growth, driven by increasing environmental regulations and the urgent need to reduce greenhouse gas emissions. As of 2023, the market was valued at approximately $7.5 billion and is projected to reach $15.3 billion by 2030, representing a compound annual growth rate of 10.7%. This growth trajectory is particularly notable in regions with stringent carbon emission policies, such as Europe and North America.
The market for solid sorbents specifically within the carbon capture technology landscape is expanding rapidly due to their superior performance characteristics compared to traditional liquid-based capture methods. Solid sorbents currently account for about 28% of the total carbon capture technology market, with projections indicating this share could increase to 35-40% by 2028 as functionalization techniques advance.
Industrial sectors represent the largest end-users of carbon capture technologies, with power generation, cement production, and chemical manufacturing collectively accounting for over 65% of market demand. The power generation sector alone constitutes approximately 40% of the current market for advanced solid sorbent technologies, driven by regulatory pressures to decarbonize electricity production.
Geographically, North America leads the market with a 38% share, followed by Europe at 32% and Asia-Pacific at 22%. China and India are emerging as high-growth markets, with annual growth rates exceeding 15% as these nations implement more aggressive carbon reduction strategies while balancing economic development priorities.
Investment in carbon capture technologies has seen a remarkable surge, with venture capital funding increasing by 150% between 2020 and 2023. Corporate investments in solid sorbent research and development reached $1.2 billion in 2023, reflecting growing confidence in the commercial viability of these technologies.
Market barriers include high implementation costs, with current carbon capture costs ranging from $40-$120 per ton of CO2 depending on the technology and application. However, enhanced surface chemistry and functionalization of solid sorbents are expected to reduce these costs by 30-45% over the next decade, potentially accelerating market adoption.
Customer segments are diversifying beyond traditional heavy industry, with emerging interest from sectors such as data centers, transportation infrastructure, and commercial real estate seeking to achieve carbon neutrality goals. This broadening customer base is expected to create new market opportunities valued at approximately $3.8 billion by 2027.
The market for solid sorbents specifically within the carbon capture technology landscape is expanding rapidly due to their superior performance characteristics compared to traditional liquid-based capture methods. Solid sorbents currently account for about 28% of the total carbon capture technology market, with projections indicating this share could increase to 35-40% by 2028 as functionalization techniques advance.
Industrial sectors represent the largest end-users of carbon capture technologies, with power generation, cement production, and chemical manufacturing collectively accounting for over 65% of market demand. The power generation sector alone constitutes approximately 40% of the current market for advanced solid sorbent technologies, driven by regulatory pressures to decarbonize electricity production.
Geographically, North America leads the market with a 38% share, followed by Europe at 32% and Asia-Pacific at 22%. China and India are emerging as high-growth markets, with annual growth rates exceeding 15% as these nations implement more aggressive carbon reduction strategies while balancing economic development priorities.
Investment in carbon capture technologies has seen a remarkable surge, with venture capital funding increasing by 150% between 2020 and 2023. Corporate investments in solid sorbent research and development reached $1.2 billion in 2023, reflecting growing confidence in the commercial viability of these technologies.
Market barriers include high implementation costs, with current carbon capture costs ranging from $40-$120 per ton of CO2 depending on the technology and application. However, enhanced surface chemistry and functionalization of solid sorbents are expected to reduce these costs by 30-45% over the next decade, potentially accelerating market adoption.
Customer segments are diversifying beyond traditional heavy industry, with emerging interest from sectors such as data centers, transportation infrastructure, and commercial real estate seeking to achieve carbon neutrality goals. This broadening customer base is expected to create new market opportunities valued at approximately $3.8 billion by 2027.
Surface Chemistry Challenges in CO2 Sorption
Carbon dioxide capture technologies face significant challenges related to surface chemistry in solid sorbents. The interaction between CO2 molecules and sorbent surfaces is governed by complex physicochemical processes that determine capture efficiency, selectivity, and regeneration capabilities. Current solid sorbents exhibit limitations in binding energy optimization, with many materials demonstrating either too weak interactions (resulting in poor capture rates) or excessively strong bonds (leading to high regeneration energy requirements).
Surface heterogeneity presents another major challenge, as non-uniform binding sites create variable adsorption energies across the sorbent surface. This heterogeneity often results in unpredictable performance under fluctuating operational conditions, particularly in the presence of moisture, SOx, and NOx contaminants that compete for active sites. The chemical stability of functionalized surfaces under repeated adsorption-desorption cycles remains problematic, with many promising materials showing significant performance degradation after multiple cycles.
Mass transfer limitations at the molecular level constitute a critical barrier to efficient CO2 capture. The diffusion of CO2 molecules to active binding sites is frequently hindered by surface congestion, pore blocking, and inadequate pore connectivity. These limitations become particularly pronounced at higher CO2 loadings, resulting in diminishing returns as capture progresses. Additionally, the kinetics of surface reactions often slow down as adsorption sites become saturated, reducing overall capture rates.
Water sensitivity represents a persistent challenge for many solid sorbents. The presence of moisture can dramatically alter surface chemistry through competitive adsorption, hydrolysis of functional groups, or formation of stable hydrates that block access to binding sites. This is especially problematic for flue gas applications where water vapor is invariably present. Developing water-resistant or water-tolerant surface chemistries without compromising CO2 selectivity remains an elusive goal.
The scalability of surface functionalization techniques poses significant industrial implementation challenges. Laboratory-scale functionalization methods often employ expensive reagents, complex procedures, or harsh conditions that are difficult to scale up economically. Furthermore, achieving uniform surface modification across large quantities of sorbent material requires precise process control that is difficult to maintain in industrial settings.
Characterization and modeling of surface interactions present methodological challenges that impede rapid development. Current analytical techniques often provide incomplete information about the dynamic behavior of CO2 molecules on functionalized surfaces under realistic operating conditions. This knowledge gap hinders the rational design of improved sorbent materials and optimization of surface chemistry for specific capture applications.
Surface heterogeneity presents another major challenge, as non-uniform binding sites create variable adsorption energies across the sorbent surface. This heterogeneity often results in unpredictable performance under fluctuating operational conditions, particularly in the presence of moisture, SOx, and NOx contaminants that compete for active sites. The chemical stability of functionalized surfaces under repeated adsorption-desorption cycles remains problematic, with many promising materials showing significant performance degradation after multiple cycles.
Mass transfer limitations at the molecular level constitute a critical barrier to efficient CO2 capture. The diffusion of CO2 molecules to active binding sites is frequently hindered by surface congestion, pore blocking, and inadequate pore connectivity. These limitations become particularly pronounced at higher CO2 loadings, resulting in diminishing returns as capture progresses. Additionally, the kinetics of surface reactions often slow down as adsorption sites become saturated, reducing overall capture rates.
Water sensitivity represents a persistent challenge for many solid sorbents. The presence of moisture can dramatically alter surface chemistry through competitive adsorption, hydrolysis of functional groups, or formation of stable hydrates that block access to binding sites. This is especially problematic for flue gas applications where water vapor is invariably present. Developing water-resistant or water-tolerant surface chemistries without compromising CO2 selectivity remains an elusive goal.
The scalability of surface functionalization techniques poses significant industrial implementation challenges. Laboratory-scale functionalization methods often employ expensive reagents, complex procedures, or harsh conditions that are difficult to scale up economically. Furthermore, achieving uniform surface modification across large quantities of sorbent material requires precise process control that is difficult to maintain in industrial settings.
Characterization and modeling of surface interactions present methodological challenges that impede rapid development. Current analytical techniques often provide incomplete information about the dynamic behavior of CO2 molecules on functionalized surfaces under realistic operating conditions. This knowledge gap hinders the rational design of improved sorbent materials and optimization of surface chemistry for specific capture applications.
Current Surface Functionalization Approaches
01 Metal-organic frameworks (MOFs) for CO2 capture
Metal-organic frameworks (MOFs) are crystalline porous materials composed of metal ions or clusters coordinated with organic ligands. They exhibit high surface areas, tunable pore sizes, and chemical functionality, making them effective solid sorbents for CO2 capture. MOFs can be designed with specific binding sites for CO2 molecules, enhancing selectivity and capacity. Their performance can be further improved through post-synthetic modifications and incorporation of functional groups that increase CO2 affinity.- Metal-organic frameworks (MOFs) for CO2 capture: Metal-organic frameworks (MOFs) are crystalline porous materials composed of metal ions or clusters coordinated to organic ligands. These materials have shown exceptional performance as solid sorbents for CO2 capture due to their high surface area, tunable pore size, and chemical functionality. MOFs can be designed with specific binding sites for CO2 molecules, enhancing selectivity and capacity. Their modular nature allows for customization to optimize adsorption-desorption cycles and improve energy efficiency in carbon capture processes.
- Amine-functionalized sorbents for enhanced CO2 adsorption: Amine-functionalized solid sorbents incorporate various amine groups onto support materials to enhance CO2 capture performance. These materials work through chemical adsorption mechanisms where the amine groups react with CO2 to form carbamates or bicarbonates. Common support materials include silica, activated carbon, and polymeric substrates. The amine functionalization significantly increases CO2 selectivity and capacity, particularly at lower CO2 concentrations relevant to flue gas conditions. These materials can be regenerated at lower temperatures compared to traditional liquid amine scrubbing, reducing the energy penalty for carbon capture.
- Zeolite-based materials for selective CO2 adsorption: Zeolites are microporous aluminosilicate minerals that serve as effective solid sorbents for CO2 capture. Their crystalline structure contains uniform pores and cavities that can selectively adsorb CO2 molecules based on size and polarity. Zeolites can be modified by ion exchange, introducing cations that enhance CO2 binding strength. These materials demonstrate good thermal stability, allowing for multiple adsorption-desorption cycles without significant degradation. Zeolite-based sorbents are particularly effective for pressure swing adsorption (PSA) and temperature swing adsorption (TSA) processes in industrial carbon capture applications.
- Carbon-based sorbents with enhanced surface properties: Carbon-based materials, including activated carbon, carbon nanotubes, and graphene derivatives, serve as versatile solid sorbents for CO2 capture. These materials can be engineered with high surface areas and optimized pore structures to maximize CO2 adsorption capacity. Surface modification techniques, such as nitrogen doping or incorporation of basic functional groups, enhance CO2 binding affinity. Carbon-based sorbents offer advantages including low cost, high thermal stability, and hydrophobicity that reduces competition from water vapor. Their regeneration typically requires less energy compared to chemical sorbents, making them promising candidates for energy-efficient carbon capture systems.
- Hybrid and composite sorbents for optimized CO2 capture performance: Hybrid and composite sorbents combine multiple materials to leverage complementary properties for enhanced CO2 capture performance. These materials typically integrate physical adsorbents (like zeolites or MOFs) with chemical sorbents (like amines) to achieve both high capacity and selectivity. Layered structures can be designed to optimize gas flow and minimize pressure drop. Some composites incorporate phase-change materials to manage heat during adsorption-desorption cycles. These hybrid approaches often result in improved stability under humid conditions, better mechanical properties, and enhanced cycling performance compared to single-component sorbents, addressing multiple challenges in practical carbon capture applications.
02 Amine-functionalized sorbents for enhanced CO2 adsorption
Amine-functionalized solid sorbents utilize the chemical reactivity between amine groups and CO2 to achieve high capture efficiency. These materials typically consist of amines grafted onto porous supports such as silica, activated carbon, or polymeric substrates. The amine groups form carbamates or bicarbonates upon reaction with CO2, enabling selective capture even at low CO2 concentrations. Various amine types (primary, secondary, tertiary) and loadings can be optimized to balance adsorption capacity, kinetics, and regeneration energy requirements.Expand Specific Solutions03 Zeolite-based materials for CO2 separation
Zeolites are crystalline aluminosilicate materials with well-defined microporous structures that can selectively adsorb CO2 based on molecular sieving and electrostatic interactions. Their performance for CO2 capture depends on factors such as Si/Al ratio, cation type, pore size, and surface area. Modified zeolites with enhanced hydrophobicity or incorporated functional groups show improved CO2 selectivity and capacity, particularly in the presence of moisture. These materials offer advantages including thermal stability, mechanical strength, and regenerability under various operating conditions.Expand Specific Solutions04 Carbon-based sorbents for CO2 capture
Carbon-based sorbents including activated carbons, carbon molecular sieves, graphene-based materials, and carbon nanotubes demonstrate promising CO2 capture performance. These materials feature high surface areas, tunable pore structures, and surface chemistry that can be modified to enhance CO2 adsorption. Nitrogen-doped carbon materials show particularly improved CO2 capture due to the creation of basic sites that interact favorably with acidic CO2 molecules. Carbon-based sorbents offer advantages including low cost, high thermal stability, and resistance to degradation in various operating environments.Expand Specific Solutions05 Regeneration methods and cyclic performance of CO2 sorbents
The practical application of solid sorbents for CO2 capture depends significantly on their regeneration efficiency and long-term cyclic stability. Various regeneration methods including temperature swing adsorption (TSA), pressure swing adsorption (PSA), vacuum swing adsorption (VSA), and combinations thereof are employed to release captured CO2 and restore sorbent capacity. Advanced regeneration techniques aim to minimize energy requirements while maintaining sorbent integrity over multiple adsorption-desorption cycles. Innovations in sorbent design focus on reducing degradation mechanisms such as amine leaching, pore blocking, and structural collapse to ensure consistent performance over thousands of cycles.Expand Specific Solutions
Leading Organizations in CO2 Capture Research
The solid sorbent CO2 capture technology market is currently in a growth phase, with increasing focus on surface chemistry and functionalization to enhance performance. The global carbon capture market is projected to reach $7-10 billion by 2030, driven by climate policies and industrial decarbonization efforts. Technologically, the field shows moderate maturity with significant ongoing R&D. Leading players include established corporations like Climeworks AG and Global Thermostat Operations LLC, who have deployed commercial-scale direct air capture facilities, alongside research-intensive organizations such as MIT, Arizona State University, and Dalian Institute of Chemical Physics. Industrial players like Corning, W.L. Gore & Associates, and Wacker Chemie AG are advancing material science aspects, while energy companies including Korea Electric Power Corp. and China Petroleum & Chemical Corp. are implementing capture technologies at emissions sources.
Global Thermostat Operations LLC
Technical Solution: Global Thermostat has developed a proprietary amine-based solid sorbent technology that chemically binds CO2 when air passes over their specially designed structures. Their approach utilizes a honeycomb ceramic monolith structure coated with amine-functionalized materials that can capture CO2 directly from ambient air or from concentrated sources. The company's technology employs aminosilicone polymers grafted onto porous supports, creating high-density amine sites that enhance CO2 adsorption capacity. Their process operates at relatively low temperatures (85-100°C) for regeneration, which significantly reduces energy requirements compared to liquid amine systems[1]. Global Thermostat's sorbents demonstrate exceptional stability through thousands of adsorption-desorption cycles and maintain performance even in humid conditions, addressing a common challenge with many solid sorbents[3].
Strengths: Low regeneration energy requirements, high selectivity for CO2 even at atmospheric concentrations, and excellent stability through multiple cycles. Their monolith structure provides favorable mass transfer properties and low pressure drop. Weaknesses: Higher manufacturing costs compared to conventional sorbents and potential amine degradation over extended operation in the presence of oxygen or contaminants.
Susteon, Inc.
Technical Solution: Susteon has pioneered advanced metal-organic framework (MOF) based sorbents with tailored surface chemistry for CO2 capture. Their technology focuses on creating hierarchical pore structures with optimized amine functionalization to maximize CO2 adsorption capacity while maintaining rapid kinetics. Susteon's approach involves precise control of the MOF synthesis conditions to create open metal sites that serve as primary binding locations for CO2 molecules. These sites are then strategically functionalized with various amine groups to enhance selectivity and capacity. Their proprietary "dual-site" binding mechanism allows for both physical adsorption at metal centers and chemical adsorption through amine-CO2 interactions[2]. Susteon has demonstrated sorbent materials achieving CO2 capacities exceeding 4 mmol/g under relevant flue gas conditions with remarkably fast adsorption kinetics, reaching 80% of equilibrium capacity within minutes[4]. Their materials maintain performance even in the presence of moisture and show minimal degradation over hundreds of cycles.
Strengths: Exceptionally high CO2 capacity, tunable pore structures allowing for application-specific optimization, and excellent selectivity over other flue gas components. Weaknesses: Higher production costs compared to conventional sorbents, potential challenges in large-scale manufacturing consistency, and sensitivity of some MOF structures to water and impurities in industrial gas streams.
Key Patents in Sorbent Surface Chemistry
Layered Solid Sorbents For Carbon Dioxide Capture
PatentActiveUS20140127104A1
Innovation
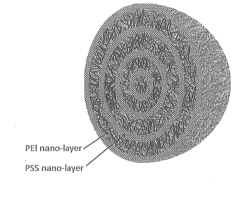
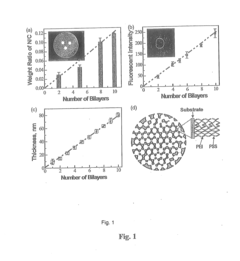
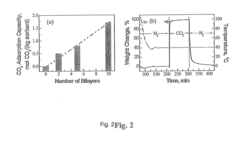
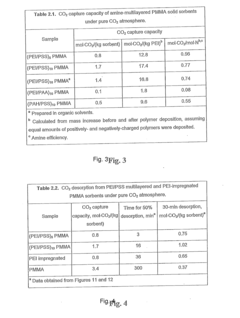
- Development of nano-layered solid sorbents using electrostatic layer-by-layer nanoassembly, where CO2-adsorbing polymers like polyethylenimine and oppositely charged polyelectrolytes are alternately deposited on porous substrates, creating bilayers that enhance CO2 capture and transport efficiency.
Sorbents, systems including sorbents, and methods using the sorbents
PatentPendingUS20240335784A1
Innovation
- Development of sorbents comprising a CO2-philic phase with a combination of polypropylenimine and polyethylenimine, which provides improved oxidative stability and hydrophilicity, allowing for efficient CO2 capture and regeneration, and are integrated into a structured support for enhanced performance.
Environmental Impact Assessment
The deployment of solid sorbents for CO2 capture represents a significant intervention in environmental systems, necessitating comprehensive assessment of both positive and negative impacts. The primary environmental benefit is the reduction of atmospheric CO2 concentrations, directly addressing climate change mitigation goals. Studies indicate that advanced functionalized solid sorbents can potentially reduce CO2 emissions from stationary sources by 85-95%, substantially exceeding the performance of conventional capture technologies.
However, the environmental footprint of sorbent production warrants careful consideration. Manufacturing processes for specialized materials like metal-organic frameworks (MOFs) and functionalized silica often involve energy-intensive synthesis routes and potentially hazardous chemicals. Life cycle assessments reveal that the environmental benefits of CO2 capture must be balanced against the embodied energy and emissions associated with sorbent production, which can range from 0.2 to 0.5 tons of CO2 equivalent per ton of capture capacity.
Water consumption represents another critical environmental parameter. While solid sorbents generally demonstrate superior water efficiency compared to liquid amine systems, certain hydrophilic functionalized materials may still require significant water inputs for regeneration cycles. This poses particular challenges in water-stressed regions where cooling systems for capture facilities compete with other essential water uses.
The fate of captured CO2 introduces additional environmental considerations. Permanent sequestration in geological formations presents minimal environmental risk when properly managed, but utilization pathways such as enhanced oil recovery may partially offset the climate benefits through induced fossil fuel production. Advanced functionalization techniques enabling direct conversion of captured CO2 to value-added products offer promising alternatives with potentially lower environmental impacts.
Waste management concerns emerge at the end of sorbent life cycles. Functionalized materials containing heavy metals or synthetic organic compounds may require specialized disposal protocols to prevent environmental contamination. Recent innovations in green chemistry approaches to surface functionalization have demonstrated progress in developing environmentally benign sorbents with biodegradable functional groups and reduced toxicity profiles.
Land use impacts of solid sorbent deployment are generally favorable compared to alternative capture technologies, with modular systems requiring 30-50% less physical footprint than equivalent liquid absorption systems. This advantage becomes particularly significant in retrofit applications where space constraints often present implementation barriers.
However, the environmental footprint of sorbent production warrants careful consideration. Manufacturing processes for specialized materials like metal-organic frameworks (MOFs) and functionalized silica often involve energy-intensive synthesis routes and potentially hazardous chemicals. Life cycle assessments reveal that the environmental benefits of CO2 capture must be balanced against the embodied energy and emissions associated with sorbent production, which can range from 0.2 to 0.5 tons of CO2 equivalent per ton of capture capacity.
Water consumption represents another critical environmental parameter. While solid sorbents generally demonstrate superior water efficiency compared to liquid amine systems, certain hydrophilic functionalized materials may still require significant water inputs for regeneration cycles. This poses particular challenges in water-stressed regions where cooling systems for capture facilities compete with other essential water uses.
The fate of captured CO2 introduces additional environmental considerations. Permanent sequestration in geological formations presents minimal environmental risk when properly managed, but utilization pathways such as enhanced oil recovery may partially offset the climate benefits through induced fossil fuel production. Advanced functionalization techniques enabling direct conversion of captured CO2 to value-added products offer promising alternatives with potentially lower environmental impacts.
Waste management concerns emerge at the end of sorbent life cycles. Functionalized materials containing heavy metals or synthetic organic compounds may require specialized disposal protocols to prevent environmental contamination. Recent innovations in green chemistry approaches to surface functionalization have demonstrated progress in developing environmentally benign sorbents with biodegradable functional groups and reduced toxicity profiles.
Land use impacts of solid sorbent deployment are generally favorable compared to alternative capture technologies, with modular systems requiring 30-50% less physical footprint than equivalent liquid absorption systems. This advantage becomes particularly significant in retrofit applications where space constraints often present implementation barriers.
Scalability and Industrial Implementation
The scalability of solid sorbent technologies for CO2 capture represents a critical bridge between laboratory success and commercial viability. Current industrial implementation faces several challenges related to the mass production of functionalized materials with consistent surface chemistry properties. Manufacturing processes must be optimized to maintain the precise chemical modifications that enhance CO2 capture performance while being economically viable at scale.
Fixed-bed and fluidized-bed configurations have emerged as the most promising reactor designs for industrial deployment of solid sorbents. Fixed-bed systems offer simplicity and reliability but face pressure drop limitations when scaled up. Fluidized-bed reactors provide superior heat and mass transfer characteristics essential for the adsorption-desorption cycles, though they introduce challenges related to particle attrition and mechanical stability of functionalized surfaces.
The economic feasibility of scaled production depends heavily on the cost and availability of raw materials used for surface functionalization. Amine-based modifications, while effective for CO2 capture, often involve expensive precursors and complex synthesis procedures. Recent advances in using industrial by-products and sustainable biomass-derived compounds as functionalization agents show promise for reducing production costs while maintaining performance.
Regeneration energy requirements represent another significant hurdle for industrial implementation. Surface chemistry modifications must be designed not only to enhance adsorption capacity but also to minimize the energy penalty during the desorption phase. Temperature-swing and vacuum-swing processes are being optimized through surface engineering to lower the energy threshold required for sorbent regeneration.
Longevity of surface functionalization under industrial conditions remains a key concern. Repeated adsorption-desorption cycles, exposure to flue gas contaminants, and mechanical stress can degrade the chemical modifications that enhance capture performance. Recent developments in covalent attachment methods and protective coatings have improved the durability of functionalized surfaces, extending operational lifetimes from hundreds to thousands of cycles.
Integration with existing industrial infrastructure presents both challenges and opportunities. Retrofitting capabilities are being enhanced through modular designs that can accommodate various solid sorbent technologies with minimal disruption to existing operations. The adaptability of surface chemistry to specific industrial environments (varying CO2 concentrations, presence of contaminants, temperature profiles) is becoming a focal point for targeted functionalization strategies.
Fixed-bed and fluidized-bed configurations have emerged as the most promising reactor designs for industrial deployment of solid sorbents. Fixed-bed systems offer simplicity and reliability but face pressure drop limitations when scaled up. Fluidized-bed reactors provide superior heat and mass transfer characteristics essential for the adsorption-desorption cycles, though they introduce challenges related to particle attrition and mechanical stability of functionalized surfaces.
The economic feasibility of scaled production depends heavily on the cost and availability of raw materials used for surface functionalization. Amine-based modifications, while effective for CO2 capture, often involve expensive precursors and complex synthesis procedures. Recent advances in using industrial by-products and sustainable biomass-derived compounds as functionalization agents show promise for reducing production costs while maintaining performance.
Regeneration energy requirements represent another significant hurdle for industrial implementation. Surface chemistry modifications must be designed not only to enhance adsorption capacity but also to minimize the energy penalty during the desorption phase. Temperature-swing and vacuum-swing processes are being optimized through surface engineering to lower the energy threshold required for sorbent regeneration.
Longevity of surface functionalization under industrial conditions remains a key concern. Repeated adsorption-desorption cycles, exposure to flue gas contaminants, and mechanical stress can degrade the chemical modifications that enhance capture performance. Recent developments in covalent attachment methods and protective coatings have improved the durability of functionalized surfaces, extending operational lifetimes from hundreds to thousands of cycles.
Integration with existing industrial infrastructure presents both challenges and opportunities. Retrofitting capabilities are being enhanced through modular designs that can accommodate various solid sorbent technologies with minimal disruption to existing operations. The adaptability of surface chemistry to specific industrial environments (varying CO2 concentrations, presence of contaminants, temperature profiles) is becoming a focal point for targeted functionalization strategies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!