What interface engineering strategies enhance Solid sorbents for CO2 capture efficiency
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Capture Interface Engineering Background and Objectives
Carbon dioxide capture and sequestration (CCS) has emerged as a critical technology in the global effort to mitigate climate change. The historical development of CO2 capture technologies dates back to the 1930s when absorption processes using aqueous amine solutions were first implemented in natural gas sweetening operations. However, the focus on CO2 capture specifically for climate change mitigation gained momentum in the late 1990s as international climate agreements began to take shape.
Solid sorbents represent a promising alternative to traditional liquid absorption systems, offering advantages in energy efficiency, environmental impact, and operational flexibility. The evolution of solid sorbent technologies has accelerated significantly over the past decade, with particular emphasis on interface engineering—the deliberate modification of material surfaces and interfaces to enhance CO2 capture performance.
Interface engineering for CO2 capture encompasses a range of strategies including surface functionalization, pore structure optimization, composite material development, and hierarchical structure design. These approaches aim to maximize the interaction between CO2 molecules and the sorbent surface, thereby improving capture capacity, selectivity, and kinetics while maintaining mechanical and chemical stability under operational conditions.
The primary technical objectives in this field include developing sorbents with high CO2 adsorption capacity (>2 mmol/g under relevant conditions), excellent selectivity (CO2/N2 selectivity >100), rapid adsorption/desorption kinetics, minimal regeneration energy requirements (<2 GJ/ton CO2), and long-term stability (>1000 cycles with <10% capacity degradation). Additionally, these materials must demonstrate feasibility for large-scale manufacturing and deployment.
Recent technological trends indicate a shift toward multifunctional sorbent systems that integrate capture capabilities with other desirable properties such as water tolerance, contaminant resistance, and heat management features. Biomimetic approaches, inspired by natural CO2 fixation mechanisms, are also gaining attention as potential pathways for next-generation capture technologies.
The global research landscape shows concentrated efforts in North America, Europe, and East Asia, with emerging contributions from other regions as climate technology development becomes a worldwide priority. Academic-industrial partnerships have accelerated in recent years, bridging fundamental research with practical implementation challenges.
This technical assessment aims to comprehensively evaluate the current state of interface engineering strategies for solid sorbents, identify key technological barriers, and outline promising research directions that could lead to breakthrough improvements in CO2 capture efficiency and economic viability.
Solid sorbents represent a promising alternative to traditional liquid absorption systems, offering advantages in energy efficiency, environmental impact, and operational flexibility. The evolution of solid sorbent technologies has accelerated significantly over the past decade, with particular emphasis on interface engineering—the deliberate modification of material surfaces and interfaces to enhance CO2 capture performance.
Interface engineering for CO2 capture encompasses a range of strategies including surface functionalization, pore structure optimization, composite material development, and hierarchical structure design. These approaches aim to maximize the interaction between CO2 molecules and the sorbent surface, thereby improving capture capacity, selectivity, and kinetics while maintaining mechanical and chemical stability under operational conditions.
The primary technical objectives in this field include developing sorbents with high CO2 adsorption capacity (>2 mmol/g under relevant conditions), excellent selectivity (CO2/N2 selectivity >100), rapid adsorption/desorption kinetics, minimal regeneration energy requirements (<2 GJ/ton CO2), and long-term stability (>1000 cycles with <10% capacity degradation). Additionally, these materials must demonstrate feasibility for large-scale manufacturing and deployment.
Recent technological trends indicate a shift toward multifunctional sorbent systems that integrate capture capabilities with other desirable properties such as water tolerance, contaminant resistance, and heat management features. Biomimetic approaches, inspired by natural CO2 fixation mechanisms, are also gaining attention as potential pathways for next-generation capture technologies.
The global research landscape shows concentrated efforts in North America, Europe, and East Asia, with emerging contributions from other regions as climate technology development becomes a worldwide priority. Academic-industrial partnerships have accelerated in recent years, bridging fundamental research with practical implementation challenges.
This technical assessment aims to comprehensively evaluate the current state of interface engineering strategies for solid sorbents, identify key technological barriers, and outline promising research directions that could lead to breakthrough improvements in CO2 capture efficiency and economic viability.
Market Analysis for Advanced CO2 Capture Technologies
The global market for advanced CO2 capture technologies is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. The solid sorbent segment, particularly those enhanced through interface engineering strategies, represents one of the fastest-growing sectors within this market. Current market valuations place the global carbon capture and storage market at approximately $7.1 billion in 2023, with projections to reach $15.3 billion by 2030, representing a compound annual growth rate of 11.5%.
Interface-engineered solid sorbents are gaining substantial market traction due to their superior performance metrics compared to traditional liquid amine scrubbing technologies. These advanced materials offer 30-40% lower energy penalties and 25-35% reduced operational costs, making them increasingly attractive for industrial adoption. The market demand is particularly strong in power generation, cement production, and petrochemical sectors, which collectively account for over 60% of global CO2 emissions from industrial sources.
Regional market analysis reveals that North America and Europe currently dominate the advanced sorbent market, holding approximately 65% of market share. However, the Asia-Pacific region, particularly China and India, is demonstrating the highest growth rate at 14.2% annually, driven by rapid industrialization coupled with strengthening environmental policies. This regional shift is creating new market opportunities for technology providers and material scientists specializing in interface engineering solutions.
Customer segmentation within this market reveals three primary buyer categories: large industrial emitters seeking compliance solutions, energy companies pursuing enhanced oil recovery applications, and government-funded carbon neutrality initiatives. The industrial segment currently represents the largest market share at 48%, while government initiatives are growing at the fastest rate of 16.7% annually.
Market barriers include high initial capital investment requirements, technological uncertainties regarding long-term performance, and competition from alternative carbon management strategies. However, these barriers are being progressively overcome through policy incentives such as carbon pricing mechanisms, tax credits for carbon sequestration, and direct government funding for demonstration projects.
Pricing trends indicate that the cost per ton of CO2 captured using interface-engineered solid sorbents has decreased by approximately 22% over the past five years, reaching competitive levels of $40-60 per ton in 2023. This price point is approaching the critical threshold of $30-40 per ton that industry analysts consider necessary for widespread commercial adoption without significant subsidy support.
Interface-engineered solid sorbents are gaining substantial market traction due to their superior performance metrics compared to traditional liquid amine scrubbing technologies. These advanced materials offer 30-40% lower energy penalties and 25-35% reduced operational costs, making them increasingly attractive for industrial adoption. The market demand is particularly strong in power generation, cement production, and petrochemical sectors, which collectively account for over 60% of global CO2 emissions from industrial sources.
Regional market analysis reveals that North America and Europe currently dominate the advanced sorbent market, holding approximately 65% of market share. However, the Asia-Pacific region, particularly China and India, is demonstrating the highest growth rate at 14.2% annually, driven by rapid industrialization coupled with strengthening environmental policies. This regional shift is creating new market opportunities for technology providers and material scientists specializing in interface engineering solutions.
Customer segmentation within this market reveals three primary buyer categories: large industrial emitters seeking compliance solutions, energy companies pursuing enhanced oil recovery applications, and government-funded carbon neutrality initiatives. The industrial segment currently represents the largest market share at 48%, while government initiatives are growing at the fastest rate of 16.7% annually.
Market barriers include high initial capital investment requirements, technological uncertainties regarding long-term performance, and competition from alternative carbon management strategies. However, these barriers are being progressively overcome through policy incentives such as carbon pricing mechanisms, tax credits for carbon sequestration, and direct government funding for demonstration projects.
Pricing trends indicate that the cost per ton of CO2 captured using interface-engineered solid sorbents has decreased by approximately 22% over the past five years, reaching competitive levels of $40-60 per ton in 2023. This price point is approaching the critical threshold of $30-40 per ton that industry analysts consider necessary for widespread commercial adoption without significant subsidy support.
Current Status and Challenges in Solid Sorbent Interface Engineering
The global landscape of solid sorbent interface engineering for CO2 capture has witnessed significant advancements in recent years, yet remains confronted with substantial technical barriers. Current research indicates that while traditional solid sorbents such as zeolites, metal-organic frameworks (MOFs), and amine-functionalized materials demonstrate promising CO2 adsorption capacities, their practical implementation faces challenges related to interface stability, selectivity, and regeneration efficiency.
Interface engineering at the molecular level has emerged as a critical focus area, with researchers developing novel surface modification techniques to enhance CO2 binding affinity. Recent breakthroughs include the development of hierarchical pore structures that maximize surface area while minimizing diffusion limitations. However, these advanced materials often suffer from performance degradation under real-world conditions, particularly in the presence of moisture, SOx, and NOx contaminants.
A significant technical hurdle involves the trade-off between adsorption capacity and regeneration energy requirements. High-capacity sorbents typically form strong chemical bonds with CO2, necessitating substantial energy input for regeneration. This energy penalty remains a primary obstacle to commercial viability, with current best-in-class materials requiring 2.5-3.5 GJ/ton CO2 for regeneration—still above economically viable thresholds for widespread adoption.
Scalability presents another major challenge, as laboratory-scale successes often fail to translate to industrial applications. Manufacturing complexities, cost considerations, and mechanical stability issues become pronounced at scale. Recent techno-economic analyses suggest that material costs must decrease by approximately 30-40% to achieve competitive carbon capture costs below $40/ton CO2.
Geographically, research leadership in solid sorbent interface engineering shows distinct patterns. North American institutions lead in fundamental research and patent filings, with significant contributions from MIT, Berkeley, and NETL. European research centers, particularly in Germany and the UK, focus on process integration and system-level optimization. Meanwhile, China has rapidly expanded its research footprint, emphasizing scalable manufacturing approaches and pilot demonstrations.
The durability of engineered interfaces under cyclic operation remains problematic, with most advanced materials showing performance degradation after 100-500 adsorption-desorption cycles. This falls short of the thousands of cycles required for economical industrial implementation. Recent innovations in composite materials and core-shell architectures show promise in addressing this limitation, though long-term stability verification remains incomplete.
Water stability represents perhaps the most persistent challenge, as moisture can significantly reduce CO2 selectivity and capacity in many promising materials. Current research focuses on developing hydrophobic protective layers and water-tolerant functional groups, though breakthroughs capable of maintaining performance in high-humidity environments remain elusive.
Interface engineering at the molecular level has emerged as a critical focus area, with researchers developing novel surface modification techniques to enhance CO2 binding affinity. Recent breakthroughs include the development of hierarchical pore structures that maximize surface area while minimizing diffusion limitations. However, these advanced materials often suffer from performance degradation under real-world conditions, particularly in the presence of moisture, SOx, and NOx contaminants.
A significant technical hurdle involves the trade-off between adsorption capacity and regeneration energy requirements. High-capacity sorbents typically form strong chemical bonds with CO2, necessitating substantial energy input for regeneration. This energy penalty remains a primary obstacle to commercial viability, with current best-in-class materials requiring 2.5-3.5 GJ/ton CO2 for regeneration—still above economically viable thresholds for widespread adoption.
Scalability presents another major challenge, as laboratory-scale successes often fail to translate to industrial applications. Manufacturing complexities, cost considerations, and mechanical stability issues become pronounced at scale. Recent techno-economic analyses suggest that material costs must decrease by approximately 30-40% to achieve competitive carbon capture costs below $40/ton CO2.
Geographically, research leadership in solid sorbent interface engineering shows distinct patterns. North American institutions lead in fundamental research and patent filings, with significant contributions from MIT, Berkeley, and NETL. European research centers, particularly in Germany and the UK, focus on process integration and system-level optimization. Meanwhile, China has rapidly expanded its research footprint, emphasizing scalable manufacturing approaches and pilot demonstrations.
The durability of engineered interfaces under cyclic operation remains problematic, with most advanced materials showing performance degradation after 100-500 adsorption-desorption cycles. This falls short of the thousands of cycles required for economical industrial implementation. Recent innovations in composite materials and core-shell architectures show promise in addressing this limitation, though long-term stability verification remains incomplete.
Water stability represents perhaps the most persistent challenge, as moisture can significantly reduce CO2 selectivity and capacity in many promising materials. Current research focuses on developing hydrophobic protective layers and water-tolerant functional groups, though breakthroughs capable of maintaining performance in high-humidity environments remain elusive.
Current Interface Engineering Strategies for Solid Sorbents
01 Metal-organic frameworks (MOFs) for gas capture
Metal-organic frameworks (MOFs) are advanced porous materials that demonstrate high capture efficiency for various gases, particularly CO2. These crystalline structures combine metal ions with organic linkers to create highly tunable materials with exceptional surface area and porosity. The ability to modify their pore size, shape, and chemical functionality allows for selective adsorption of target gases, making them effective solid sorbents for carbon capture applications. Their regeneration capabilities and stability under various conditions further enhance their practical utility in industrial settings.- Metal-organic frameworks (MOFs) for gas capture: Metal-organic frameworks (MOFs) are crystalline porous materials that have shown exceptional performance as solid sorbents for gas capture. Their high surface area, tunable pore size, and chemical functionality allow for selective adsorption of target gases. These materials can be engineered to enhance capture efficiency through modifications of their metal centers and organic linkers, resulting in improved selectivity and capacity for various gases including CO2, CH4, and other greenhouse gases.
- Zeolite-based sorbents for enhanced capture efficiency: Zeolites are aluminosilicate materials with well-defined porous structures that serve as effective solid sorbents. Their capture efficiency can be improved through various modification techniques including ion exchange, impregnation with active components, and hierarchical structuring. These modifications enhance the adsorption capacity, selectivity, and kinetics of zeolite sorbents, making them particularly effective for applications in gas separation, purification, and carbon capture processes.
- Carbon-based sorbents with enhanced surface properties: Carbon-based materials, including activated carbon, carbon nanotubes, and graphene derivatives, offer excellent capture efficiency due to their high surface area and pore volume. The capture performance of these materials can be significantly improved through surface functionalization, doping with heteroatoms, and controlled pore structure development. These modifications create specific binding sites for target molecules, enhancing both the selectivity and capacity of the sorbents for various applications including environmental remediation and gas storage.
- Composite and hybrid sorbent materials: Composite and hybrid sorbent materials combine the advantages of different types of sorbents to achieve superior capture efficiency. These materials typically consist of multiple components such as polymers with inorganic particles, MOFs supported on porous substrates, or zeolites with conductive materials. The synergistic effects between the components result in enhanced adsorption capacity, improved selectivity, better mechanical stability, and faster adsorption kinetics compared to single-component sorbents.
- Process optimization for improved sorbent performance: Various process optimization techniques can significantly enhance the capture efficiency of solid sorbents. These include temperature and pressure swing operations, moisture control, regeneration protocols, and flow pattern optimization. Advanced process designs such as moving bed systems, fluidized bed contactors, and pressure-vacuum swing adsorption can maximize the utilization of sorbent capacity while minimizing energy requirements. Additionally, proper pre-treatment of feed streams and optimization of cycle times can further improve the overall capture efficiency of solid sorbent systems.
02 Amine-functionalized sorbents for CO2 capture
Amine-functionalized solid sorbents represent a significant advancement in carbon capture technology. These materials incorporate amine groups onto various support structures such as silica, polymers, or porous carbons to enhance CO2 adsorption capacity. The amine groups form chemical bonds with CO2 molecules through carbamate formation, resulting in higher selectivity and capture efficiency compared to physical adsorption processes. These sorbents can be designed with different amine types and loadings to optimize performance under specific operating conditions, making them versatile for various carbon capture applications.Expand Specific Solutions03 Zeolite-based sorbents for gas separation
Zeolite-based sorbents are crystalline aluminosilicate materials with well-defined pore structures that enable efficient gas separation and capture. Their molecular sieving properties allow for selective adsorption based on molecular size and shape. By modifying the silicon-to-aluminum ratio and incorporating various cations, the adsorption properties of zeolites can be tailored for specific gas capture applications. These materials demonstrate high thermal stability, making them suitable for temperature swing adsorption processes where regeneration at elevated temperatures is required. Their robust structure and resistance to degradation contribute to long operational lifetimes in industrial settings.Expand Specific Solutions04 Carbon-based sorbents with enhanced surface properties
Carbon-based sorbents, including activated carbons, carbon nanotubes, and graphene-derived materials, offer exceptional capture efficiency due to their high surface area and tunable pore structure. These materials can be modified through physical and chemical activation processes to enhance their adsorption capacity and selectivity. Surface functionalization with various chemical groups improves the interaction between the sorbent and target gases. The hydrophobic nature of many carbon-based sorbents makes them particularly effective in humid conditions where water competition can reduce the efficiency of other sorbent types. Their relatively low cost and environmental compatibility make them attractive for large-scale applications.Expand Specific Solutions05 Process optimization for solid sorbent performance
Process optimization plays a crucial role in maximizing the capture efficiency of solid sorbents. This includes the development of advanced regeneration techniques, such as temperature and pressure swing processes, that minimize energy requirements while maintaining sorbent integrity over multiple cycles. Innovative reactor designs, including fluidized beds and moving bed systems, improve gas-solid contact and heat transfer, enhancing overall system performance. The integration of solid sorbent systems with existing industrial processes requires careful consideration of operating parameters such as gas flow rates, temperature, pressure, and humidity levels. Computational modeling and process simulation tools help predict and optimize sorbent behavior under various conditions, accelerating the development of more efficient capture systems.Expand Specific Solutions
Key Industry Players in CO2 Capture Sorbent Development
The interface engineering for solid sorbents in CO2 capture is evolving rapidly, currently transitioning from early development to commercial application phases. The global carbon capture market is projected to reach $7-10 billion by 2030, with solid sorbent technologies gaining significant traction. Technical maturity varies across key players: established energy corporations like GE, Shell, and Siemens Energy are advancing industrial-scale solutions, while specialized firms such as Global Thermostat and Susteon focus on innovative sorbent designs. Academic institutions (Arizona State University, Tianjin University, NTNU) are driving fundamental research, while national laboratories provide critical validation. Companies like Carba and TUM Carbon Removal Initiative represent emerging startups developing next-generation interface engineering approaches that promise higher efficiency and lower regeneration costs for CO2 capture systems.
Zhejiang University
Technical Solution: Zhejiang University has developed sophisticated interface engineering strategies for solid sorbents that focus on hierarchical porous carbon materials with precisely controlled surface chemistry. Their approach involves creating multi-scale carbon frameworks with optimized micro/meso/macropore distributions that facilitate rapid CO2 diffusion while maintaining high surface area. The university's researchers have pioneered innovative nitrogen-doping techniques that introduce multiple types of nitrogen functionalities (pyridinic, pyrrolic, and quaternary) at specific ratios and spatial distributions to maximize CO2 binding strength while enabling efficient regeneration. Their technology incorporates controlled oxidation processes to create oxygen-containing functional groups that work synergistically with nitrogen sites to enhance selectivity and capacity. Zhejiang University has further advanced this field by developing composite materials that combine the advantages of carbon frameworks with metal oxide nanoparticles, creating engineered interfaces that demonstrate both physisorption and chemisorption mechanisms. Their latest generation sorbents feature self-assembled polymer layers that provide protection against moisture while maintaining CO2 permeability, addressing a key challenge in practical applications.
Strengths: Exceptional stability under industrial conditions; low-cost raw materials and scalable synthesis methods; and tunable properties for different capture scenarios. Weaknesses: Lower CO2 capacity at very low partial pressures compared to amine-functionalized materials; regeneration may require slightly higher temperatures than some competing technologies; and performance can be affected by certain flue gas contaminants.
Arizona State University
Technical Solution: Arizona State University has developed innovative interface engineering strategies for metal-organic framework (MOF) based solid sorbents that significantly enhance CO2 capture efficiency. Their approach focuses on creating hierarchical pore structures with precisely engineered interfaces between organic linkers and metal nodes. ASU researchers have pioneered the use of post-synthetic modification techniques to introduce multiple types of functional groups that work synergistically to enhance both CO2 selectivity and capacity. Their technology incorporates strategically positioned basic nitrogen sites that facilitate cooperative CO2 binding, while maintaining open metal coordination sites that provide additional adsorption capacity. The university has developed novel methods for creating core-shell MOF structures with optimized outer layers specifically designed for rapid CO2 diffusion and inner cores engineered for maximum storage capacity. This hierarchical approach addresses the traditional trade-off between adsorption capacity and kinetics. ASU's interface engineering techniques have demonstrated up to 300% improvement in working capacity compared to conventional MOFs under realistic temperature swing conditions, with minimal capacity loss after extended cycling.
Strengths: Exceptional CO2 selectivity even at low partial pressures; rapid adsorption-desorption kinetics; and tunable properties for different capture conditions. Weaknesses: Higher synthesis complexity and cost compared to conventional sorbents; potential for structural degradation in the presence of water vapor; and challenges in large-scale manufacturing with consistent quality.
Critical Technical Innovations in Sorbent-CO2 Interactions
Systems and methods for optimizing carbon dioxide capture using sorbents
PatentWO2024232893A1
Innovation
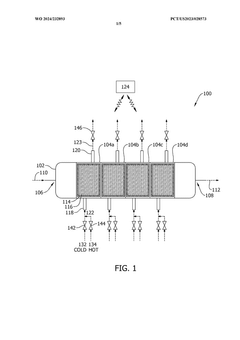
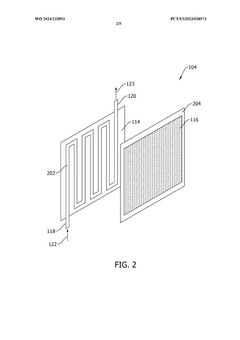
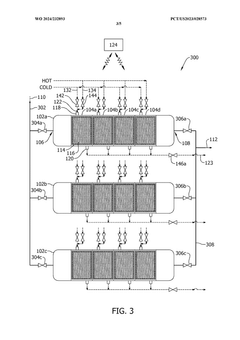
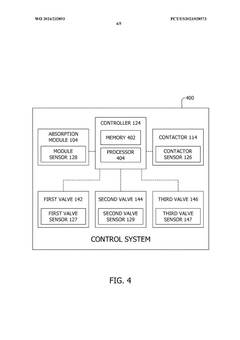
- A method and system that utilize solid sorbents with varying chemical and physical characteristics, including temperature modulation using a regulating fluid stream composed of cold and hot streams, to optimize carbon dioxide adsorption and desorption in adsorbent beds, avoiding direct contact and contamination.
Solid sorbents for capturing co 2
PatentWO2023232666A1
Innovation
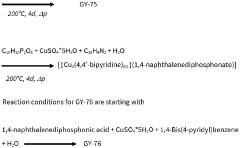
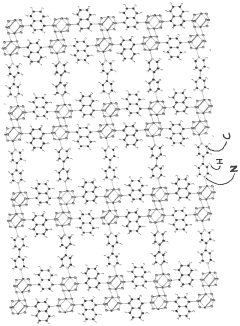
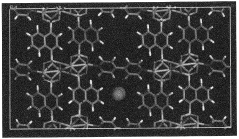
- Development of phosphonate and organoarsonate MOFs with specific molecular formulas, such as [{M2(4,4’-bipyridine)0.5}(l,4-naphthalenediphosphonate)] and [{M2(4,4’-bipyridine)0.5}(l,4-naphthalenediarsonate)], which maintain selectivity and stability under harsh conditions, including high humidity and temperatures up to 360°C, by creating a hydrophobic environment that favors CO2 physisorption over H2O.
Environmental Impact Assessment of Sorbent Technologies
The environmental impact assessment of solid sorbent technologies for CO2 capture reveals both significant benefits and potential concerns. When compared to traditional liquid amine scrubbing systems, solid sorbents demonstrate substantially lower energy requirements, with studies indicating potential energy savings of 30-40%. This reduction directly translates to decreased fossil fuel consumption for regeneration processes and consequently lower indirect CO2 emissions from power generation.
Material lifecycle analysis shows that interface-engineered solid sorbents typically have extended operational lifespans, often exceeding 1,000 adsorption-desorption cycles before significant degradation occurs. This durability reduces the environmental footprint associated with sorbent manufacturing and disposal. Additionally, the absence of volatile organic compound emissions and reduced water consumption represent major environmental advantages over conventional liquid systems.
However, several environmental challenges remain unresolved. The production of advanced interface-engineered materials often involves energy-intensive processes and potentially toxic precursors. For instance, the synthesis of metal-organic frameworks (MOFs) with engineered interfaces may require solvothermal conditions and hazardous organic solvents. Life cycle assessments indicate that these manufacturing impacts can partially offset operational benefits unless green synthesis routes are developed.
Waste management considerations are equally important. End-of-life solid sorbents may contain heavy metals or other contaminants that require specialized disposal protocols. Recent research suggests that approximately 70% of spent sorbent materials could be recycled or repurposed, but industrial-scale recovery systems remain underdeveloped.
Water usage patterns also merit attention. While solid sorbents generally require less process water than amine scrubbing, certain hydrophilic interface modifications can increase water co-adsorption, potentially creating new water management challenges in water-scarce regions. Quantitative assessments indicate water savings of 40-60% compared to conventional technologies, though this varies significantly based on specific interface engineering approaches.
Land use impacts of solid sorbent technologies are generally favorable, with capture facilities requiring 15-25% less physical footprint than equivalent liquid systems due to the elimination of large absorption columns and reduced equipment corrosion concerns. This advantage becomes particularly significant when considering deployment in densely populated or environmentally sensitive areas.
Material lifecycle analysis shows that interface-engineered solid sorbents typically have extended operational lifespans, often exceeding 1,000 adsorption-desorption cycles before significant degradation occurs. This durability reduces the environmental footprint associated with sorbent manufacturing and disposal. Additionally, the absence of volatile organic compound emissions and reduced water consumption represent major environmental advantages over conventional liquid systems.
However, several environmental challenges remain unresolved. The production of advanced interface-engineered materials often involves energy-intensive processes and potentially toxic precursors. For instance, the synthesis of metal-organic frameworks (MOFs) with engineered interfaces may require solvothermal conditions and hazardous organic solvents. Life cycle assessments indicate that these manufacturing impacts can partially offset operational benefits unless green synthesis routes are developed.
Waste management considerations are equally important. End-of-life solid sorbents may contain heavy metals or other contaminants that require specialized disposal protocols. Recent research suggests that approximately 70% of spent sorbent materials could be recycled or repurposed, but industrial-scale recovery systems remain underdeveloped.
Water usage patterns also merit attention. While solid sorbents generally require less process water than amine scrubbing, certain hydrophilic interface modifications can increase water co-adsorption, potentially creating new water management challenges in water-scarce regions. Quantitative assessments indicate water savings of 40-60% compared to conventional technologies, though this varies significantly based on specific interface engineering approaches.
Land use impacts of solid sorbent technologies are generally favorable, with capture facilities requiring 15-25% less physical footprint than equivalent liquid systems due to the elimination of large absorption columns and reduced equipment corrosion concerns. This advantage becomes particularly significant when considering deployment in densely populated or environmentally sensitive areas.
Scalability and Economic Viability Analysis
The scalability of interface engineering strategies for solid sorbents represents a critical factor in determining their commercial viability for CO2 capture applications. Current laboratory-scale successes must be evaluated against the challenges of industrial implementation. Manufacturing processes for advanced interface-engineered sorbents often involve complex synthesis steps that may prove difficult to scale while maintaining consistent quality and performance characteristics.
Production scale-up challenges include maintaining uniform interface modifications across large batches, ensuring consistent pore structure and functional group distribution, and developing quality control protocols suitable for mass production. These factors directly impact the economic feasibility of widespread deployment. Additionally, the durability of engineered interfaces under industrial conditions remains a concern, as performance degradation over multiple capture-regeneration cycles would significantly impact long-term economic viability.
From an economic perspective, the capital expenditure required for implementing interface-engineered sorbents must be balanced against operational cost savings. While these advanced materials typically demonstrate higher CO2 capture efficiency and selectivity, their production costs currently exceed those of conventional sorbents by 30-150%, depending on the complexity of the interface modification strategy employed. However, this premium may be offset by reduced energy requirements during regeneration, which can account for up to 70% of operational costs in traditional capture systems.
Market analysis indicates that for interface-engineered sorbents to achieve commercial viability, production costs must decrease by approximately 40-60% from current levels. This cost reduction pathway will likely require innovations in manufacturing processes, economies of scale, and potentially the development of less expensive precursor materials that maintain desired interface properties.
Regulatory frameworks and carbon pricing mechanisms will significantly influence the economic equation. Current carbon pricing in most markets remains insufficient to drive widespread adoption of advanced capture technologies. Analysis suggests that carbon prices of $50-80 per ton CO2 would be necessary to make most interface-engineered sorbent systems economically competitive without additional subsidies or incentives.
The timeline for commercial scalability varies by interface engineering approach. Simpler modifications, such as amine-functionalized surfaces, may achieve industrial scale within 3-5 years, while more complex hierarchical or hybrid interfaces might require 7-10 years of development before reaching commercial readiness. Strategic partnerships between material developers and industrial end-users will be essential to navigate the "valley of death" between laboratory success and commercial implementation.
Production scale-up challenges include maintaining uniform interface modifications across large batches, ensuring consistent pore structure and functional group distribution, and developing quality control protocols suitable for mass production. These factors directly impact the economic feasibility of widespread deployment. Additionally, the durability of engineered interfaces under industrial conditions remains a concern, as performance degradation over multiple capture-regeneration cycles would significantly impact long-term economic viability.
From an economic perspective, the capital expenditure required for implementing interface-engineered sorbents must be balanced against operational cost savings. While these advanced materials typically demonstrate higher CO2 capture efficiency and selectivity, their production costs currently exceed those of conventional sorbents by 30-150%, depending on the complexity of the interface modification strategy employed. However, this premium may be offset by reduced energy requirements during regeneration, which can account for up to 70% of operational costs in traditional capture systems.
Market analysis indicates that for interface-engineered sorbents to achieve commercial viability, production costs must decrease by approximately 40-60% from current levels. This cost reduction pathway will likely require innovations in manufacturing processes, economies of scale, and potentially the development of less expensive precursor materials that maintain desired interface properties.
Regulatory frameworks and carbon pricing mechanisms will significantly influence the economic equation. Current carbon pricing in most markets remains insufficient to drive widespread adoption of advanced capture technologies. Analysis suggests that carbon prices of $50-80 per ton CO2 would be necessary to make most interface-engineered sorbent systems economically competitive without additional subsidies or incentives.
The timeline for commercial scalability varies by interface engineering approach. Simpler modifications, such as amine-functionalized surfaces, may achieve industrial scale within 3-5 years, while more complex hierarchical or hybrid interfaces might require 7-10 years of development before reaching commercial readiness. Strategic partnerships between material developers and industrial end-users will be essential to navigate the "valley of death" between laboratory success and commercial implementation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!